Abstract
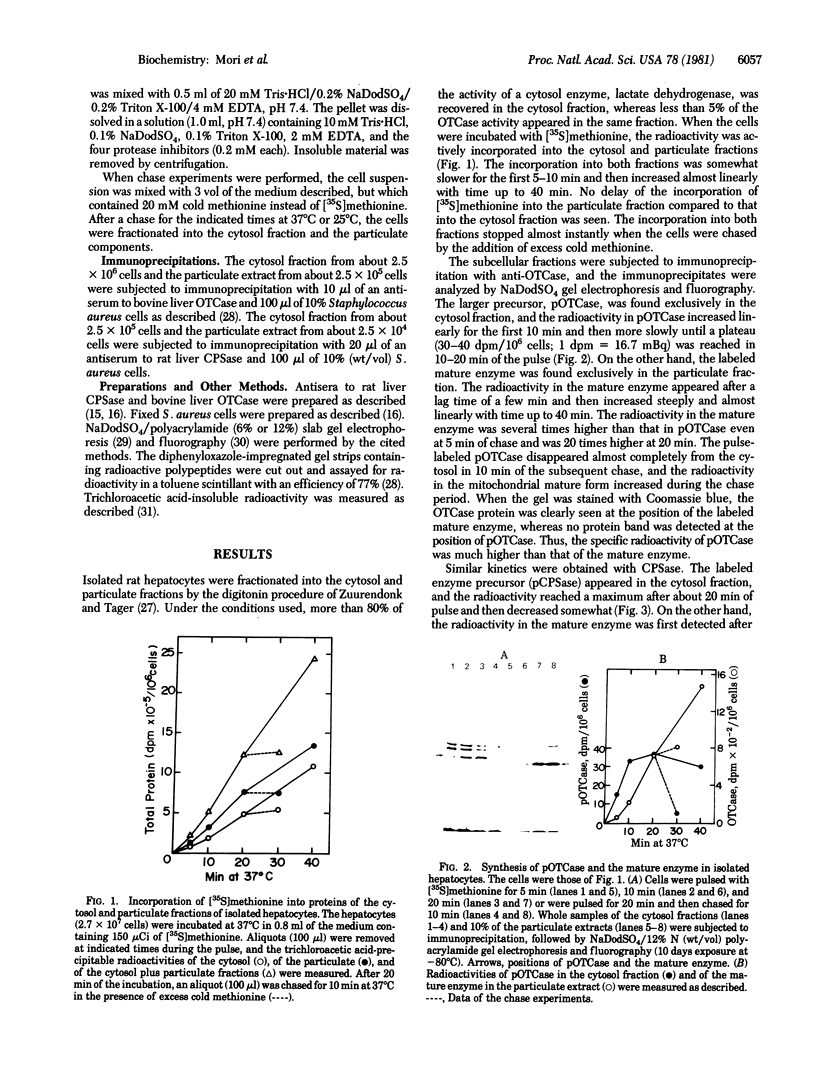
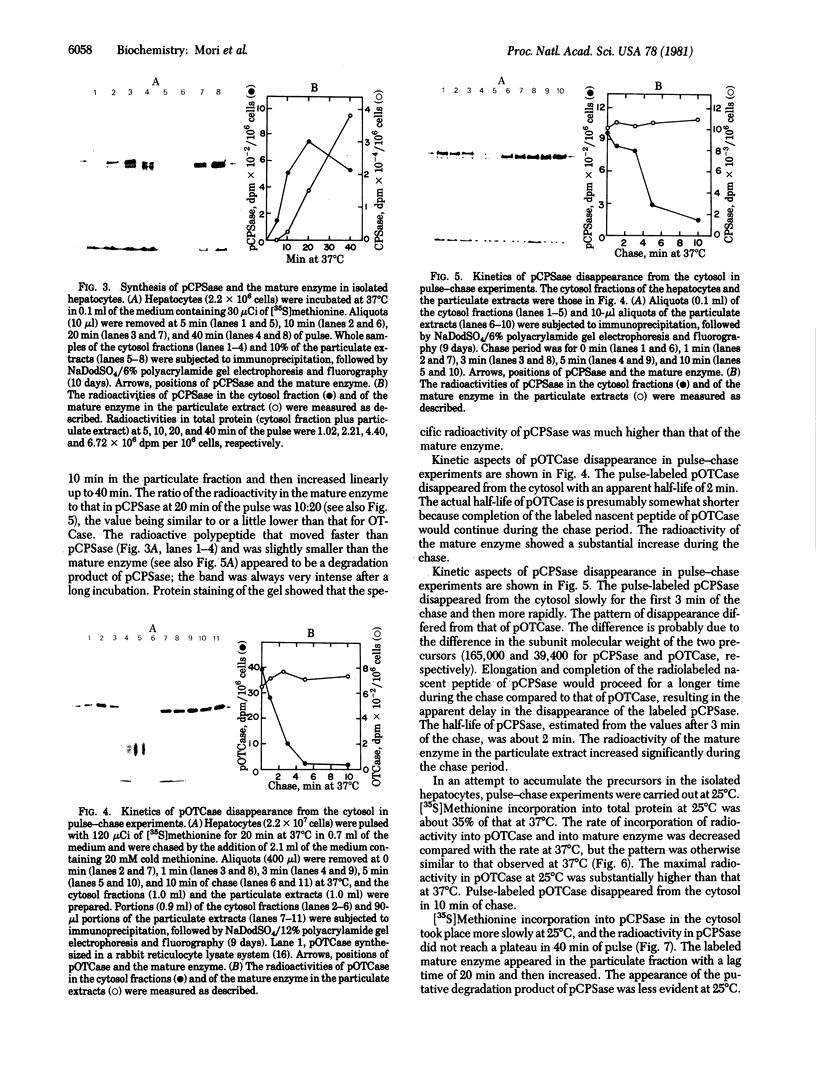
The synthesis and intracellular transport of the mitochondrial matrix enzymes ornithine transcarbamylase (carbamoylphosphate: L-ornithine carbamoyltransferase, EC 2.1.3.3.) and carbamoyl-phosphate synthetase (ammonia) I [carbon-dioxide:ammonia ligase (ADP-forming, carbamate-phosphorylating), EC 6.3.4.16] were studied in isolated rat hepatocytes. In pulse experiments at 37 degrees C, the larger precursors of the two enzymes appeared in the cytosol of the liver cells, where radioactivity levels of the precursors reached a plateau in 10-20 min after the pulse. The pulse-labeled mature enzymes appeared in the particulate fraction (containing mitochondria) after a time lag and increased almost linearly with time up to 40 min. The specific radioactivities of the precursors in the cytosol were much higher than those of the mature enzymes in the particulate fraction. In pulse--chase experiments, the labeled precursors disappeared from the cytosol with estimated half-lives of about 1-2 min. These results indicate that ornithine transcarbamylase and carbamoyl-phosphate synthetase I are initially synthesized as larger precursors and exist in a cytosolic pool from which they are transported into mitochondria and processed there to the mature enzymes concomitantly with or immediately after transport. Although the rates of synthesis, transport, and processing were decreased about 3-fold at 25 degrees C (as compared to incubation at 37 degrees C), the pool size of the precursors in the cytosol were somewhat larger at this temperature.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berry M. N., Friend D. S. High-yield preparation of isolated rat liver parenchymal cells: a biochemical and fine structural study. J Cell Biol. 1969 Dec;43(3):506–520. doi: 10.1083/jcb.43.3.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Clarke S. A major polypeptide component of rat liver mitochondria: carbamyl phosphate synthetase. J Biol Chem. 1976 Feb 25;251(4):950–961. [PubMed] [Google Scholar]
- Conboy J. G., Kalousek F., Rosenberg L. E. In vitro synthesis of a putative precursor of mitochondrial ornithine transcarbamoylase. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5724–5727. doi: 10.1073/pnas.76.11.5724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamble J. G., Lehninger A. L. Transport of ornithine and citrulline across the mitochondrial membrane. J Biol Chem. 1973 Jan 25;248(2):610–618. [PubMed] [Google Scholar]
- Guthöhrlein G., Knappe J. Structure and function of carbamoylphosphate synthase. I. Transitions between two catalytically inactive forms and the active form. Eur J Biochem. 1968 Dec;7(1):119–127. doi: 10.1111/j.1432-1033.1968.tb19582.x. [DOI] [PubMed] [Google Scholar]
