Abstract
Current ovarian cancer treatments based on surgery/chemotherapy show limited efficacy. Targeting immunosuppression is a requirement for the effectiveness of novel promising anti-tumor immunotherapies. Our latest work in preclinical models shows that nanoparticle-mediated delivery of immunostimulatory microRNAs specifically to tumor-associated leukocytes is sufficient to re-program immunological control of metastatic ovarian cancers.
Keywords: dendritic cell, Immunosuppression, microRNA, ovarian cancer, tumor immunology
microRNAs (miRNAs) are genome-encoded regulatory RNAs of 18 to 25 nucleotides that are processed by RNase type III Dicer from precursor stem-loop RNA hairpins. miRNAs loaded in Argonaute-containing multi-protein complexes (RNA-induced silencing complex; RISC) act as guides that bind to complementary sequences in the 3′ untranslated region of target mRNAs and thereby control gene expression via mRNA degradation and/or translational repression. miRNA-mediated regulation can coordinately affect hundreds of targets and drive distinct phenotypes. miRNAs, therefore, can have an enormous impact on genomic regulation and play a crucial role in carcinogenesis. In ovarian cancer, miRNA dysregulation correlates with clinical parameters and histopathological subtypes.1 However, little is known about how alteration of miRNAs expression in non-cancer cells embedded in the tumor microenvironment (TME) influence ovarian cancer progression.
We have demonstrated that myeloid cells with functional activities and markers of tolerogenic dendritic cells (DCs) are the most abundant leukocyte subset infiltrating solid ovarian cancer masses. These cells, rather than supporting anti-tumor immunity, drive neo-vascularization and immunosuppression, thereby promoting malignant progression.2,3 More relevant for translational purposes, we also showed that tumor-associated DCs can be transformed into effective antigen-presenting cells in the right milieu.4 We therefore hypothesized that increasing the levels of immunostimulatory miRNAs could trigger a cascade of genome-wide changes in these plastic cells, re-programing their capacity to boost protective immunity. To test this, we generated double-stranded RNA oligonucleotides mimicking the sequence of endogenous miR-155 precursor RNA hairpin. We focused on supplementing miR-155 because it is required for effective antigen presentation.5 In addition, its negligible expression in tumor-associated DCs can be upregulated with synergistic CD40 and TLR agonists.6
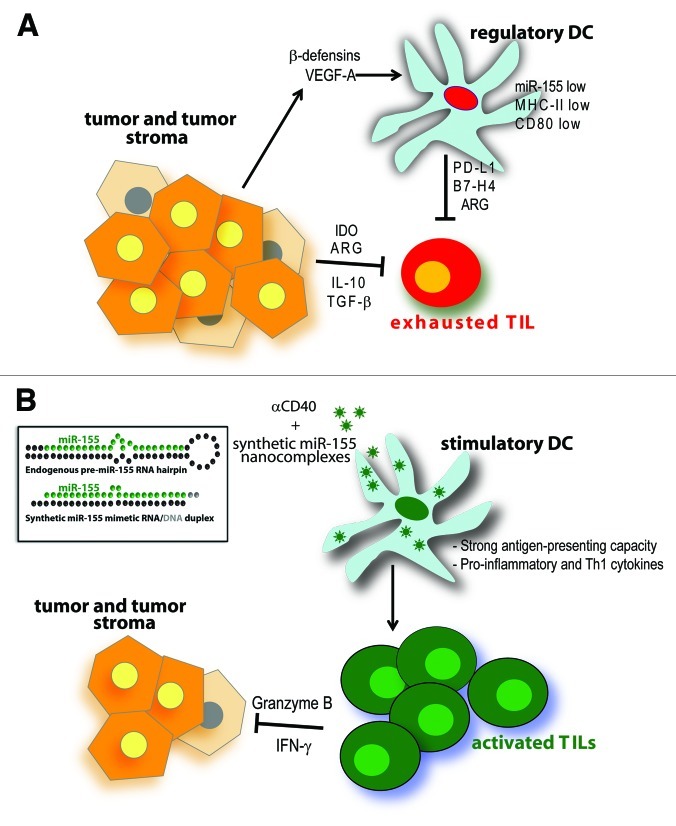
In our hands, delivery of functional RNA specifically to cancer cells in vivo has been extremely challenging due to low bioavailability and poor cellular uptake. In contrast, the enhanced endocytic pathways and accessibility of ovarian cancer-associated myeloid leukocytes make them ideal targets for nanocarrier-mediated delivery.7 Correspondingly, we confirmed that nanocomplexes of synthetic miR-155 and biocompatible polyethylenimine (PEI) are selectively engulfed by immunosuppressive myeloid leukocytes in vivo, in the absence of targeting motifs.6 miRNA mimetic compounds were processed