Abstract
Regulatory T cells (Tregs) represent a major obstacle of cancer immunotherapy. We reviewed here our discovery that Class I histone deacetylase (HDAC) inhibition can functionally target Tregs and help break the immune tolerance. We also discuss the effects of different classes of HDAC inhibitors on Tregs and the underline mechanisms, which may have a direct impact on designing cancer immunotherapy trials involving HDAC inhibitors.
Keywords: Foxp3, HDAC inhibitors, STAT3, immunotherapy, regulatory T cells
During the last decade, immunotherapy has become one of the most attractive and extensively studied approaches for the treatment of solid tumors. However, current clinical studies of cancer immunotherapy still show very limited efficacy. Immune tolerance to cancer has been shown to be a major barrier. Tumor growth and stromal establishment modulate not only the local microenvironment but also peripheral components of the immune system to induce multiple levels of tolerance mechanisms: immunosuppressive cells such as Tregs, myeloid derived suppressor cells and tumor-associated macrophages; immunological checkpoints; and abnormal levels of circulating cytokines. Among these immunosuppressive factors, Tregs have been identified as one of the major players. The number or function of Tregs is usually promoted in cancer patients.1 Preclinical data also suggest the role of Tregs in inducing tolerance for tumor associated antigens.2 More importantly, immunotherapies such as cytokines and vaccines themselves may induce promotion of Treg number or function in the patient.3 Taken together, there is strong evidence that targeting Tregs can improve the efficacy of immunotherapy.
Most current strategies to target Tregs aim at depletion of Tregs with monoclonal antibodies or ligand-directed toxins that bind to the cell-surface receptor, CD25, or with metronomic cyclophosphamide treatment.4 These depletion approaches have limited clinical benefit, probably due to their side effect to eliminate activated T effector cells (Teffs) and induction of Tregs replenishment. Strategies targeting other surface markers also have specificity problem. More methods have been tested in animals to affect Treg intracellular protein expression, function or signaling, such as siRNA and miRNA approaches, which usually have restrictions and a big gap toward possible clinical applications.
Recently, we have reported that a class I HDAC inhibitor, entinostat, suppressed Treg function, enhanced anti-tumor immune response, and facilitated cytokine and vaccine immunotherapy in murine renal cell carcinoma and prostate cancer models, respectively.5 This Treg suppression action was not through a depletion mechanism. Instead, entinostat targeted the function of Tregs by downregulating Foxp3 gene expression. Importantly, the low (5 mg/kg), immune-promoting dose of entinostat did not affect the proliferation capacity of Teffs and did not have a cytotoxic effect against tumor cells.
HDAC inhibitors induce acetylation of many histone and non-histone proteins, which contributes to a wide spectrum of anti-tumor and immunomodulatory activities of HDAC inhibitors. Pan HDAC inhibitors have shown either immunosuppressive or immunopromoting properties through modulating cytokine expression, affecting macrophage and dendirtic cells, or regulating costimulation molecules. Interestingly, previous studies reported that HDAC inhibitors promote the generation or suppressive function of Tregs. Treatment of a class I/II HDAC inhibitor, Trichostatin A (TSA), led to increased numbers of Tregs with improved suppressive activity.6 The study demonstrated that Foxp3 interacts with class II HDAC 7 and 9, and the histone acetyltransferase (HAT) TIP60, and TSA treatment induced Foxp3 hyperacetylation. These observations suggest that Foxp3 hyperacetylation is correlated with improved functionality. Similarly, a recent study showed that Foxp3 acetylation can be reciprocally regulated by the HAT p300 and the class III HDAC SIRT1.7 The authors demonstrated that Foxp3 hyperacetylation stabilized Foxp3 protein by preventing polyubiquitination and proteasomal degradation. Therefore, a SIRT1 inhibitor has similar Tregs promoting effect as TSA. On the contrary, our study clearly shows that the class I HDAC inhibitor, entinostat, suppresses Foxp3 expression at a transcriptional or post-transcriptional level, which leads to a decreased Foxp3 protein level, as well as impaired suppressive function in Treg populations without affecting peripheral Treg numbers.5 In our study we investigated the potential mechanism for Foxp3 downregulation by entinostat and found out that STAT3 signaling is involved in this mechanism. A highly specific peptide STAT3 inhibitor partially reversed the Foxp3 downregulation by entinostat treatment.5 This observation is consistent with previous reports, showing that suppression of Tregs development by IL-6 and IL-27 is STAT3 dependent.8 It is well established that STAT3 can form a complex with class I HDAC 1 and 3 and HAT p300, which are responsible for the regulation of STAT3 acetylation.9 STAT3 hyperacetylation facilitates dimerization and nuclear transportation of STAT3 protein, and consequently promotes the activation of STAT3 signaling. Our study confirmed that STAT3 is a target of entinostat, as entinostat induced STAT3 acetylation. Taken together, these results suggest that entinostat induces STAT3 acetylation and activation of the pathway, which lead to suppression of Foxp3 gene expression.