- Hallermayer G., Zimmermann R., Neupert W. Kinetic studies on the transport of cytoplasmically synthesized proteins into the mitochondria in intact cells of Neurospora crassa. Eur J Biochem. 1977 Dec;81(3):523–532. doi: 10.1111/j.1432-1033.1977.tb11978.x. [DOI] [PubMed] [Google Scholar]
- Harmey M. A., Hallermayer G., Korb H., Neupert W. Transport of cytoplasmically synthesized proteins into the mitochondria in a cell free system from Neurospora crassa. Eur J Biochem. 1977 Dec;81(3):533–544. doi: 10.1111/j.1432-1033.1977.tb11979.x. [DOI] [PubMed] [Google Scholar]
- Hoogenraad N. J., Sutherland T. M., Howlett G. J. Purification of ornithine transcarbamylase from rat liver by affinity chromatography with immobilized transition-state analog. Anal Biochem. 1980 Jan 1;101(1):97–102. doi: 10.1016/0003-2697(80)90045-7. [DOI] [PubMed] [Google Scholar]
- Kalousek F., François B., Rosenberg L. E. Isolation and characterization of ornithine transcarbamylase from normal human liver. J Biol Chem. 1978 Jun 10;253(11):3939–3944. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lusty C. J. Carbamoylphosphate synthetase I of rat-liver mitochondria. Purification, properties, and polypeptide molecular weight. Eur J Biochem. 1978 Apr 17;85(2):373–383. doi: 10.1111/j.1432-1033.1978.tb12249.x. [DOI] [PubMed] [Google Scholar]
- Lusty C. J., Jilka R. L., Nietsch E. H. Ornithine transcarbamylase of rat liver. Kinetic, physical, and chemical properties. J Biol Chem. 1979 Oct 25;254(20):10030–10036. [PubMed] [Google Scholar]
- Marshall M., Cohen P. P. Ornithine transcarbamylase from Streptococcus faecalis and bovine liver. I. Isolation and subunit structure. J Biol Chem. 1972 Mar 25;247(6):1641–1653. [PubMed] [Google Scholar]
- Mori M., Miura S., Tatibana M., Cohen P. P. Cell-free synthesis and processing of a putative precursor for mitochondrial carbamyl phosphate synthetase I of rat liver. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5071–5075. doi: 10.1073/pnas.76.10.5071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori M., Miura S., Tatibana M., Cohen P. P. Cell-free translation of carbamyl phosphate synthetase I and ornithine transcarbamylase messenger RNAs of rat liver. Effect of dietary protein and fasting on translatable mRNA levels. J Biol Chem. 1981 Apr 25;256(8):4127–4132. [PubMed] [Google Scholar]
- Mori M., Miura S., Tatibana M., Cohen P. P. Characterization of a protease apparently involved in processing of pre-ornithine transcarbamylase of rat liver. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7044–7048. doi: 10.1073/pnas.77.12.7044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori M., Miura S., Tatibana M., Cohen P. P. Processing of a putative precursor of rat liver ornithine transcarbamylase, a mitochondrial matrix enzyme. J Biochem. 1980 Dec;88(6):1829–1836. doi: 10.1093/oxfordjournals.jbchem.a133158. [DOI] [PubMed] [Google Scholar]