into functional mature miR-155, which elicited dramatic changes in nearly half of transcripts.6 Therapeutic supplementation of miR-155 re-programmed tolerogenic DCs into immunostimulatory cells capable of triggering robust T cell-mediated anti-tumor immunity (Fig. 1). Interestingly, only RNA duplexes mimicking the bulged structure of endogenous miR-155 precursor elicited optimal silencing of known targets and, subsequently, therapeutically significant immune protection.6 This suggests that weakened thermodynamic stability of RNA duplexes (as in endogenous miRNAs) is critical for the activation (selective incorporation of the mature strand into specific Argonaute isoform-containing RISCs) of synthetic miRNAs, which has important implications for the design of therapeutic compounds.
Figure 1. Re-programming tumor-associated DCs using miR-155 nanoparticles. (A) Ovarian cancers can directly control the cytotoxic activity of tumor-infiltrating T cells (TILs) by producing IL-10, TGF-β, indoleamine 2,3-dioxygenase (IDO) and arginase (ARG). In addition, they express β-defensins and VEGF-A, which recruit and transform immature DCs into pro-angiogenic and immunosuppressive cells. These atypical DCs show defective antigen-presenting capacity and express immunosuppressive molecules such as PD-L1, ARG and B7-H4, among many others. Overall, the immunosuppressive molecules produced in the TME inhibit the activity of anti-tumor T cells, thus contributing to tumor growth and progression. (B) Intraperitoneally-injected nanoparticles encapsulating synthetic miR-155 mimetic compound (inset) are preferentially engulfed by ovarian cancer-associated DCs. In combination with CD40 stimulation, miR-155 supplementation induces potent activation/maturation of tumor-associated DC, a process that transforms them into immunostimulatory cells that promote the expansion and function of anti-tumor T cells.
Because immune responses – including those against tumor antigens – depend on rapid phenotypic changes in multiple cell types, the overall effect of supplementing a single miRNA is not totally surprising. However, we were astonished that synthetic miR-155 could directly or indirectly alter the expression of thousands of transcripts. Among the multiple genes silenced, some were previously described immunosuppressive targets, including C/epbβ and multiple members of the Tgf-β pathway.6 Our study also identified Cd200, a known mediator of DC-induced tolerance, along with the master genomic organizer Satb1, as direct targets of miR-155.6 Correspondingly, the optimized design of our miRNA-mimicking oligonuleotides can be widely applied to understand the role of miRNAs in phagocytic immune cells in different processes. For instance, immunoprecipitation of cross-linked labeled sequences coupled to deep sequencing analyses is allowing us to define the repertoire of mRNA targets physically binding to this miRNA in vivo (unpublished results). We propose that identifying bona fide targets of individual miRNAs would overcome one of the most significant hurdles in the field, as different bioinformatical algorithms typically generate non-overlapping predictions.
