Abstract
Myeloid derived suppressor cells (MDSC) suppress anti-tumor immune responses. Our recent publication provides evidence that SHIP-1 plays a prominent role in pancreatic tumor development by regulating MDSC. Therefore, SHIP-1 may be a potential therapeutic target for the treatment of MDSC-related hematological malignancies and solid tumors.
Keywords: Src homology inositol phosphatase, anti-tumor immunity, immune suppression, myeloid-derived suppressor cells, pancreatic cancer
It is paramount to have functional T cell immunity prior to implementing immunotherapy strategies to eradicate tumors in cancer patients. In patients with solid tumors, there is the expansion of a plethora of immunosuppressive leukocytes that contributes to the significant suppression of CD8+ T cell (anti-tumor) immune responses. Ineffective cancer therapies against solid tumors are partially due to the expansion of regulatory immunosuppressive cells known as Myeloid Derived Suppressor Cells (MDSC). These regulatory cells have the capacity to suppress innate and adaptive immune responses. MDSC are a heterogeneous population of immature macrophages, granulocytes and dendritic cells (DC) found in mice and humans.1 In mice, MDSC are generally characterized as Gr-1+CD11b+, whereas human MDSC are characterized as CD33+HLA-DR-Lin-.1 Tumor-induced MDSC are expanded in the peripheral blood, lymphoid organs, and also in the tumor where they reduce CD8+ T cell responses.2 Accumulation of these MDSC is triggered by tumor-derived soluble factors that stimulate myelopoiesis.1 These immature MDSC can differentiate into mature immune cells depending on the cytokine milieu.1 MDSC are essential for immune homeostasis and should not be completely eliminated from the microenvironment. Therefore, novel therapeutic strategies, that can specifically restore MDSC immune homeostasis and enhance immunotherapy responses in patients with MDSC-related cancers, are warranted.
Src Homology Inositol Phosphatase (SHIP-1) is a 5′-inositol phosphatase and adaptor protein that negatively regulates the PI3K/AKT downstream signaling that regulates numerous cellular processes.3 More importantly, SHIP-1 is primarily expressed in hematopoietic cells and responds to inflammatory cytokines.4 Therefore SHIP-1 is important for innate as well as adaptive immune responses.4 SHIP-1 may act as a tumor suppressor during leukemogenesis and lymphomagenesis in humans, whereas SHIP deficiency results in myeloproliferative diseases (MPD) in mice.4 However, SHIP deficient (SHIPKO) mice have a significant expansion of MDSC in their lymphoid compartments, which contributes to the immunosuppression of allogeneic T cell responses in vitro and in vivo.5 In addition, SHIPKO mice have altered immunosuppressive Regulatory T cells (Treg), natural killer (NK), B and mast cells, monocytes and macrophage development and function.3 Thus far, SHIP-1 mutations and loss of expression, have only been described in blood malignancies, not solid tumors.3
Lakhanpal et al. (2010) reported that the lack of SHIP-1 does not lead to tumor development, per se, but accelerates tumor progression.6 We were the first to demonstrate, in our recent PLoS ONE publication,7 that SHIP-1 has a critical role in “solid tumor” progression, in particular, murine pancreatic cancer. Our results strongly suggest that SHIP-1 may be a potential therapeutic target for combating pancreatic tumor progression. We observed the presence of pro-inflammatory cytokines (IL-6, MCP-1 and IL-10) released from murine Panc02 cells in vitro. The subcutaneous injections of murine Panc02 cells into immunocompetent C57BL/6 mice, led to tumor progression as well as enlarged spleens (splenomegaly). This splenomegaly phenotype is also observed in SHIPKO mice. This prompted us to investigate SHIP-1 expression in our pancreatic tumor-bearing (TB) mice. We observed a reduction in SHIP-1 mRNA and protein expression but no differences in SHIP-2 and PTEN protein expression in splenocytes from TB compared with wild-type (WT) mice. More importantly, we also observed that soluble factors from murine Panc02 cells cause the downregulation of SHIP-1 in splenocytes in vitro. In addition to the downregulation of SHIP-1 expression in vivo and in vitro, we observed altered PI3K/AKT downstream signaling events in spleens and sorted MDSC from TB compared with WT mice. Functional analyses revealed that sorted TB MDSC significantly suppressed antigen–specific CD8+ T cell immune responses compared with sorted WT-MDSC in vitro (Fig. 1). We also observed an increase in Tregs but a decrease in B cell percentages in our TB mice.7 It is evident that our pancreatic cancer mouse model has a similar phenotype to SHIPKO mice with perturbed myelopoiesis, which negatively influences the development and function of immune leukocytes, especially regulatory MDSC.
