Abstract
Incomplete differentiation of CD8+ cytotoxic T-lymphocytes (CTLs) in the tumor microenvironment is associated with cancer progression. We describe a new type of tumor-infiltrating CD8+CD57+ T cell in cancer with hybrid phenotypic and functional properties of both an early effector-memory cell and a terminally-differentiated effector cell. These cells behave as incompletely-differentiated CTLs.
Keywords: CD57, CD8+ effector-memory, CTL, melanoma, tumor-infiltrating lymphocytes
Melanomas are considered to be one of the most immunogenic cancers and are frequently infiltrated with CD8+ and CD4+ T lymphocytes specific for major tumor antigens. CD8+ cytotoxic T lymphocytes (CTL) mediate antigen-specific lysis of tumor cells through cytolytic granule proteins, such as granzymes, granulysin, and perforin. Previously, other investigators have shown a lack of complete CTL differentiation in solid tumors.1 However, the exact stage of differentiation affected was unclear. We recently reported in Clinical Cancer Research a novel subset of CD8+ tumor-infiltrating lymphocytes (TILs) that co-expressed early effector-memory markers (CD27, CD28) and a marker for end-stage, senescent T cells (CD57).2
CD28 and CD27 are markers of early CD8+ effector-memory T (TEM) cells. Based on studies on virus-specific T cells in humans, it was postulated that CD8+ TEM cells differentiate in a linear pathway from CD28+CD27+ (early-differentiated) to CD28-CD27+ (intermediate-differentiated) to CD28-CD27- (late-differentiated).3 As CD8+ TEM transitions to end-stage effector (TEFF), the loss of CD28 and gain of CD57 is an immunological characteristic of humans and non-human primates, but not of mice.3 CD57 is a marker on highly differentiated CD8+CD27-CD28- T cells needed to control CMV and other endemic viruses in humans.3,4 CD57 was also proposed as a marker of end-stage, senescent CD8+ T cells in HIV patients exhibiting highly cytotoxic potential.3,5 However, in HIV progressors, a failure to coordinately downregulate CD27 and upregulate CD57 resulted in an accumulation of an unusual subset of HIV-specific CD8+CD27+CD57+ cells.4 CD8+CD27+CD57+ T lymphocytes have also been observed in the peripheral blood of melanoma patients after vaccination with gp100 tumor antigen.6
We set out to determine the role of CD8+CD57+ T cells in the melanoma. By performing flow cytometry staining on TIL isolated from 44 metastatic tumors, we found that the CD8+CD57+ subset was 16.2 ± 3.5% of the total tumor-infiltrating CD8+ T cells. The vast majority of these CD8+ T cells were also “locked” in the TEM stage and, on average, > 20% of the CD8+CD57+ subset co-expressed CD27 and CD28. These T cells were GBhi but Perflo/-, and they recognized melanoma tumor antigens, MART-1 and gp100 in HLA-A2+ patients. In contrast, only a few (< 5%) of the CD8+CD57+ T cells in the PBMC of the same melanoma patients co-expressed CD27 and CD28, but they were GBhi and Perfhi. We also found a similar population in pleural effusions from metastatic breast cancer. Notably, this TIL subset was different from the Foxp3+ CD8+ early effector TILs, which did not express CD57, as reported earlier.7
Whether CD8+CD57+ T cells are really “senescent” has become a controversial issue.3 We addressed this issue in the CD8+CD27+CD57+ TIL subset and found that they indeed proliferated in response to IL-2. We then purified this subset from IL-2-cultured TILs by cell sorting. Interestingly, we found that the CD27+CD57+ TILs were able to proliferate and produce high levels of IFN-γ upon TCR stimulation, which was inconsistent with CD57 as a general marker for T-cell senescence. We also found that PD-1 expression was higher in the CD27+CD57+ subset compared with the CD27+CD57- subset. Since CD8+ T cells naturally express PD-1 as a result of TCR activation, where it plays a regulatory mechanism to prevent over-reactivity,3 it is possible that the CD8+CD27+CD57+ subset are more highly activated T cells in the tumor microenvironment.
