Abstract
Mimetics of second mitochondria-derived activator of caspases (SMAC) enhance tumor cell death in a variety of cancers. Several molecular mechanisms of action have been identified. However, it was only recently that the modus of action was linked to stimulation of anti-tumor immunity. Here we comment on these findings, highlighting several remaining questions.
Keywords: SMAC, lentiviral vector, dendritic cell, T cell, cancer
Second mitochondria-derived activator of caspases (SMAC) is an endogenous pro-apoptotic protein, which functions as an antagonist of inhibitor of apoptosis proteins (IAPs). Under normal conditions SMAC is confined to the mitochondria. However, upon receiving apoptogenic stimuli, SMAC is translocated to the cytosol where it binds to IAPs, resulting in the release of caspases. Deregulation of this mechanism for instance by immobilization of SMAC in the mitochondria or overexpression of IAPs is reported in several cancer types and is linked to evasion of tumor cells from apoptosis both during tumor development and during treatment with death inducing anti-cancer drugs. Therefore, it is not surprising that several strategies have been developed to mimic the action of SMAC (referred to as SMAC mimetics), demonstrating that SMAC mimetics have therapeutic potential either as a single agent or in combination with chemotherapeutics.1
It is generally accepted that successful death inducing agents need to prompt tumor cell death in such a way that it instigates a potent anti-tumor immune response. The concept of immunogenic tumor killing was introduced over a decade ago.2 Herein it is postulated that the mode of tumor cell killing and linked herewith the levels of cell death and components present within the dying tumor, determines whether or not tumor-resident immune cells, such as dendritic cells (DCs) engulf dying cells and become activated, hence are able to stimulate tumor-specific CD8+ cytotoxic T lymphocytes (CTLs). Today, several molecular mechanisms are linked to immunogenic cell death, including exposure of calreticulin (CRT), release of high mobility group box 1 (HMGB1) proteins and adenosine triphosphate (ATP) by dying tumor cells.3 Of these, CRT dictates the uptake of dying cells by DCs, whereas HMGB1 and ATP are involved in the DC’s activation.
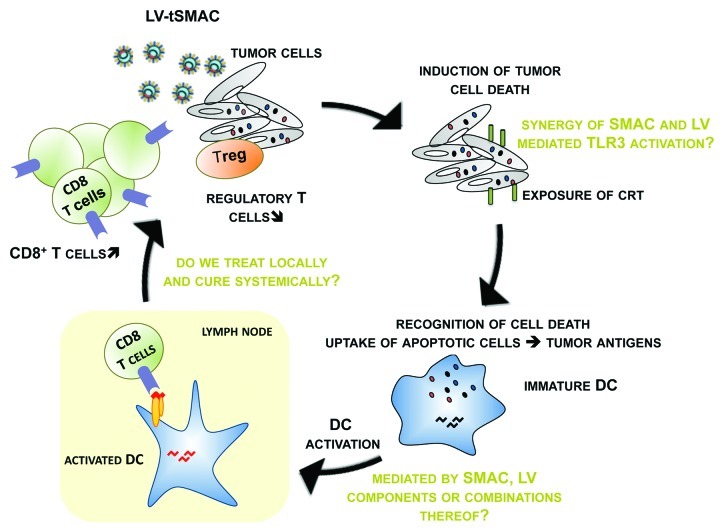
Based on this knowledge, we evaluated whether lentiviral delivery of the cytosolic form of SMAC (LV-tSMAC) resulted in immunogenic tumor cell killing.4 We demonstrated that treatment of tumor-bearing mice with a single intratumoral injection of only 107 transducing units of LV-tSMAC resulted in a significantly reduced tumor outgrowth in two melanoma models and even total tumor regression in a mastocytoma model. This favorable outcome was linked to the induction of apoptosis in situ and more importantly to the activation of anti-tumor immunity. The latter was mediated by DCs that upon uptake of dying tumor cells were activated. The net effect of this immune activation was an increase in functional CTL numbers and a decrease in regulatory T cell (Treg) numbers in the tumor. Surprisingly, dying tumor cells did not release HMGB1 or ATP, although they expressed CRT, suggesting alternative DC activating stimuli in this system (Fig. 1). These observations led us to hypothesize that the efficacy of our model might be due to the choice of delivery system, i.e., the use of lentiviral vectors (LVs), as these were previously shown to be a versatile tool to fight cancer.
Figure 1. Schematic representation of the findings published by Emeagi et al. on the link between SMAC mimetics, induction of tumor cell death and anti-tumor immunotherapy.4
A characteristic of LVs that has proven to be useful in LV-based cancer therapy is their ability to activate the immune system through interaction with among others toll-like receptor (TLR) 3 and 7.5 This characteristic might explain (1) the efficacy of LV-tSMAC to induce tumor cell death even at low multiplicity of infection, (2) the activation of DCs in the absence of HMGB1 and ATP, and linked herewith (3) the activation of CTLs and reduction in Treg.