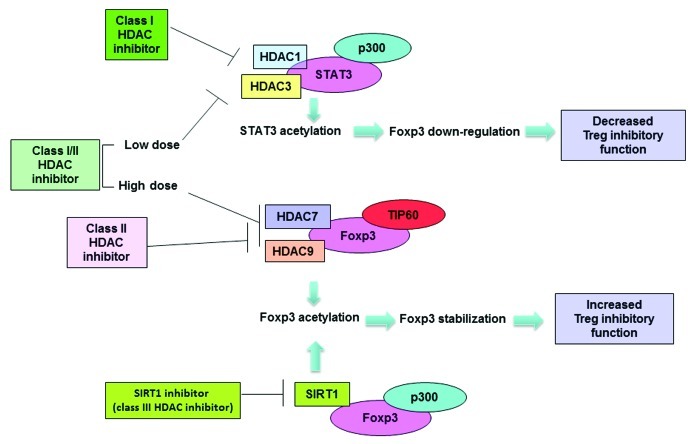
In order to clarify the reason for the discrepancy between our observation and previous reports, we tested HDAC inhibitors with different specificities in an in vitro experiment. Tregs were measured by flow cytometry after exposure to HDAC inhibitors. Inhibitors that can modify class I HDACs (class I specific and pan) decreased Foxp3 levels in Tregs in a dose-dependent manner, whereas class II specific HDAC inhibitors did not affect Foxp3 levels. This suggests that only class I HDAC inhibition results in Treg suppression. In addition, we observed that in vivo treatment of a pan HDAC inhibitor LBH589 showed opposite effects when applied at different doses: low dose inhibited Tregs suppressive function whereas high dose treatment promoted Treg function (unpublished). LBH589 has much higher affinity with class I HDACs than that with class II HDACs.10 We reasoned that LBH589 primarily inhibits class I HDAC at low doses, which shows Treg suppressive effect similar to class I specific inhibitors. At a high dose, LBH589 saturates class I HDACs and targets class II and the Treg-promoting effect resulting from class II HDACs inhibition becomes dominant. Here we summarize in Figure 1 the effects of different types of HDAC inhibitors on Treg function, as well as provide a putative underlining mechanism.
Figure 1. Modulation of Treg function with class-specific HDAC inhibitors. Class I HDAC inhibitors induce acetylation of STAT3 by inhibiting HDAC3 or HDAC1, downregulate Foxp3 gene expression, and suppress Treg function. Class II HDAC inhibitor treatment induces Foxp3 hyperacetylation by targeting HDAC7 and HDAC 9, which leads to stabilization of Foxp3 protein and enhanced Treg function. SIRT1 (class III specific) inhibitor also induces hyperacetylation and stabilization of Foxp3 protein, and enhances the Treg function. A pan inhibitor may target class I HDACs at a low dose and impair Treg function. At a higher dose, the pan inhibitor may target class II HDAC and show a dominant Treg promoting effect.
