Abstract
Compelling evidence indicates Type I CD20 immunotherapeutic mAbs promote targeted tumor cell elimination exclusively via immune effector functions, which can be exhausted/saturated. mAb dosing paradigms should therefore take into account the capacity of these cytotoxic mechanisms, leading to the conclusion that lower doses, given frequently, may be far more effective.
Keywords: CD20 mAbs, immunomonitoring, therapeutic antibodies, therapeutic trials, trogocytosis
Our in vitro investigations and studies by others have demonstrated that without exception type I CD20 mAbs rituximab (RTX) and ofatumumab (OFA) promote robust killing of B cell lines and primary chronic lymphocytic leukemia (CLL) cells only via immune effector mechanisms.1,2 These mechanisms include antibody-dependent cytotoxicity (ADCC) mediated by NK cells and macrophages, complement-dependent cytotoxicity (CDC), and phagocytosis by macrophages. OFA promotes much higher levels of CDC of CLL cells, which are generally refractory to RTX-mediated CDC.1 This finding likely explains the enhanced clinical efficacy of OFA as a single agent in the immunotherapy of CLL.3 Numerous mouse model studies confirm that RTX and OFA require the same effector mechanisms for therapeutic efficacy.2,4,5 In addition, simple cross-linking of target cell-bound mAbs, promoted by effector cells which express Fc receptors, does not induce target cell killing;5 downstream signaling by chelated and fully functioning Fc receptors on effector cells is absolutely required to mediate killing of mAb-opsonized cells. Moreover, NK cell-induced ADCC generates an “apoptotic phenotype” in mAb-opsonized cells which is due to the action of injected granzymes that activate caspases in targeted cells.6 Thus, it is our contention that there is no reliable evidence to support apoptosis as an independent stand-alone cytotoxic mechanism for Type I CD20 mAbs.
Therefore, based on our studies in CLL, we stipulate that the key limiting factor for mAb-mediated elimination of tumor cells is effector function capacity, rather than mAb dose or concentration. After effector mechanisms are saturated/exhausted in patients with circulating target cells, additional mAb does not increase efficacy. Our in vivo studies strongly support this conclusion and demonstrate the adverse consequences of excessive doses of mAb: high doses of mAb actually promote CD20 loss.
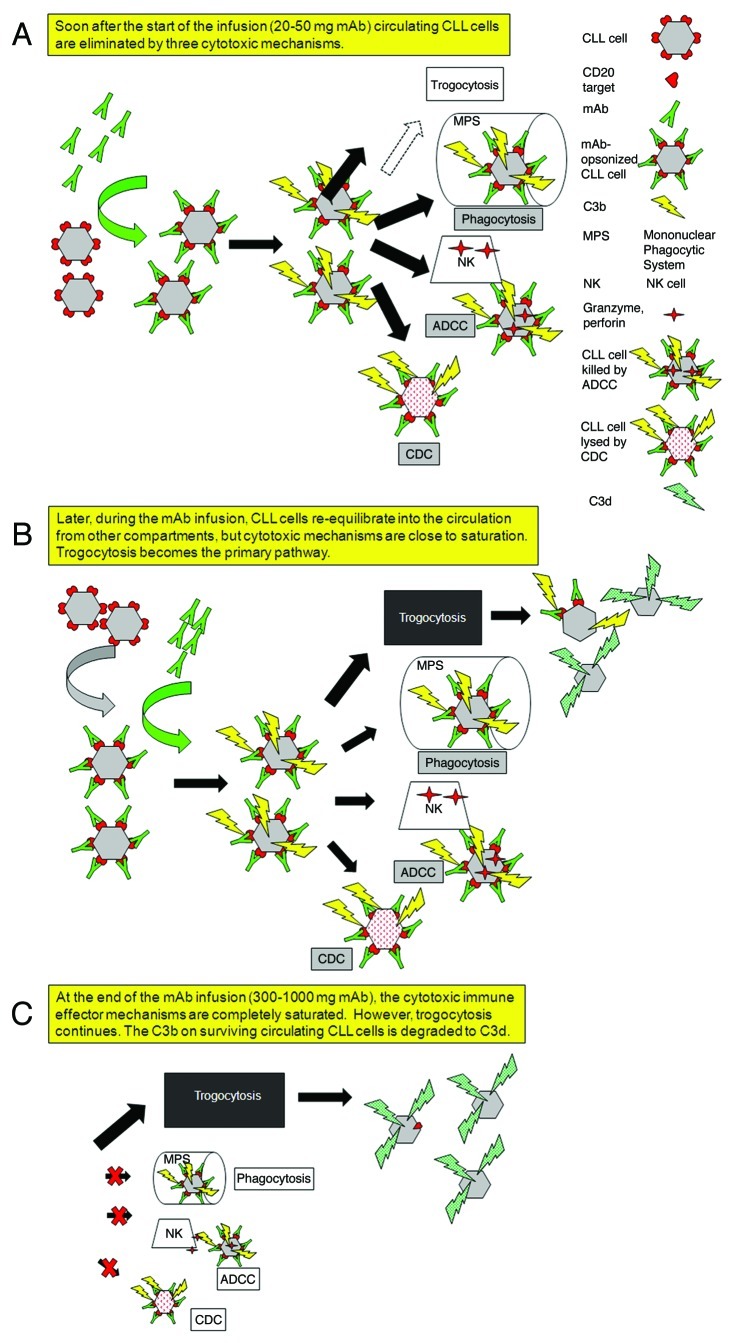
Clearance of circulating CLL cells is observed after infusion of ~30 mg of RTX or OFA.1,7,8 However, after the first wave of target cells has cleared there is a recurrence of tumor cells in the circulation, likely reflecting re-entry into circulation from other compartments. These cells rapidly lose CD20 and are resistant to clearance. This sequence of events is based on our examination of blood samples taken from CLL patients who received RTX infusions at the usual 375 mg/m2 dose. Within less than 24 h CD20 loss from circulating CLL cells was virtually complete and there was substantial complement consumption to the point of exhaustion of this effector mechanism.7 Based on these studies, we initiated clinical trials, which included correlative measurements. Findings in these trials are consistent with our initial observations and the reproducible results demonstrate general patterns.1,8,9 After infusion of only 20–50 mg of RTX or OFA, large amounts of mAb bind