Abstract
Hepatocellular carcinoma (HCC) commonly arises in chronically inflamed livers, but may also provoke (anti-tumoral) immune responses. Using non-inflammatory diethylnitrosamine (DEN)-induced liver cancer in mice, we demonstrate that distinct axes of the adaptive immune system, which are also prognostic in human HCC, actively suppress hepatocarcinogenesis by controlling tumor formation and progression.
Keywords: adaptive immunity, DEN, liver cancer, mouse models, tumor
Hepatocellular carcinoma (HCC) is one of the most frequent malignancies worldwide. While cases of primary liver cancer in seemingly healthy livers do exist, hepatic cirrhosis and its underlying chronic inflammation is the major risk factor for HCC development. Our group has a long-standing interest in delineating inflammatory mechanisms in liver diseases with a focus on monocytes and macrophages.1,2 When we investigated altered functional properties of macrophages in humans, many immunological features in both blood and liver clearly distinguished between healthy volunteers, patients with chronic liver disease and with established cirrhosis,3 but distinct immunological features of cirrhotic patients with concomitant HCC were more subtle. Given the vast body of current literature concerning the crosstalk between cancer and the immune system, it was likely that liver cancer would affect the host’s immune system. However, those signals would be difficult to dissect in the presence of severe liver inflammation. We therefore decided to investigate immunological alterations induced by HCC in the diethylnitrosamine (DEN) model of liver cancer, whose most prominent weakness, the absence of a typical inflammation-carcinogenesis sequence, would tremendously favor our project.4 DEN injected 14 d after birth i.p. reliably induces liver tumors in male mice (detectable at 40 weeks of age) without a general, lasting liver inflammation and fibrosis.5 Although this feature is an obstacle in translating results from this model into human pathogenesis, it allowed to study effects of hepatic tumor growth on the immune system and vice versa without interference from underlying inflammation.
We thus established liver tumors in wildtype mice and four different knockout strains: D6−/−, Rag1−/−, Igh6−/− and MHCII−/− mice, each of which offered a unique immunologically relevant phenotype. Deficiency of the chemokine scavenger receptor D6 leads to enhanced accumulation of macrophages, Rag1−/− mice lack B and T cells. Igh6−/− mice have no B cells, MHC II-deficient mice lack functional CD4 T cells or dendritic cells.
In wildtype mice, we detected a significant hepatic inflammation in response to tumor progression, mirrored by the influx of leukocytes into the liver. This infiltrate predominantly consisted of macrophages and CD8+ T cells, which was well in accordance with the literature and our own observations from human HCC samples.4 Even before visible tumor nodules could be detected, an increase in liver chemokine levels (i.e., monocyte-attractant CCL2 and T-cell-attractive chemokines CCL3, CCL4, CCL5, CXCL9) was observed, which further rose along with growing tumor burden. Furthermore, tumor growth induced myeloid derived suppressor cells (MDSC) systemically.4
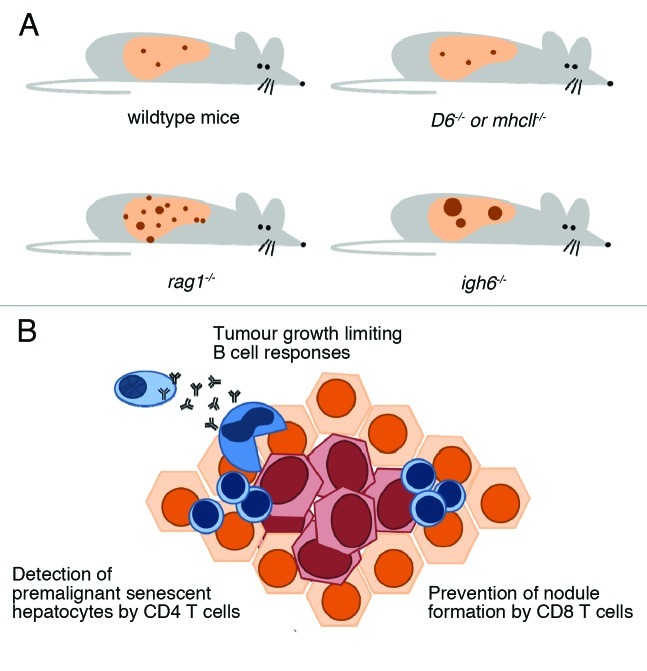
While an increased influx of macrophages into the livers in the D6−/− mice did not influence the phenotype regarding tumor burden in comparison to their wildtype counterparts, the lack of B and T cells in Rag1−/− mice dramatically increased the number of nodules (Fig. 1). Rag1−/− mice not only suffered higher tumor loads, but also a more aggressive phenotype with earlier nodule formation. The lack of B cells in the Igh6−/− mice related to increased individual neoplastic nodules and thereby also raised tumor burden. MHCII−/− mice, however, did not show a significantly altered phenotype,4 but when interpreting these results, one has to bear in mind the complex nature of their impaired immune system, with many cell populations affected by the disruption of MHC class II-based antigen presentation.
