Abstract
The therapeutic efficacy of oncolytic viruses including adenovirus has been thought to depend mostly on direct viral destruction of tumor cells. However, this view has changed with the discovery that oncolysis can also induce innate and antigen-specific adaptive immunity against the tumor. Here we summarize our findings from cancer patients.
Keywords: adenoviruses, immunotherapy, immunovirotherapy, oncoimmunology, oncolytic adenoviruses
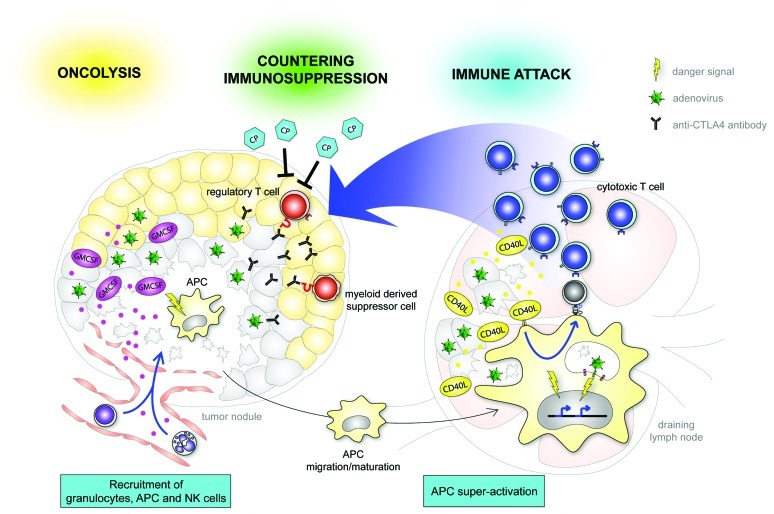
Oncolytic virotherapy for cancer is currently under investigation in phase I-III clinical trials. Oncolytic viruses (OVs) are specifically engineered to preferentially infect, replicate in, and kill tumor cells. For the past few decades, the therapeutic efficacy of these agents has been thought to depend mostly on the direct tumor cell destructive effect of replication . This thinking has changed with the discovery that oncolytic viruses not only elicit bystander anti-tumor effects through ‘generic’ inflammation but may also induce antigen-specific adaptive immunity against the tumor. As adenovirus interacts with a variety of receptors of the innate immune system mainly expressed in dendritic cells (DCs) and other antigen presenting cells (APCs), we hypothesized that by arming our oncolytic viruses with immunostimulatory transgenes we could increase the magnitude of the ensuing immune response (Fig. 1).
Figure 1. A schematic overview of the main tools and strategies used in our laboratory and of their interactions with the tumor and host. In the vanguard, fiber chimeric oncolytic adenovirus encoding GMCSF, Ad5/3-D24-GMCSF (CGTG-102), has demonstrated promising potency in both preclinical and clinical use.1,3 This virus is now entering Phase I clinical trials in the EU and US (sponsored by Oncos Therapeutics Ltd). Another promising clinical candidate, Ad5/3-D24-CD40L (CGTG-401), functions both at the tumor site and at the stromal interface to promote anti-tumor immunity.6,7Adenovirus expressing anti-CTLA4 antibody, Ad5/3-D24-aCTLA4 (CGTG-701), and/or metronomic dosing of cyclophosphamide9 can be used to reduce tumor immunosuppression.