Most importantly, synthetic miR-155-carrying nanoparticles induced significant survival increases in ovarian cancer-bearing mouse models. Furthermore, tumor rejection was evident in ~30% of animals as concurrently administered CD40 agonists synergized with miR-155-mediated anti-tumor effects.6 As iterations of “surgical debulking+chemotherapy” interventions have resulted in very modest improvements in the dismal prognosis of ovarian cancer patients in the past 40 y, complementary immunotherapies could offer significant hope. Supporting this proposition, T cells exert spontaneous pressure against the progression of ovarian cancer,8 which is eventually overcome by immunosuppressive mechanisms primarily orchestrated by microenvironmental DCs.3 Thus, ovarian cancers remain significantly antigenic, but eliciting immune control of advanced tumors will likely require concurrent targeting of tumor-induced immunosuppression. Our platform could be applied as an individual intervention or as part of combinatorial approaches. Since we bypassed the use of virus-based vectors by transiently and reversibly enhancing miRNA activity in short-lived tumor-associated DCs, our therapy could be implemented in the clinic more easily. In fact, both PEI-based nanocomplexes and RNA duplexes can be produced under Good Manufacturing Practices (GMP) and are being separately tested through clinical trials. Moreover, a fully human anti-CD40 antibody is commercially available and has also being used in cancer patients as a potent immunotherapeutic agent.9 Although the compartmentalized (peritoneal) nature of ovarian cancer makes it an ideal disease for nanomaterial-based interventions, synthetic miRNAs could also have significant effects upon intratumoral administration against other types of metastatic cancer. Ongoing experiments are testing this possibility. Given that virtually all solid tumors induce the mobilization of immunosuppressive myeloid leukocytes to the TME, modulating miRNA activity in these cell types could become a widely applicable and effective intervention.
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/20020
References
- 1.Zhang L, Volinia S, Bonome T, Calin GA, Greshock J, Yang N, et al. Genomic and epigenetic alterations deregulate microRNA expression in human epithelial ovarian cancer. Proc Natl Acad Sci U S A. 2008;105:7004–9. doi: 10.1073/pnas.0801615105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cubillos-Ruiz JR, Rutkowski M, Conejo-Garcia JR. Blocking ovarian cancer progression by targeting tumor microenvironmental leukocytes. Cell Cycle. 2010;9:260–8. doi: 10.4161/cc.9.2.10430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scarlett UK, Rutkowski MR, Rauwerdink AM, Fields J, Escovar-Fadul X, Baird J, et al. Ovarian cancer progression is controlled by phenotypic changes in dendritic cells. J Exp Med. 2012 doi: 10.1084/jem.20111413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cubillos-Ruiz JR, Engle X, Scarlett UK, Martinez D, Barber A, Elgueta R, et al. Polyethylenimine-based siRNA nanocomplexes reprogram tumor-associated dendritic cells via TLR5 to elicit therapeutic antitumor immunity. J Clin Invest. 2009;119:2231–44. doi: 10.1172/JCI37716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rodriguez A, Vigorito E, Clare S, Warren MV, Couttet P, Soond DR, et al. Requirement of bic/microRNA-155 for normal immune function. Science. 2007;316:608–11. doi: 10.1126/science.1139253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cubillos-Ruiz JR, Baird JR, Tesone AJ, Rutkowski MR, Scarlett UK, Camposeco-Jacobs AL, et al. Reprogramming tumor-associated dendritic cells in vivo using microRNA mimetics triggers protective immunity against ovarian cancer. Cancer Res. 2012 doi: 10.1158/0008-5472.CAN-11-3160. Epub ahead of press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cubillos-Ruiz JR, Fiering S, Conejo-Garcia JR. Nanomolecular targeting of dendritic cells for ovarian cancer therapy. Future Oncol. 2009;5:1189–92. doi: 10.2217/fon.09.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang L, Conejo-Garcia JR, Katsaros D, Gimotty PA, Massobrio M, Regnani G, et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med. 2003;348:203–13. doi: 10.1056/NEJMoa020177. [DOI] [PubMed] [Google Scholar]
- 9.Beatty GL, Chiorean EG, Fishman MP, Saboury B, Teitelbaum UR, Sun W, et al. CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science. 2011;331:1612–6. doi: 10.1126/science.1198443. [DOI] [PMC free article] [PubMed] [Google Scholar]