Abstract
To improve multimodal glioblastoma treatment strategies, it appears useful to integrate a selective inhibitor of the TβR-I kinase, which may be able to potentiate radiation responses by increasing apoptosis and cancer-stem-like cell targeting while blocking DNA damage repair, invasion, mesenchymal transition and angiogenesis.1
Keywords: TGFβ, combination therapy, glioblastoma, radiotherapy, stem cells
Transforming growth factor β (TGF-β) is recognized as a tumor-promoting mediator in glioblastoma. In a recently published paper, we show that the combination of radiotherapy and blocking TGF-β signaling is an effective combination in terms of slowing down orthotopic glioblastoma growth and prolonging survival of mice.1 While we used LY2109761 to characterize the effect of inhibiting TGF-β in this preclinical research paper, a similar compound (LY2157299 monohydrate) is currently being clinically investigated in patients receiving chemo radiation with temozolomide (TMZ). LY2109761 is a specific inhibitor of the serine/threonine kinase associated with the TGF-β type I receptor (TβRI) and thus, blocks signals transmitted by the TGF-βRI. LY2157299 monohydrate (LY2157299) is a similar serine/threonine kinase inhibitor which appears to have a safe profile in patients with malignant glioma.2 The immunomodulatory effect of LY2157299 has not been published, but ongoing work will be released at the end of the First-in-Human Dose Study of the compound LY2157299.
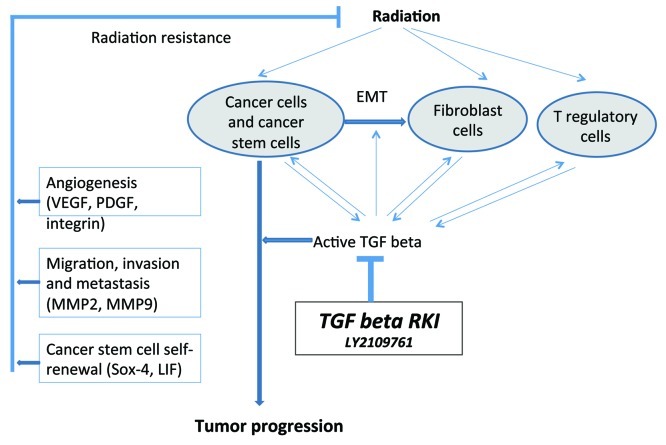
Glioblastoma multiforme (GBM) continues to be the most common primary malignant brain tumor in adults and carries a dismal prognosis with median survival of 14.6 mo. Virtually all patients suffer tumor recurrence despite the therapeutic efforts based on the aggressive conventional anticancer therapies, emphasizing the treatment resistant nature of GBMs. A number of studies in recent years have found that response to radiation therapy in various cancers may be improved when certain growth factors are blocked at the same time. Glioblastoma cells often produce large amounts of TGF-β, a family of polypeptides that regulates a wide variety of biological functions including cell proliferation, survival, apoptosis and immunosurveillance. High levels of TGF-β in these tumors or blood are correlated with particularly aggressive growth and a poor prognosis.3 TGF-β also seems to support the self-renewal capability of glioblastoma stem cells,4 a crucial subset of tumor cells that are supposed to be responsible for both refractory to most traditional therapies and capable of regenerating the tumor following treatment. Indeed, we found in glioblastoma stem cells isolated from human GBM surgical samples, that TGF-β signaling blocking by LY2109761 reduced the self-renewal capability of tumor stem cells and significantly reduced cell proliferation and clonogenic survival. Interestingly, when combined with ionizing radiation, which is a mainstay of glioblastoma treatment in patients, the antitumor effects of TGF-β blockade + radiotherapy were supra-additive. When CD133+ glioblastoma stem cells were injected into immunodeficient mice, large, highly infiltrative and vascularized tumors developed. Both LY2109761 and radiotherapy induced a marked survival benefit in mice, which was enhanced if both treatments were given concurrently. Moreover, tissue studies and magnetic resonance imaging showed that the combination therapy reduced tumor growth, reduced invasiveness, and reduced tumor angiogenesis. Paradoxically, radiation therapy appeared to be able to provoke aggressive tumor behavior, while LY2109761 prevented this unwanted interaction with radiation (Fig 1).
Figure 1. Schematic diagram of TGF-β relevant interactions and adaptive responses of radiation, tumor cells and T regulatory cells in the context of the potential use of TGF-β blockade for the treatment of tumors in combination with radiotherapy.
Epithelial to mesenchymal transition (EMT) is a hypothesized program characterized by loss of cell adhesion, repression of E-cadherin expression, and increased cell mobility. The mesenchymal change of promoting invasion, treatment response and even cancer stem cell function may play a fundamental role for human carcinoma and GBM invasion.5 TGF-β is considered a master regulator of EMT in carcinoma. Strikingly, we found that the blockade of TGF-β signaling using LY2109761 markedly reduced the expression of mesenchymal markers in glioblastoma.1 It is possible that the inhibitory effect on mesenchymal change by TGF-β signal inhibition contributes to its anti-migratory capacity and subsequently to its enhancement of treatment response.