- Morita T., Mori M., Tatibana M., Cohen P. P. Site of synthesis and intracellular transport of the precursor of mitochondrial ornithine carbamoyltransferase. Biochem Biophys Res Commun. 1981 Mar 31;99(2):623–629. doi: 10.1016/0006-291x(81)91790-3. [DOI] [PubMed] [Google Scholar]
- Pierson D. L., Brien J. M. Human carbamylphosphate synthetase I. Stabilization, purification, and partial characterization of the enzyme from human liver. J Biol Chem. 1980 Aug 25;255(16):7891–7895. [PubMed] [Google Scholar]
- Pierson D. L., Cox S. L., Gilbert B. E. Human ornithine transcarbamylase. Purification and characterization of the enzyme from normal liver and the liver of a Reye's syndrome patient. J Biol Chem. 1977 Sep 25;252(18):6464–6469. [PubMed] [Google Scholar]
- Raymond Y., Shore G. C. Processing of the precursor for the mitochondrial enzyme, carbamyl phosphate synthetase. Inhibition by rho-aminobenzamidine leads to very rapid degradation (clearing) of the precursor. J Biol Chem. 1981 Mar 10;256(5):2087–2090. [PubMed] [Google Scholar]
- Raymond Y., Shore G. C. The precursor for carbamyl phosphate synthetase is transported to mitochondria via a cytosolic route. J Biol Chem. 1979 Oct 10;254(19):9335–9338. [PubMed] [Google Scholar]
- Roberts B. E., Paterson B. M. Efficient translation of tobacco mosaic virus RNA and rabbit globin 9S RNA in a cell-free system from commercial wheat germ. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2330–2334. doi: 10.1073/pnas.70.8.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatz G. How mitochondria import proteins from the cytoplasm. FEBS Lett. 1979 Jul 15;103(2):203–211. doi: 10.1016/0014-5793(79)81328-9. [DOI] [PubMed] [Google Scholar]
- Shore G. C., Carignan P., Raymond Y. In vitro synthesis of a putative precursor to the mitochondrial enzyme, carbamyl phosphate synthetase. J Biol Chem. 1979 May 10;254(9):3141–3144. [PubMed] [Google Scholar]
- Virden R. The molecular weights of two forms of carbamoyl phosphate synthase from rat liver. Biochem J. 1972 Apr;127(3):503–508. doi: 10.1042/bj1270503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamauchi K., Hayashi N., Kikuchi G. Translocation of delta-aminolevulinate synthase from the cytosol to the mitochondria and its regulation by hemin in the rat liver. J Biol Chem. 1980 Feb 25;255(4):1746–1751. [PubMed] [Google Scholar]
- Zahlten R. N., Kneer N. M., Stratman F. W., Lardy H. A. The influence of ammonium and calcium lons on gluconeogenesis in isolated rat hepatocytes and their response to glucagon and epinephrine. Arch Biochem Biophys. 1974 Apr 2;161(2):528–535. doi: 10.1016/0003-9861(74)90335-x. [DOI] [PubMed] [Google Scholar]