The complexity of immunomodulatory properties of HDAC inhibitors, such as their “double edged sword” effect on Tregs, needs to be taken into account when designing combinational pre-clinical or clinical studies with immunotherapies and HDAC inhibitors study. In addition, when targeting Tregs with class I specific HDAC inhibitors, the dose of the agents needs to be chosen carefully, since our results suggest that the benefit of Treg suppression is lost through Teff damage with higher doses of selective class I HDAC inhibitors.
Glossary
Abbreviations:
- Tregs
regulatory T cells
- HDAC
histone deacetylase
- HDACi
histone deacetylase inhibitor
- Teffs
effector T cells
- TSA
Trichostatin A
- HAT
histone acetyltransferase
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/20306
References
- 1.Yokokawa J, Cereda V, Remondo C, Gulley JL, Arlen PM, Schlom J, et al. Enhanced functionality of CD4+CD25(high)FoxP3+ regulatory T cells in the peripheral blood of patients with prostate cancer. Clin Cancer Res. 2008;14:1032–40. doi: 10.1158/1078-0432.CCR-07-2056. [DOI] [PubMed] [Google Scholar]
- 2.Antony PA, Do Restifo NP. CD4+ CD25+ immunoregulatory T cells hinder tumor immunotherapy? J Immunother. 2002;25:202–6. doi: 10.1097/00002371-200205000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahmadzadeh M, Rosenberg SA. IL-2 administration increases CD4+ CD25(hi) Foxp3+ regulatory T cells in cancer patients. Blood. 2006;107:2409–14. doi: 10.1182/blood-2005-06-2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Colombo MP, Piconese S. Regulatory-T-cell inhibition versus depletion: the right choice in cancer immunotherapy. Nat Rev Cancer. 2007;7:880–7. doi: 10.1038/nrc2250. [DOI] [PubMed] [Google Scholar]
- 5.Shen L, Ciesielski M, Ramakrishnan S, Miles KM, Ellis L, Sotomayor P, et al. Class I histone deacetylase inhibitor entinostat suppresses regulatory T cells and enhances immunotherapies in renal and prostate cancer models. PLoS ONE. 2012;7:e30815. doi: 10.1371/journal.pone.0030815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tao R, de Zoeten EF, Ozkaynak E, Chen C, Wang L, Porrett PM, et al. Deacetylase inhibition promotes the generation and function of regulatory T cells. Nat Med. 2007;13:1299–307. doi: 10.1038/nm1652. [DOI] [PubMed] [Google Scholar]
- 7.van Loosdregt J, Vercoulen Y, Guichelaar T, Gent YY, Beekman JM, van Beekum O, et al. Regulation of Treg functionality by acetylation-mediated Foxp3 protein stabilization. Blood. 2010;115:965–74. doi: 10.1182/blood-2009-02-207118. [DOI] [PubMed] [Google Scholar]
- 8.Huber M, Steinwald V, Guralnik A, Brüstle A, Kleemann P, Rosenplänter C, et al. IL-27 inhibits the development of regulatory T cells via STAT3. Int Immunol. 2008;20:223–34. doi: 10.1093/intimm/dxm139. [DOI] [PubMed] [Google Scholar]
- 9.Yuan ZL, Guan YJ, Chatterjee D, Chin YE. Stat3 dimerization regulated by reversible acetylation of a single lysine residue. Science. 2005;307:269–73. doi: 10.1126/science.1105166. [DOI] [PubMed] [Google Scholar]
- 10.Atadja P. Development of the pan-DAC inhibitor panobinostat (LBH589): successes and challenges. Cancer Lett. 2009;280:233–41. doi: 10.1016/j.canlet.2009.02.019. [DOI] [PubMed] [Google Scholar]