Glioblastoma in humans are also highly angiogenic tumors. Although we found that LY2109761 reduced newly formed blood vessels, the mechanisms of the antiangiogenicity are less understood. In addition to recruiting vessels from outside, GBM may produce endothelial cells for vessel formation by transdifferentiation from stem-like cells into endothelial cells, thereby generating tumor vasculature.6 This phenomenon describes a novel link between glioblastoma stem-like cells and endothelial cells and a yet incomplete understood new mechanism for tumor vascularization. Our present work indicates that TGF-β signaling may play a critical role both in the regulation of glioblastoma stem-like cells function and angiogenesis. In light of the published literature, it seems possible that TGF-β signaling is involved in the regulation of endothelial cell production derived from glioblastoma stem-like cells.
Lastly, the immunomodulatory effect of TGF-β and its isoforms have long been recognized in glioblastoma, especially their effects on T regulatory cells.7 The isoform TGF-β2 had been originally described as “glioblastoma-derived T-cell suppressor factor,” which is associated with an immuno-suppressed status in patients and thus responsible for loss of tumor immune surveillance. Only recently, the role of T regulatory cell activation by stem cells has been recognized.8 Because T regulatory cells produce TGF-β and are susceptible to TGF-β activation, TGF-β blockers may reduce the T regulatory cell population and enhance the cytotoxic T cell response to glioblastoma. Because our models were based on standard murine xenograft models, in which the immune response mechanism is severely impaired, future studies in immunocompetent mice may further expand our observation on the use of TβR-I kinase inhibitors.
Together, we conclude that a selective inhibitor of the TβR-I kinase can potentiate radiation responses in glioblastoma by increasing apoptosis and CSLC targeting while blocking DNA damage repair, invasion, mesenchymal transition and angiogenesis. Hence, our preclinical results including microarray-based gene expression studies are encouraging the clinical investigation of such TβR-I kinase inhibitors in patients, especially when they are integrated with multimodal therapy regimens containing chemotherapy.9 The immunological response should also be evaluated in patients to further understand the immunomodulatory effect of such TβR-I kinase inhibitors.
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/19789
References
- 1.Zhang M, Kleber S, Röhrich M, Timke C, Han N, Tuettenberg J, et al. Blockade of TGF-β signaling by the TGFβR-I kinase inhibitor LY2109761 enhances radiation response and prolongs survival in glioblastoma. Cancer Res. 2011;71:7155–67. doi: 10.1158/0008-5472.CAN-11-1212. [DOI] [PubMed] [Google Scholar]
- 2.Jordi Rodon, Jose Baselga, Emiliano Calvo, Joan Seoane, Irene Brana, Elisabet Sicart, Ivelina Gueorguieva, Ann Cleverly, Michael Lahn, N. Sada Pillay, Matthias Holdoff, Jaishri Blakeley and Michael Carducci. First Human Dose (FHD) Study of the oral transforming growth factor-beta (TGF-β) receptor I kinase inhibitor LY2157299 in patients with treatment-refractory malignant glioma. ASCO MEETING ABSTRACTS, June 9, 2011:3011.
- 3.Bruna A, Darken RS, Rojo F, Ocaña A, Peñuelas S, Arias A, et al. High TGFbeta-Smad activity confers poor prognosis in glioma patients and promotes cell proliferation depending on the methylation of the PDGF-B gene. Cancer Cell. 2007;11:147–60. doi: 10.1016/j.ccr.2006.11.023. [DOI] [PubMed] [Google Scholar]
- 4.Peñuelas S, Anido J, Prieto-Sánchez RM, Folch G, Barba I, Cuartas I, et al. TGF-beta increases glioma-initiating cell self-renewal through the induction of LIF in human glioblastoma. Cancer Cell. 2009;15:315–27. doi: 10.1016/j.ccr.2009.02.011. [DOI] [PubMed] [Google Scholar]
- 5.Phillips HS, Kharbanda S, Chen R, Forrest WF, Soriano RH, Wu TD, et al. Molecular subclasses of high-grade glioma predict prognosis, delineate a pattern of disease progression, and resemble stages in neurogenesis. Cancer Cell. 2006;9:157–73. doi: 10.1016/j.ccr.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 6.Wang R, Chadalavada K, Wilshire J, Kowalik U, Hovinga KE, Geber A, et al. Glioblastoma stem-like cells give rise to tumour endothelium. Nature. 2010;468:829–33. doi: 10.1038/nature09624. [DOI] [PubMed] [Google Scholar]
- 7.Sonabend AM, Rolle CE, Lesniak MS. The role of regulatory T cells in malignant glioma. Anticancer Res. 2008;28(2B):1143–50. [PubMed] [Google Scholar]
- 8.Wei J, Barr J, Kong LY, Wang Y, Wu A, Sharma AK, et al. Glioma-associated cancer-initiating cells induce immunosuppression. Clin Cancer Res. 2010;16:461–73. doi: 10.1158/1078-0432.CCR-09-1983. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 9.Zhang M, Herion TW, Timke C, Han N, Hauser K, Weber KJ, et al. Trimodal glioblastoma treatment consisting of concurrent radiotherapy, temozolomide, and the novel TGF-β receptor I kinase inhibitor LY2109761. Neoplasia. 2011;13:537–49. doi: 10.1593/neo.11258. [DOI] [PMC free article] [PubMed] [Google Scholar]