Figure 1. Functional role of anti-tumoral adaptive immunity in experimental liver cancer. (A) Diethylnitrosamine (DEN), injected i.p. fourteen days of age, reliably induced liver tumors in male mice. While a pronounced infiltration of macrophages in D6−/− mice or the knockout of MHC class II did not alter the phenotype, the absence of T and B cells in Rag1−/− mice caused aggressive tumor growth with earlier onset of nodule formation and greater numbers of tumors. The lack of B cells in Igh6−/− mice was accompanied by increased nodule size. (B) Taking the current literature into account, liver tumor provoked immune responses may be outlined as such: nodule formation is limited by CD8 and CD4 positive T cells, which eliminate (pre-)malignant cells and keep transformed cells in equilibrium. Established tumors are contained by B cell dependent mechanisms, possibly through Fc-gamma-receptor dependent M2-macrophages.
When taking a closer look at B cell functionality, tumor bearing wildtype mice displayed activated B cells as shown by induction of B cell activation related genes and by elevated antibody production specific for liver-expressed model antigen (ovalbumine). We concluded that adaptive immune mechanisms play an essential role in controlling tumor initiation and growth within the liver. Interestingly, liver cancer apparently promotes B cell activation, which in turn contributes to control established tumor nodules.
In order to translate our findings to human HCC, we analyzed gene array data sets from 139 human HCC samples6 with respect to pathways of innate and adaptive immunity (http://www.genome.jp.kegg). T and B cell related genes from these pathways clustered in HCC samples and defined distinct groups of favorable or poor prognosis.4 Downregulation of B cell related genes was associated with poor outcome, indicating that immunological mechanisms identified in the murine DEN model may also be relevant in human liver cancer.
Together, our findings and the current literature revealed important aspects on the complex role of the adaptive immune system in the establishment and growth of liver cancer (Fig. 1): On the one hand, adaptive immune mechanisms facilitate high cell turn over in inflamed livers by deleting e.g., infected hepatocytes and thereby boost the risk of malignant transformation. This is suggested by the abrogated tumor formation in inflammation-based liver cancer models through immune suppression.7 On the other hand, once cells are transformed, adaptive immune mechanisms play an important role in restricting liver cancer, as described in the concept of tumor equilibrium which is CD4 and CD8 T cell dependent8 and emphasized by the recent work of Kang et al.,9 which elucidated the critical role of CD4 T cells for detecting and clearing precancerous senescent hepatocytes. This is in accordance with our observations of enhanced numbers of neoplastic nodules in Rag1-deficient mice,4 because high cell turnover is not needed for malignant transformation in the genotoxic DEN-model and the amount of transformed cells giving rise to a tumor nodule is expected to be higher due to the lack of tumor suppressive immunological surveillance mechanisms. With respect to the role of B cells, one has to keep in mind that, despite a common presence of antibodies directed against tumor-associated antigens, B cells are widely regarded as tumor promoting. This function is thought to be facilitated by inhibition of Th1-mediated anti-tumor immunity and, depending on M2-like macrophages, through Fc-gamma receptor-dependent signaling.10 However, the latter B cell function was demonstrated in an inflammatory tumor model. Our data indicate that (hepatic) B cells are clearly activated in the tumor environment and exert suppressive functions on tumor growth in liver cancer, once a nodule is established.4
In conclusion, our work revealed that liver cancer provokes distinct adaptive immune responses, which functionally limit tumor formation and progression. Thus, future therapeutic approaches for HCC may not only target tumor-promoting inflammatory reactions, but should also augment HCC-induced anti-tumoral adaptive immune cell functions.