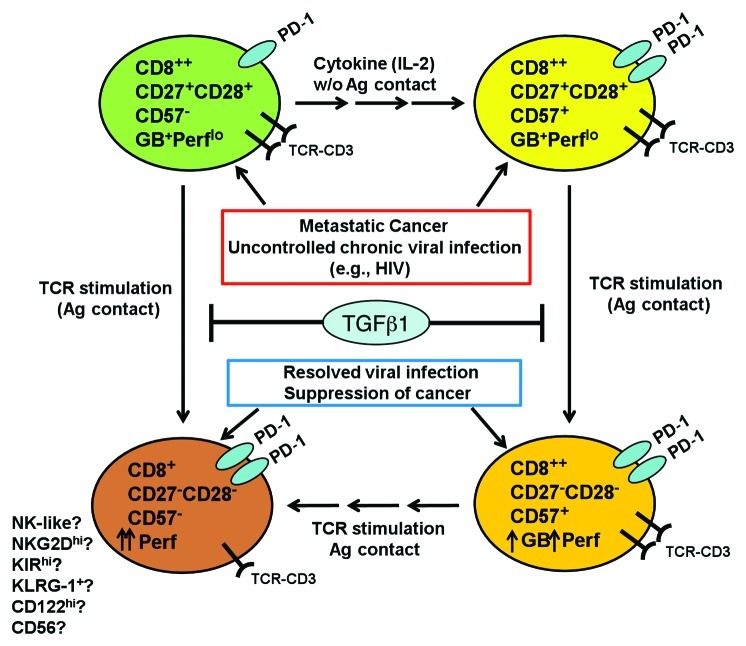
Building on our observation that CD8+CD57+ TILs were non-senescent, we hypothesized that these T cells were in a transition state and could be induced to differentiate further to cytotoxic end-stage effectors. Indeed, we found that the sorted CD8+CD27+CD57+ TILs, which were Perflo and poorly cytotoxic, could differentiate into a Perfhi, highly cytotoxic CD27-CD57+ or CD27-CD57- subset after TCR stimulation (Fig. 1). IL-2 treatment alone induced a minor fraction of CD27+ precursor cells to become CD27+CD57+, which suggested that IL-2 was sufficient to expand the CD27+CD57+ subset from CD27+ precursors (Fig. 1). TCR-stimulation induced the differentiation of sorted CD8+CD27+CD57- TILs into a Perfhi, CD27-CD57- subset, but the cells differentiating from the CD27+CD57+ subset on average acquire higher perforin levels and killing function (Fig 1). We also found that TGF-β1, produced by many melanomas, arrested the differentiation and cytotoxic activities of both the CD27+CD57- and CD27+CD57+ subsets at the CD27+ stage. Taken together, these findings may help explain why metastatic melanomas are often infiltrated with only early TEM CD8+ T cells that have limited ability to kill tumor cells. Thus, TGF-β1 may be a key suppressor of CTL differentiation in the tumor microenvironment. However, other factors such as PGE2, indoleamine 2,3-diooxygenase (IDO), IL-10, or inhibitory signaling through PD-1, may also play a role.8
Figure 1. Differentiation pathway of the tumor-infiltrating CD8+ T cells in metastatic cancer. In situations where CD8+ T cells encounter persistent, chronic antigenic stimulation such as metastatic cancer or uncontrolled chronic viral infections, CD8+ effector-memory T (TEM) cells fail to coordinate downregulation of CD27 with upregulation of an end-stage CTL marker, CD57 and acquire a more cytolytic phenotype. Thus TEM fail to transition from a granzyme B (GB+) perforinlo (Perflo) cells into Perfhi, highly cytotoxic end-stage CTL. This resulted in accumulation of CD8+ T cells at a transitional stage where markers for early TEM (CD27, CD28) are co-expressed with CD57, even though the cells remain Perflo. We also found that TGF-β1, an immunosuppressive cytokine frequently found in the microenvironment of metastatic cancer, could also contribute to the arrested differentiation and accumulation of CD27+CD57- precursor T cells and CD27+CD57+ T cells. We found that PD-1 was expressed more in the CD27+CD57+ T cells, which implied that they may exhibit a higher level of effector activity. When these tumor-infiltrating lymphocytes (TIL) are expanded with IL-2, a minor fraction (~30%) of CD27+CD57- subset differentiated into CD27+CD57+ T cells. After TCR stimulation ex vivo, the CD27+CD28+CD57- subset directly differentiated to become CD27-CD57- T cells. On the other hand, CD27+CD57+ differentiated to become CD27-CD57+, and, in some patients, CD27-CD57-. These phenotypic changes were accompanied by increased perforin expression and acquisition of potent cytotoxicity against tumor cells. We think that CD57 is not a marker for T-cell senescence, but rather marks highly differentiated T cells that are in the process of transitioning into a truly end-stage, effector CTL. Currently it is not known which set of markers define truly senescent, end-stage, highly cytolytic CTL. We also propose that ultimately, both the CD8+CD57+ and CD8+CD57- subsets may ultimately differentiate into a subset of CD8+CD27-CD28-CD57- cells that may be more NK-like, expressing higher levels of CD122, KLRG1, NKG2D and other NK receptors.
