Abstract
The immune milieu in malignant tumors can influence the prognosis of patients. However, little is known about the effect the antitumoral therapy has on the inflammatory infiltrate. In head and neck squamous cell carcinoma we found evidence that a neoadjuvant radiochemotherapy could modulate the composition of the intratumoral inflammation into an anti-tumoral pattern.
Keywords: cytotoxic T-cells, head and neck squamous cell carcinomas, radiochemotherapy, regulatory T cells
Radiochemotherapy (RCT) is nowadays a standard procedure in the treatment of a large variety of malignant tumors. The therapeutic regimens which have been developed over the past decades are so potent, that in many cases the bulk of the tumor cells can be eliminated. However, a few tumor cells, e.g., proliferative inactive ones, survive which can later be the source of tumor relapse and metastases. Therefore, it appears vital for a successful tumor therapy that those RCT-insensitive cells are eliminated by other means. One potential mediator of such an anti-tumoral response is the immune-system of the patient. In fact, it has already been reported for head and neck squamous cell carcinomas (HNSSC) that in cases with a very limited inflammatory infiltrate at the invasive front the overall survival is decreased and locoregional recurrences are increased arguing for a role of the immune reaction in anti-tumoral mechanisms.1 Moreover, an accumulation of regulatory T cells in HNSCC has been found to be associated with an unfavorable prognosis.2 As regulatory T cells (Treg) inhibit the function of effector T cells this would also go in line with the idea that a specific anti-tumoral immune reaction takes place in HNSCC, which can be suppressed by Treg.
Little is known of the effect RCT has on the intra-tumoral inflammatory infiltrate. RCT might have an impact on the number or composition of the inflammatory cells in the treated tumors and, hypothetically, this effect might drive the local immune-milieu in either an anti-tumoral or pro-tumoral direction. In an earlier investigation of HNSSC, indeed, we found that factors like the tumor stage and the therapeutic scheme can influence the composition and even the prognostic impact of tumor infiltrating lymphocytes. Whereas in a group of patients with early disease treated by surgery and adjuvant radiotherapy high B cell counts were associated with a better prognosis. The opposite effect was seen in patients with advanced disease treated by RCT.3
These first findings prompted us to investigate the effect RCT has on the composition of the tumor infiltrating inflammatory cell profile by comparing HNSCC before and after RCT.4 A group of patients was selected which was diagnosed with HNSCC and treated by neoadjuvant RCT followed by surgical removal of the remaining tumor. Pretherapeutic biopsies were compared with resected tumor tissue after RCT. The stromal and intraepithelial infiltration of different inflammatory cells subtypes in the tumor was quantified. Moreover, cases with residual tumor after RCT (postRCT+) and without tumor rests (postRCT-) were compared. CD3 was used as a pan-T cell marker, CD4 for T helper cells, CD8 and GranzymeB as markers of cytotoxic T cells (CTL), FoxP3 for regulatory T cells (Treg), CD20 for B cells, CD25 as a lymphocytic activation marker, CD68 as a macrophage marker and CD1a as a marker of immature dendritic cells.
The neoadjuvant RCT lead to a general decrease of the number of tumor infiltrating cells and to a decrease of the proliferative fraction of tumor cells as measured by Ki-67 expression. Interestingly, this effect was much more pronounced on the number of Treg, than on the CTL number (CD8 and GranzymeB). Therefore, the ratio of CTL/Treg increased by a factor 2 to 3 after RCT and, indeed, this shift toward a more cytotoxic response was associated with a better event free survival in cases with a high CTL/Treg ratio, going in line with the idea that CTL mediate an anti-tumoral response that is inhibited by Treg.
Remarkably, the effect of Treg on the prognosis of HNSCC patients diametrically changed after RCT. Before RCT high numbers of Treg were associated with a better chance of complete tumor remission which was linked to a better survival. After RCT high Treg numbers were prognostically unfavorable. This dual effect of Treg on survival at first glance appears paradoxical. One possible explanation, however, could be that in the untreated tumor high numbers of Treg suppress the general inflammatory intratumoral reaction. Chronic inflammation has been widely accepted as an important factor in tumorigenesis.5 So Treg in the pre RCT context might attenuate the harmful oncogenic effect of chronic inflammation. In contrast, the unfavorable effect of Treg after RCT might indeed be a sign of a suppression of the specific anti-tumoral immune response.
The biggest proportion of inflammatory cells was localized in the tumor stroma, whereas the number of intraepithelial cells was small in comparison. After RCT the relative number of intraepithelial inflammatory cells compared with stromal inflammatory cells was increased. Immature CD1a expressing dendritic cells were significantly increased in the remaining scar after RCT in cases without residual tumor compared with the sclerotic stroma in cases with remaining tumor cells. High numbers of CD1a expressing cells after RCT had a beneficial effect on the prognosis, maybe indicating an on-going antigen-presentation to prime effector cells able to eliminate the remaining tumor cells.