to circulating CLL cells, inducing rapid complement activation and covalent deposition of substantial amounts of C3 fragments on the cells. CLL cell counts drop precipitously, reflecting CDC, NK cell-mediated ADCC, and clearance of cells (opsonized with both IgG and C3b/iC3b fragments) by fixed tissue macrophages in liver and spleen which have receptors specific for IgG and for C3b/iC3b (Fig. 1A). Such clearance is readily predictable based on pioneering investigations of Frank and coworkers, who examined clearance of IgG-opsonized erythrocytes in experimental animals and in humans.10
Figure 1. Schematic illustration of the sequence of events that occurs when CLL patients receive intravenous infusions of large quantities of Type I CD20 mAbs. (A) Several of the body’s immune effector mechanisms promote a high level of clearance and destruction of circulating CLL cells after infusion of the first 20–50 mg of the Type I CD20 mAb. (B) Later, after a first wave of clearance, a substantial number of CD20+ CLL cells have re-equilibrated into the bloodstream from other compartments. The cells are opsonized by mAb, but the cytotoxic mechanisms are less effective, and an alternative reaction predominates: trogocytosis (shaving) of bound mAb and CD20 by fixed cells that express Fcγ receptors. (C) After the infusion is complete, the effector mechanisms are nearly exhausted or saturated, but trogocytosis continues. Although the complement titer is substantially reduced, there is sufficient residual complement activity that the cells are covalently opsonized with C3 activation fragments (which decay to C3d) before they lose CD20. These C3d-opsonized “low CD20” CLL cells are not cleared, and can remain in the circulation for weeks to more than one month.
Our correlative measurements were made at multiple times during and after infusions, and the results after infusion of only 15–30 mg of mAb are consistent with quantitative expectations.1,8,9 Based on estimates of the number of CD20 molecules expressed on circulating CLL cells, a 20 mg mAb dose will easily saturate available CD20 sites for even the high burdens of circulating cells commonly seen in CLL. Indeed, after this low mAb dose, the heavily opsonized cells are subject to successful killing and/or clearance by the three effector mechanisms.1,2 However, as circulating CLL cells are cleared, additional CLL cells re-equilibrate into the bloodstream (Fig. 1B). As mAb infusion continues (reaching cumulative doses of ~300–1000 mg), then although ample mAb is available to opsonize cells, they are not cleared, because effector mechanisms are at this time exhausted. Instead, > 90% of cell-associated CD20 is trogocytosed (shaved) from circulating CLL cells by fixed effector cells that express Fc receptors (Fig. 1C). Compelling evidence that shaved cells were first opsonized with mAb is based on the observation that “CD20-low” cells are covalently tagged with C3d, the final breakdown product of C3b.1,7 That is, infused RTX/OFA bound to cells, activated complement and promoted deposition of C3b, which is degraded to C3d within hours. Bound mAb and CD20 were shaved from target cells, but these cells remained viable and continued to circulate for weeks to > one month despite the presence of mAb in the bloodstream. The C3d deposited on the cells provides unambiguous evidence that the cells had been opsonized by RTX or OFA, but not cleared.
