Abstract
Monoclonal antibodies (mAb) induce tumor regression through antibody-dependant cellular cytotoxicity (ADCC). We recently showed that an agonistic anti-CD137 mAb stimulates natural killer (NK) cells which have been activated by a tumor-specific mAb, resulting in increased ADCC against cancer cells.
Keywords: ADCC, antibody, CD137, NK Cell, synergy
Monoclonal antibody technology is among the most important developments in the field of cancer therapy in the last quarter century. Monoclonal antibodies (mAbs) against CD20 and HER2, rituximab and trastuzumab have been important additions to our therapeutic armentarium for patients with lymphoma and breast cancer, respectively. One of the primary mechanisms of anti-tumor activity of monoclonal antibodies is antibody dependent cell-mediated cytotoxicity (ADCC) whereby a natural killer (NK) cell or macrophage/monocyte bearing an Fc receptor binds to the antibody-targeted tumor cell and mediates the killing function. In xenotransplant models, therapy with rituximab and trastuzumab have both been shown to be dependent on NK cell activation through the Fcγ-receptor(FcγR).1 Additionally, patients harbouring a polymorphism in the gene encoding FcγRIII (CD16) which leads to a higher affinity of binding of the mAb to the NK cell have higher rates and more prolonged durations of responses to mAb therapy.2-4 Therefore many different strategies are under development to stimulate immune effector cells implicated in ADCC in order to enhance the efficacy of tumor-specific mAbs. We have shown that a stimulatory mAb directed against CD137 enhances NK cell-mediated ADCC against tumor cells.5,6
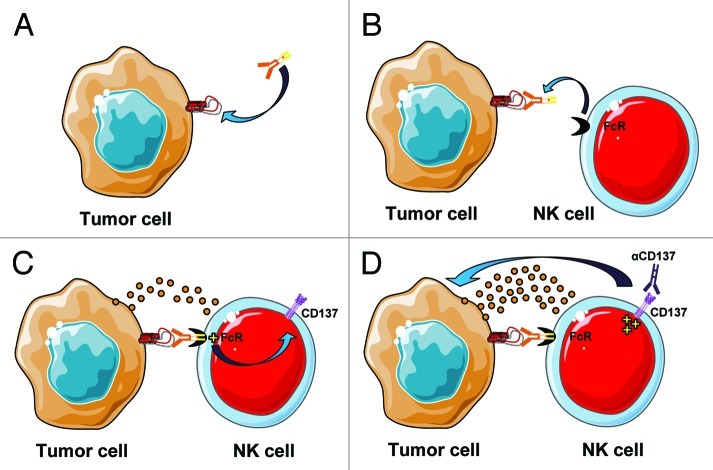
CD137 (4–1BB) is a costimulatory receptor that belongs to the tumor necrosis factor receptor superfamily. It is expressed on a variety of immune cells following activation, including T cells, dendritic cells (DC) and NK cells.7 Expression of CD137 on NK cells is minimal at baseline and increases significantly following FcR-engagement. Notably, NK cells upregulate their surface CD 137 when they encounter a mAb bound to tumor cells. We reasoned that the addition of an agonistic mAb against CD137 would further stimulate activated NK cells and result in enhanced ADCC (Fig. 1). This proved to be the case in two different tumor models, lymphoma5 and breast cancer.6 Tumor cells coated with either Rituximab and trastuzumab induced upregulation of CD137 on NK cells. Subsequent addition of an anti-CD137 mAb increased NK cell degranulation and tumor lysis. In vivo, anti-CD137 mAb potentiated the antitumor activity of anti-CD20 and anti-HER2 mAbs in syngenic and xenotransplant mouse models of lymphoma and breast cancer, respectively. This effect required specific targeting by the anti-tumor monoclonal antibody. This was demonstrated in a xenotransplant model of breast cancer where CD137 stimulation enhanced antitumor activity of trastuzumab only against HER2-overexpressing but not in HER2-negative tumors.6
Figure 1. Cooperation for tumor cell killing between a tumor-specific mAb and an anti-CD137 stimulatory mAb. (A) A mAb directed against a tumor cell target binds to the cancer cell. (B) The tumor-directed mAb then recruits NK effector cells through their Fc receptor. (C) Binding of the tumor-directed mAb to the FcR activates NK cells which results in degranulation and upregulation of CD137 on their surface. (D) Addition of an agonistic anti-CD137 mAb further activates NK cells and increases their cytotoxicity against tumor cells.