Glossary
Abbreviations:
- HCC
hepatocellular carcinoma
- DEN
diethylnitrosamine
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/20304
References
- 1.Karlmark KR, Weiskirchen R, Zimmermann HW, Gassler N, Ginhoux F, Weber C, et al. Hepatic recruitment of the inflammatory Gr1+ monocyte subset upon liver injury promotes hepatic fibrosis. Hepatology. 2009;50:261–74. doi: 10.1002/hep.22950. [DOI] [PubMed] [Google Scholar]
- 2.Zimmermann HW, Tacke F. Modification of chemokine pathways and immune cell infiltration as a novel therapeutic approach in liver inflammation and fibrosis. Inflamm Allergy Drug Targets. 2011;10:509–36. doi: 10.2174/187152811798104890. [DOI] [PubMed] [Google Scholar]
- 3.Zimmermann HW, Seidler S, Nattermann J, Gassler N, Hellerbrand C, Zernecke A, et al. Functional contribution of elevated circulating and hepatic non-classical CD14CD16 monocytes to inflammation and human liver fibrosis. PLoS One. 2010;5:e11049. doi: 10.1371/journal.pone.0011049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schneider C, Teufel A, Yevsa T, Staib F, Hohmeyer A, Walenda G et. al. Adaptive immunity suppresses formation and progression of diethylnitrosamine-induced liver cancer. Gut 2012; Epub ahead of press; PMID: 22267597. [DOI] [PMC free article] [PubMed]
- 5.Newell P, Villanueva A, Friedman SL, Koike K, Llovet JM. Experimental models of hepatocellular carcinoma. J Hepatol. 2008;48:858–79. doi: 10.1016/j.jhep.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee JS, Chu IS, Heo J, Calvisi DF, Sun Z, Roskams T, et al. Classification and prediction of survival in hepatocellular carcinoma by gene expression profiling. Hepatology. 2004;40:667–76. doi: 10.1002/hep.20375. [DOI] [PubMed] [Google Scholar]
- 7.Haybaeck J, Zeller N, Wolf MJ, Weber A, Wagner U, Kurrer MO, et al. A lymphotoxin-driven pathway to hepatocellular carcinoma. Cancer Cell. 2009;16:295–308. doi: 10.1016/j.ccr.2009.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koebel CM, Vermi W, Swann JB, Zerafa N, Rodig SJ, Old LJ, et al. Adaptive immunity maintains occult cancer in an equilibrium state. Nature. 2007;450:903–7. doi: 10.1038/nature06309. [DOI] [PubMed] [Google Scholar]
- 9.Kang TW, Yevsa T, Woller N, Hoenicke L, Wuestefeld T, Dauch D, et al. Senescence surveillance of pre-malignant hepatocytes limits liver cancer development. Nature. 2011;479:547–51. doi: 10.1038/nature10599. [DOI] [PubMed] [Google Scholar]
- 10.Andreu P, Johansson M, Affara NI, Pucci F, Tan T, Junankar S, et al. FcRgamma activation regulates inflammation-associated squamous carcinogenesis. Cancer Cell. 2010;17:121–34. doi: 10.1016/j.ccr.2009.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]