On the basis of these findings we suggest that future clinical trials in CLL which employ these mAbs should be based on lower, more frequent mAb dosing schedules which allow for recovery of effector function between doses and also minimize CD20 loss. These trials should include correlative measurements which evaluate patient effector functions (complement titers, levels of CD16 on NK cells), to provide additional guidance. Treatment schedules based on subcutaneous doses (~40–50 mg/day, 3 or more days/week) could provide a new paradigm that may be more effective and more convenient for patients. Finally, although our focus herein is on CD20 mAbs in CLL, this approach may be effective in other mAb-based cancer immunotherapies which require effector functions to eliminate tumor cells.
Glossary
Abbreviations:
- ADCC
antibody-dependent cellular cytotoxicity
- CDC
complement-dependent cytotoxicity
- CLL
chronic lymphocytic leukemia
- mAb
monoclonal antibody
- NK cell
natural killer cell
- OFA
ofatumumab
- RTX
rituximab
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/20368
References
- 1.Beurskens FJ, Lindorfer MA, Farooqui M, Beum PV, Engelberts P, Mackus WJM, et al. Exhaustion of cytotoxic effector systems may limit monoclonal antibody-based immunotherapy in cancer patients. J Immunol. 2012;188:3532–41. doi: 10.4049/jimmunol.1103693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Golay J, Introna M. Mechanism of action of therapeutic monoclonal antibodies: Promises and pitfalls of in vitro and in vivo assays. Arch Biochem Biophys. 2012;••• doi: 10.1016/j.abb.2012.02.011. [DOI] [PubMed] [Google Scholar]
- 3.Coiffier B, Lepretre S, Pedersen LM, Gadeberg O, Fredriksen H, van Oers MHJ, et al. Safety and efficacy of ofatumumab, a fully human monoclonal anti-CD20 antibody, in patients with relapsed or refractory B-cell chronic lymphocytic leukemia: a phase 1-2 study. Blood. 2008;111:1094–100. doi: 10.1182/blood-2007-09-111781. [DOI] [PubMed] [Google Scholar]
- 4.Tedder TF, Baras A, Xiu Y. Fcγ receptor-dependent effector mechanisms regulate CD19 and CD20 antibody immunotherapies for B lymphocyte malignancies and autoimmunity. Springer Seminars in Immunopathology. 2006;28:351–64. doi: 10.1007/s00281-006-0057-9. [DOI] [PubMed] [Google Scholar]
- 5.de Haij S, Jansen JHM, Boross P, Beurskens FJ, Bakema JE, Bos DL, et al. In vivo cytotoxicity of type I CD20 antibodies critically depends on Fc receptor ITAM signaling. Cancer Res. 2010;70:3209–17. doi: 10.1158/0008-5472.CAN-09-4109. [DOI] [PubMed] [Google Scholar]
- 6.Wilson NS, Yang B, Yang A, Loeser S, Marsters S, Lawrence D, et al. An Fcγ receptor-dependent mechanism drives antibody-mediated target-receptor signaling in cancer cells. Cancer Cell. 2011;19:101–13. doi: 10.1016/j.ccr.2010.11.012. [DOI] [PubMed] [Google Scholar]
- 7.Kennedy AD, Beum PV, Solga MD, DiLillo DJ, Lindorfer MA, Hess CE, et al. Rituximab infusion promotes rapid complement depletion and acute CD20 loss in chronic lymphocytic leukemia. J Immunol. 2004;172:3280–8. doi: 10.1016/j.jim.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 8.Williams ME, Densmore JJ, Pawluczkowycz AW, Beum PV, Kennedy AD, Lindorfer MA, et al. Thrice-weekly low-dose rituximab decreases CD20 loss via shaving and promotes enhanced targeting in chronic lymphocytic leukemia. J Immunol. 2006;177:7435–43. doi: 10.4049/jimmunol.177.10.7435. http://www.jimmunol.org/content/177/10/7435.full.html [DOI] [PubMed] [Google Scholar]
- 9.Zent CS, LaBlant BR, Link BKCTG, Shanafelt TD, Bowen DA, Kay NE, et al. Pentostatin, alemtuzumab, and low dose rituximab is effective therapy for relapsed/refractory chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL). Blood (ASH Annual Meeting Abstracts) 2011; 118:Abstract 1790;
- 10.Schreiber AD, Frank MM. Role of antibody and complement in the immune clearance and destruction of erythrocytes. I. In vivo effects of IgG and IgM complement-fixing sites. J Clin Invest. 1972;51:575–82. doi: 10.1172/JCI106846. [DOI] [PMC free article] [PubMed] [Google Scholar]