Other strateties to stimulate immune effector cells have been tested in order to enhance mAb-induced cytotoxicity against tumor cells. For instance, tumor-specific mAbs have been combined with toll-like receptor agonists (CpG), IMIDs (thalidomide, lenalidomide), cytokines (IL-2, IL-12, IL-21, IFN-α, G-CSF, GM-CSF), immunomodulating mAbs (anti-KIR, anti-CD47) or γδ T cells agonists (BrHPP). Some of these agents have been tested in clinical trials.8 We believe that stimulating CD137 has advantages over many of these approaches. First, CD137 is known to be a potent stimulatory receptor. Second, anti-CD137 mAb stimulates only the activated NK cells involved in ADCC while sparing resting NK cells. Only the NK cells that have encountered mAb-coated tumor cells are sensitive to CD137 stimulation. This selectivity would be expected to prevent systemic stimulation of NK cells and thus reduce non-specific toxicities. By contrast cytokines including IL-2 or anti-KIR blocking mAbs are stimulate all NK cells, because of their constitutive expression of cytokine receptors and KIRs. Third, in addition to NK cells, anti-CD137 mAb may also stimulate anti-tumor T cells and DC that take up dying tumor cells and thus the antibody might enhance an adaptive immune response against the tumor. Finally, anti-CD137 mAb can be easily combined with any tumor-specific mAb that induces ADCC. This is in contrast to “built-in” reagents such as bispecific mAbs which need to be constructed for reactivity against each tumor antigen. Clinical trials are now ongoing to test the combination of rituximab and anti-CD137 mAb in patients with lymphoma (NCT01307267).
Despite these promising advances, several questions remain to be answered. How might one improve the efficacy of anti-CD137 therapy in combination with a tumor-specific mAb? What is the optimal dose and schedule of administration of anti-CD137 mAb when given in conjunction with anti-tumor antibodies? How important is the Fc fragment of the anti CD137 antibody? Can anti-CD137 mAbs also enhance the adaptive immune response generated by a tumor-specific mAb? Can other immunostimulatory targets be identified to enhance mAb-induced cytotoxicity against tumor cells?
The recent success of a mAb against cytotoxic T lymphocyte antigen (CTLA-4) in patients with metastatic melanoma illustrates the relevance of stimulating immune cells to treat cancer.9,10 Our results suggest that the combination of a tumor-specific mAb with a stimulatory mAb directed against immune effector cells can be synergistic. This general approach, that targets simultaneously the tumor and its immune environment, opens an exciting new field in cancer immunotherapy.
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/19974
References
- 1.Clynes RA, Towers TL, Presta LG, Ravetch JV. Inhibitory Fc receptors modulate in vivo cytotoxicity against tumor targets. Nat Med. 2000;6:443–6. doi: 10.1038/74704. [DOI] [PubMed] [Google Scholar]
- 2.Musolino A, Naldi N, Bortesi B, Pezzuolo D, Capelletti M, Missale G, et al. Immunoglobulin G fragment C receptor polymorphisms and clinical efficacy of trastuzumab-based therapy in patients with HER-2/neu-positive metastatic breast cancer. J Clin Oncol. 2008;26:1789–96. doi: 10.1200/JCO.2007.14.8957. [DOI] [PubMed] [Google Scholar]
- 3.Weng WK, Levy R. Two immunoglobulin G fragment C receptor polymorphisms independently predict response to rituximab in patients with follicular lymphoma. J Clin Oncol. 2003;21:3940–7. doi: 10.1200/JCO.2003.05.013. [DOI] [PubMed] [Google Scholar]
- 4.Weiner LM, Surana R, Wang S. Monoclonal antibodies: versatile platforms for cancer immunotherapy. Nat Rev Immunol. 2010;10:317–27. doi: 10.1038/nri2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kohrt HE, Houot R, Goldstein MJ, Weiskopf K, Alizadeh AA, Brody J, et al. CD137 stimulation enhances the antilymphoma activity of anti-CD20 antibodies. Blood. 2011;117:2423–32. doi: 10.1182/blood-2010-08-301945. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 6.Kohrt HE, Houot R, Weiskopf K, Goldstein MJ, Scheeren F, Czerwinski D, et al. Stimulation of natural killer cells with a CD137-specific antibody enhances trastuzumab efficacy in xenotransplant models of breast cancer. J Clin Invest. 2012;122:1066–75. doi: 10.1172/JCI61226. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 7.Melero I, Murillo O, Dubrot J, Hervás-Stubbs S, Perez-Gracia JL. Multi-layered action mechanisms of CD137 (4-1BB)-targeted immunotherapies. Trends Pharmacol Sci. 2008;29:383–90. doi: 10.1016/j.tips.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 8.Houot R, Kohrt H, Goldstein MJ, Levy R. Immunomodulating antibodies and drugs for the treatment of hematological malignancies. Cancer Metastasis Rev. 2011;30:97–109. doi: 10.1007/s10555-011-9274-3. [DOI] [PubMed] [Google Scholar]
- 9.Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–23. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Robert C, Thomas L, Bondarenko I, O’Day S, M D JW, Garbe C, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364:2517–26. doi: 10.1056/NEJMoa1104621. [DOI] [PubMed] [Google Scholar]