Taken together these results might indicate that an adjuvant RCT not only operates via the killing of the proliferative fraction of the malignant tumor cells indicated by the reduction of the Ki-67 positive proportion of tumor cells, but also via the promotion and enhancement of an anti-tumoral immune microenvironment, which might even help to eliminate remaining, resting tumor cells, that would not be effectively killed by the radiation or chemotherapeutic agent itself.
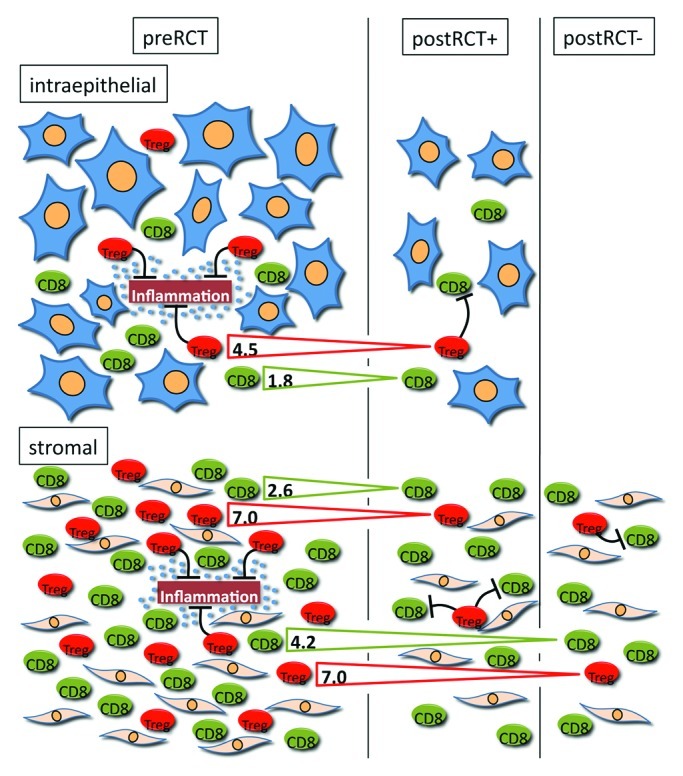
Figure 1.Radiochemotherapy induced changes of the inflammatory infiltrate in the stromal and intraepithelial compartment of HNSCC and possible functions of Treg in tumor progression: On the left side the situation in the untreated tumor is depicted with a higher number of inflammatory cells in the stromal compared with the intraepithelial compartment. In this context Treg might have anti-tumoral effects by the suppression of a potentially oncogenic chronic inflammation. On the right side two possible outcomes after RCT are shown. In some tumors after RCT residual tumor epithelia were still present (postRCT+) whereas in others no tumor rests were detectable and only a scar remained in the former localization of the carcinoma (postRCT+). Like in the preRCT state the numbers of inflammatory cells in the stroma predominated, however, the ratio was changed in favor of the intraepithelial cells. Treg and CTL numbers decreased after RCT in both compartments. The numbers in the arrows indicate the fold reduction of both cell types when comparing tumors before and after treatment (+/− residual tumor). The effect of RCT on Treg numbers was more pronounced than on CTL numbers inducing a shift toward a higher CTL/Treg ratio. In this setting low numbers of Treg might be beneficial by a reduced suppressive effect on tumor specific CTL and thereby the anti-tumoral immune response.
Glossary
Abbreviations:
- RCT
Radiochemotherapy
- HNSSC
head and neck squamous cell carcinomas
- Treg
regulatory T cells
- CTL
cytotoxic T-lymphocytes
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/20200
References
- 1.Brandwein-Gensler M, Teixeira MS, Lewis CM, Lee B, Rolnitzky L, Hille JJ, et al. Oral squamous cell carcinoma: histologic risk assessment, but not margin status, is strongly predictive of local disease-free and overall survival. Am J Surg Pathol. 2005;29:167–78. doi: 10.1097/01.pas.0000149687.90710.21. [DOI] [PubMed] [Google Scholar]
- 2.Strauss L, Bergmann C, Szczepanski M, Gooding W, Johnson JT, Whiteside TL. A unique subset of CD4+CD25highFoxp3+ T cells secreting interleukin-10 and transforming growth factor-beta1 mediates suppression in the tumor microenvironment. Clin Cancer Res. 2007;13:4345–54. doi: 10.1158/1078-0432.CCR-07-0472. [DOI] [PubMed] [Google Scholar]
- 3.Distel LV, Fickenscher R, Dietel K, Hung A, Iro H, Zenk J, et al. Tumour infiltrating lymphocytes in squamous cell carcinoma of the oro- and hypopharynx: prognostic impact may depend on type of treatment and stage of disease. Oral Oncol. 2009;45:e167–74. doi: 10.1016/j.oraloncology.2009.05.640. [DOI] [PubMed] [Google Scholar]
- 4.Tabachnyk M, Distel LV, Büttner M, Grabenbauer GG, Nkenke E, Fietkau R, et al. Radiochemotherapy induces a favourable tumour infiltrating inflammatory cell profile in head and neck cancer. Oral Oncol. 2012;••• doi: 10.1016/j.oraloncology.2012.01.024. [DOI] [PubMed] [Google Scholar]
- 5.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–7. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]