Preclinical studies are challenging with oncolytic viruses coding for human immunostimulatory molecules because of species incompatibility issues relating to both the virus and the transgene. One exception is oncolytic adenovirus coding for granulocyte-macrophage colony-stimulating factor (GMCSF), as Syrian hamsters seem semipermissive to both components. We found that Ad5-D24-GMCSF was able to induce tumor specific T cell memory that was able to fully protect Syrian Hamsters from subsequent rechallenge with the same tumor.1 In the same manuscript, we summarized immunological data obtained with Ad5-D24-GMCSF in our advanced therapy access program (ATAP). This was the first demonstration that armed OV therapy is able to elicit tumor-specific T cell responses also in human cancer patients.1
To increase gene delivery to tumor tissues we moved next to capsid-modified adenoviruses. To this end, we generated Ad5/3-D24-GMCSF, a chimera whose receptor-binding moeity (fiber) originates from adenovirus serotype 3, which enters through the tumor associated desmoglein 2 receptor,2 instead of the coxsackie-adenovirus receptor which is frequently downregulated in advanced tumors. We also constructed Ad5-RGD-D24-GMCSF, where the fiber contains an integrin-binding RGD-4C motifs. Both of these viruses showed safety and signs of efficacy in cancer patients.3,4 Anti-adenoviral and antitumoral immune responses were elicited in several patients in ELISPOT assays.
One intriguing part of these patient treatments was that ex-vivo assays were utilized to select the best virus for each patient, emphasizing the personalized medicine approach of ATAP.
GMCSF acts through several mechanisms, including direct recruitment of natural killers (NK) and DCs. GMCSF can also specifically activate DCs at the tumor site to increase expression of co-stimulatory molecules to enhance cross-priming and T cell activation rather than cross-tolerance. However, GMCSF has no direct effect on tumor cells and does not function as co-stimulatory molecule on DCs.5 Thus, next we generated an oncolytic adenovirus that expresses the soluble form of CD40 ligand (CD40L), Ad5/3-hTERT-CD40L.
CD40L binds to CD40 on DCs and functions as a potent co-activating signal during T cell stimulation and it may also induce apoptosis of cancer cells on its own. We demonstrated efficacy of the approach in both human xenograft and syngeneic mouse models6 and preclinical work led to treatment of cancer patients.7 Our findings underscored multi-modal and possibly immunologically synergistic killing of tumors through oncolysis, CD40L-mediated apoptosis, cytokine secretion and induction of anti-tumor T cell responses. Importantly, we observed increased calreticulin exposure as well as release of HMGB1 and ATP into the extracellular space, which have been proposed as signs of immunogenic cell death. Tumors treated with the CD40L-expressing virus also displayed an increase in the presence of macrophages and cytotoxic CD8+ T cells, but not B-cells.6 In cancer patients, high levels of virus, CD40L and RANTES were documented locally at the tumor site. Peripheral blood mononuclear cells were analyzed by IFN-g ELISPOT and induction of both tumor-specific and adenovirus-specific T cells was observed. Up to 83% of treated patients displayed evidence of possible therapeutic benefits7 suggesting a potent Th1 type response. Interestingly neutralizing antibodies were not induced to the same degree as with our previous viruses.
Our ATAP data are in accord with the relatively recent realization (well demonstrated by sipuleucel-T and ipilimumab) that the problem in cancer immunotherapy is not so much achieving an anti-tumor response, but dealing with tumor induced immunosuppression. To this end, we have utilized co-administration of low dose metronomic cyclophosphamide (CP) to selectively downregulate regulatory T cell (Tregs).8
Another approach not yet tested in patients is to counter tumor immunosuppression by blocking the cytotoxic T lymphocyte-associated antigen-4 (CTLA-4, CD152) by a full length human monoclonal antibody produced by tumor cells from an oncolytic adenovirus.9
In conclusion, our preclinical and clinical experience has taught us two key lessons about cancer immunotherapy:
1. Every tumor and patient is unique It is quite likely that efficient OV based therapy instead consist of a custom adaptation of an arsenal of tools. We envision a combination of two or more non-overlapping and synergistically armeded oncolytic viruses coupled with chemotherapy used in an immunostimulatory manner. The classic view of chemotherapy as immune suppressive may need updating since emerging data suggests some chemotherapeutics, when used in the right way, can be immunostimulatory.