Arguments for a potential synergy between LV triggered TLR3 activation and SMAC were provided by two independent research groups, who recently demonstrated that the synthetic TLR3 ligand polyI:C exerts pro-apoptotic activity in cancer cells, a mode of tumor cell killing that was enhanced by the combined use of SMAC mimetics.6,7
Activation of DCs through TLR3 (and 7) and its added benefit with regard to CTL activation and inhibition of Treg has been extensively described.8 Moreover, activation of DCs by LVs was described for both human and mouse DCs.5,8 Since we were unable to demonstrate HMGB1 or ATP release but observed DC activation upon uptake of dying tumor cells, we hypothesized that LV-tSMAC treated dying tumor cells might contain LV remnants, which are then responsible for DC activation. An alternative explanation might be that SMAC itself has an immune stimulatory function, as suggested by Müller-Sienerth et al.9 SMAC mimetics were shown to have complex effects on nuclear factor-κB (NF-κB) in DCs. Whereas SMAC mimetics themselves upregulated NF-κB, they reduced the activation of NF-κB by several activation stimuli. Nonetheless, treatment of human DCs with SMAC mimetics resulted in enhanced expression of NF-κB regulated stimulatory molecules, which are indispensable for potent anti-tumor immunity.
An intriguing observation in our study was the reduction in Treg numbers in the tumor. One might speculate that tumor-associated immune cells are simply eliminated as we showed that a significant percentage of these cells are also transduced by LV-tSMAC.4 However, at least for human T cells and DCs, it was shown that SMAC mimetics do not drive these into apoptosis.9 Alternatively, the reduction in Treg might be explained by the presence of TLR activating LV components. These might not only activate DCs, conditioning them to suppress Treg but might moreover have a direct negative impact on Treg function.10
In our experiments LV-tSMAC proved to be a potent anti-cancer compound. We demonstrated its direct cytotoxicity as well as its ability to instigate immune responses. Importantly, these were shown to aid initial tumor regression and to protect mice against subsequent tumor challenge.4 This suggests that local treatment of tumors with SMAC mimetics - be it alone or combined with TLR activation - might result in a systemic cure. The data presented above suggest that SMAC mimetics have immune regulatory effects, which can be exploited in immunotherapy of cancer and lead us to speculate that SMAC mimetics will become a lead compound in the treatment of cancer.
Glossary
Abbreviations:
- ATP
adenosine triphosphate
- CTL
cytotoxic T lymphocyte
- CRT
calreticulin
- DC
dendritic cell
- HMGB1
high mobility group box 1
- IAP
inhibitor of apoptosis protein
- LV
lentiviral vector
- TLR
toll-like receptor, Treg, regulatory T cell
- SMAC
second mitochondria-derived activator of caspases
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/20369
References
- 1.Chen DJ, Huerta S. Smac mimetics as new cancer therapeutics. Anticancer Drugs. 2009;20:646–58. doi: 10.1097/CAD.0b013e32832ced78. [DOI] [PubMed] [Google Scholar]
- 2.Melcher A, Gough M, Todryk S, Vile R. Apoptosis or necrosis for tumor immunotherapy: what’s in a name? J Mol Med (Berl) 1999;77:824–33. doi: 10.1007/s001099900066. [DOI] [PubMed] [Google Scholar]
- 3.Zitvogel L, Kepp O, Kroemer G. Decoding cell death signals in inflammation and immunity. Cell. 2010;140:798–804. doi: 10.1016/j.cell.2010.02.015. [DOI] [PubMed] [Google Scholar]
- 4.Emeagi PU, Van Lint S, Goyvaerts C, Maenhout S, Cauwels A, McNeish IA, et al. Proinflammatory Characteristics of SMAC/DIABLO-Induced Cell Death in Antitumor Therapy. Cancer Res. 2012;72:1342–52. doi: 10.1158/0008-5472.CAN-11-2400. [DOI] [PubMed] [Google Scholar]
- 5.Breckpot K, Escors D, Arce F, Lopes L, Karwacz K, Van Lint S, et al. HIV-1 lentiviral vector immunogenicity is mediated by Toll-like receptor 3 (TLR3) and TLR7. J Virol. 2010;84:5627–36. doi: 10.1128/JVI.00014-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weber A, Kirejczyk Z, Besch R, Potthoff S, Leverkus M, Häcker G. Proapoptotic signalling through Toll-like receptor-3 involves TRIF-dependent activation of caspase-8 and is under the control of inhibitor of apoptosis proteins in melanoma cells. Cell Death Differ. 2010;17:942–51. doi: 10.1038/cdd.2009.190. [DOI] [PubMed] [Google Scholar]
- 7.Friboulet L, Gourzones C, Tsao SW, Morel Y, Paturel C, Témam S, et al. Poly(I:C) induces intense expression of c-IAP2 and cooperates with an IAP inhibitor in induction of apoptosis in cancer cells. BMC Cancer. 2010;10:327. doi: 10.1186/1471-2407-10-327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Breckpot K, Emeagi P, Dullaers M, Michiels A, Heirman C, Thielemans K. Activation of immature monocyte-derived dendritic cells after transduction with high doses of lentiviral vectors. Hum Gene Ther. 2007;18:536–46. doi: 10.1089/hum.2007.006. [DOI] [PubMed] [Google Scholar]
- 9.Müller-Sienerth N, Dietz L, Holtz P, Kapp M, Grigoleit GU, Schmuck C, et al. SMAC mimetic BV6 induces cell death in monocytes and maturation of monocyte-derived dendritic cells. PLoS One. 2011;6:e21556. doi: 10.1371/journal.pone.0021556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang RF. Regulatory T cells and toll-like receptors in cancer therapy. Cancer Res. 2006;66:4987–90. doi: 10.1158/0008-5472.CAN-05-4676. [DOI] [PubMed] [Google Scholar]