Another interesting observation we made was that both the CD8+CD27+CD57+ and CD8+CD27+CD57- T cells seemed to ultimately differentiate into CD27-CD57- (also CD28-) cells subset with high perforin and killing activity. Are these the true end-stage or terminally-differentiated state of CTL that actually may be more NK-like, by expressing CD122, other NK receptors (NKG2D and high KIR levels)? These cells have been described before, but their origin is unknown.9
In conclusion, we characterized a novel subset of TILs in metastatic melanoma and breast cancer that seems to be locked in a transitional state between early TEM and fully-differentiated CTL. Our study also underscores the need for a greater understanding of the state of CTL differentiation and activity in patients in relation to tumor control. Overall, there has been an over-emphasis on the role of cytokine release by CD8+ T cells (e.g., IFNγ), which has generally not correlated with clinical efficacy during cancer immunotherapy. A case in point is the recent HIV vaccine trials where effective immunization correlated more with a gain of antigen-specific CTL phenotype and killing function rather than IFN-γ production.10
Glossary
Abbreviations:
- CTL
cytotoxic T lymphocytes
- TCR
T-cell receptor
- TIL
tumor-infiltrating lymphocytes
- ACT
adoptive T-cell therapy
- PBMC
peripheral blood mononuclear cells
- Perf
perforin
- GB
Granzyme B
- HNK-1
human natural killer-1
- TN
naïve T cells
- TEM
effector-memory T cells
- TTDE
terminally-differentiated effector cells
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/20307
References
- 1.Mortarini R, Piris A, Maurichi A, Molla A, Bersani I, Bono A, et al. Lack of terminally differentiated tumor-specific CD8+ T cells at tumor site in spite of antitumor immunity to self-antigens in human metastatic melanoma. Cancer Res. 2003;63:2535–45. [PubMed] [Google Scholar]
- 2.Wu RC, Liu S, Chacon JA, Wu S, Li Y, Sukhumalchandra P, et al. Detection and Characterization of a Novel Subset of CD8+CD57+ T-cells in Metastatic Melanoma with an Incompletely-Differentiated Phenotype. Clin Cancer Res. 2012;••• doi: 10.1158/1078-0432.CCR-11-2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Strioga M, Pasukoniene V, Characiejus D. CD8+ CD28- and CD8+ CD57+ T cells and their role in health and disease. Immunology. 2011;134:17–32. doi: 10.1111/j.1365-2567.2011.03470.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoji A, Connolly NC, Buchanan WG, Rinaldo CR., Jr. CD27 and CD57 expression reveals atypical differentiation of human immunodeficiency virus type 1-specific memory CD8+ T cells. Clin Vaccine Immunol. 2007;14:74–80. doi: 10.1128/CVI.00250-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brenchley JM, Karandikar NJ, Betts MR, Ambrozak DR, Hill BJ, Crotty LE, et al. Expression of CD57 defines replicative senescence and antigen-induced apoptotic death of CD8+ T cells. Blood. 2003;101:2711–20. doi: 10.1182/blood-2002-07-2103. [DOI] [PubMed] [Google Scholar]
- 6.Walker EB, Haley D, Petrausch U, Floyd K, Miller W, Sanjuan N, et al. Phenotype and functional characterization of long-term gp100-specific memory CD8+ T cells in disease-free melanoma patients before and after boosting immunization. Clin Cancer Res. 2008;14:5270–83. doi: 10.1158/1078-0432.CCR-08-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anichini A, Molla A, Vegetti C, Bersani I, Zappasodi R, Arienti F, et al. Tumor-reactive CD8+ early effector T cells identified at tumor site in primary and metastatic melanoma. Cancer Res. 2010;70:8378–87. doi: 10.1158/0008-5472.CAN-10-2028. [DOI] [PubMed] [Google Scholar]
- 8.Polak ME, Borthwick NJ, Gabriel FG, Johnson P, Higgins B, Hurren J, et al. Mechanisms of local immunosuppression in cutaneous melanoma. Br J Cancer. 2007;96:1879–87. doi: 10.1038/sj.bjc.6603763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Takayama E, Koike Y, Ohkawa T, Majima T, Fukasawa M, Shinomiya N, et al. Functional and Vbeta repertoire characterization of human CD8+ T-cell subsets with natural killer cell markers, CD56+ CD57- T cells, CD56+ CD57+ T cells and CD56- CD57+ T cells. Immunology. 2003;108:211–9. doi: 10.1046/j.1365-2567.2003.01575.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Makedonas G, Betts MR. Living in a house of cards: re-evaluating CD8+ T-cell immune correlates against HIV. Immunol Rev. 2011;239:109–24. doi: 10.1111/j.1600-065X.2010.00968.x. [DOI] [PMC free article] [PubMed] [Google Scholar]