2. The genetically unstable nature of tumors allows them to develop resistance to any drug including oncolytic viruses. When subjected to oncolytic virotherapy, tumors can learn to avoid adaptive immunity through immunoediting but may also mount antiviral defenses.10 Through an improved understanding of oncolysis we may be able to design combination therapies that can counteract or delay resistance.
Disclosure of Potential Conflicts of Interest
A.H. is a shareholder of and consultant to Oncos therapeutics, Ltd.
Glossary
Abbreviations:
- OV
oncolytic adenovirus
- DC
dendritic cell
- APC
antigen presenting cell
- GMCSF
granulocyte-macrophage colony-stimulating factor
- ATAP
advanced therapy access program
- NK
natural killer cells
- T reg
Regulatory T cell
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/20172
References
- 1.Cerullo V, Pesonen S, Diaconu I, Escutenaire S, Arstila PT, Ugolini M, et al. Oncolytic adenovirus coding for granulocyte macrophage colony-stimulating factor induces antitumoral immunity in cancer patients. Cancer Res. 2010;70:4297–309. doi: 10.1158/0008-5472.CAN-09-3567. [DOI] [PubMed] [Google Scholar]
- 2.Wang H, Li ZY, Liu Y, Persson J, Beyer I, Möller T, et al. Desmoglein 2 is a receptor for adenovirus serotypes 3, 7, 11 and 14. Nat Med. 2011;17:96–104. doi: 10.1038/nm.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koski A, Kangasniemi L, Escutenaire S, Pesonen S, Cerullo V, Diaconu I, et al. Treatment of cancer patients with a serotype 5/3 chimeric oncolytic adenovirus expressing GMCSF. Mol Ther. 2010;18:1874–84. doi: 10.1038/mt.2010.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Trottier MD, Jr., Palian BM, Reiss CS. VSV replication in neurons is inhibited by type I IFN at multiple stages of infection. Virology. 2005;333:215–25. doi: 10.1016/j.virol.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 5.Nowak AK, Lake RA, Marzo AL, Scott B, Heath WR, Collins EJ, et al. Induction of tumor cell apoptosis in vivo increases tumor antigen cross-presentation, cross-priming rather than cross-tolerizing host tumor-specific CD8 T cells. J Immunol. 2003;170:4905–13. doi: 10.4049/jimmunol.170.10.4905. [DOI] [PubMed] [Google Scholar]
- 6.Balachandran S, Barber GN. Vesicular stomatitis virus (VSV) therapy of tumors. IUBMB Life. 2000;50:135–8. doi: 10.1080/713803696. [DOI] [PubMed] [Google Scholar]
- 7.Coccia EM, Romeo G, Nissim A, Marziali G, Albertini R, Affabris E, et al. A full-length murine 2-5A synthetase cDNA transfected in NIH-3T3 cells impairs EMCV but not VSV replication. Virology. 1990;179:228–33. doi: 10.1016/0042-6822(90)90292-Y. [DOI] [PubMed] [Google Scholar]
- 8.Cerullo V, Diaconu I, Kangasniemi L, Rajecki M, Escutenaire S, Koski A, et al. Immunological effects of low-dose cyclophosphamide in cancer patients treated with oncolytic adenovirus. Mol Ther. 2011;19:1737–46. doi: 10.1038/mt.2011.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Clercq E, De Somer P. Comparative study of the efficacy of different forms of interferon therapy in the treatment of mice challenged intranassaly with vesicular stomatitis virus (VSV) Proc Soc Exp Biol Med. 1971;138:301–7. doi: 10.3181/00379727-138-35884. [DOI] [PubMed] [Google Scholar]
- 10.Liikanen I, Monsurrò V, Ahtiainen L, Raki M, Hakkarainen T, Diaconu I, et al. Induction of interferon pathways mediates in vivo resistance to oncolytic adenovirus. Mol Ther. 2011;19:1858–66. doi: 10.1038/mt.2011.144. [DOI] [PMC free article] [PubMed] [Google Scholar]