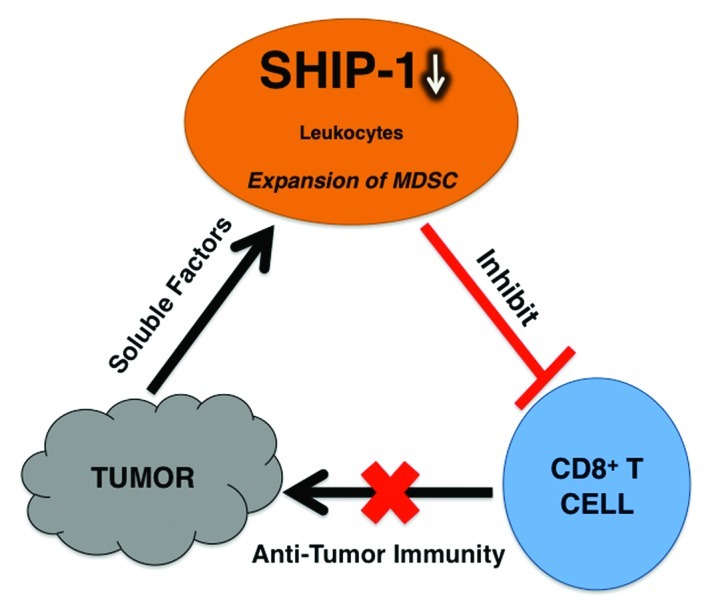
Figure 1. Murine pancreatic cancer cells release soluble factors that promote the downregulation of SHIP-1 protein expression in leukocytes from pancreatic tumor-bearing (TB) mice. In addition, TB mice have an expansion of immunosuppressive Myeloid Derived Suppressor Cells (MDSC), which suppress CD8+ T cell immune responses.7 Therefore, tumor-bearing mice have reduced anti-tumor immunity which renders cancer therapies (immunotherapy) ineffective to combat pancreatic cancer tumor progression.
Our data strongly suggest that SHIP-1 expression is dampened by pro-inflammatory cytokines from murine pancreatic cancer. Therefore, we are using this model to elucidate the molecular mechanism(s) by which soluble factors transcriptionally and translationally repress SHIP-1 expression and promote the induction of immunosuppressive leukocytes (i.e., MDSC) in a tumor microenvironment. Our results raise the question as to whether the suppression of SHIP-1 expression is specific to pancreatic cancer or translatable to other solid tumors. Solid tumors release diverse soluble factors that cause the expansion of a variation of tumor-induced MDSC subsets with different modes of suppression.
Recently, research interests have been focused on microRNAs and transcription factors and their role in cancer progression. Reports have shown that mir-155 overexpression in hematopoietic stem cells results in MPD, and represses SHIP-1 expression via cytokine signaling.8 Lakhanpal et al. reported that the Fli-1 transcription factor acts as a transcriptional repressor of SHIP-1 and dramatically reduces its expression in cancer cells.6 More recently, the Ikaros family of transcription factors, have been found to regulate SHIP expression and play a significant role in lymphoid development and blood cancers.9 Therefore, targeting cytokine-dependent microRNAs and transcription factors that regulate SHIP-1 expression and activity may be a beneficial therapeutic modality for the treatment of numerous cancers. However, a more direct therapeutic method of enhancing SHIP-1 activity may be more advantageous for combating MDSC-dependent cancers. Kennah et al. (2009) identified a class of molecules that bind to the C2 domain of SHIP-1 and increase its catalytic activity, reduce AKT phosphorylation and decrease cell proliferation of myeloma cells in vitro.10 Therefore, these compounds, potential “SHIP-1 activators,” could be useful for the treatment of other myeloid related diseases.
In conclusion, our results reveal that murine pancreatic soluble factor(s) cause a reduction in SHIP-1 expression, which can be correlated with significant expansion of highly immunosuppressive MDSC and tumor progression. Therefore, the identification of soluble factor(s) responsible for dampened SHIP-1 expression may potentially lead to the development of better therapeutic drugs to enhance SHIP-1 activity. This therapeutic strategy of enhancing or stabilizing SHIP-1 activity and expression may maintain immune balance and reduce immunosuppression, prior to immunotherapy, and may be clinically translatable to combating pancreatic cancer and other MDSC-related malignancies in humans.
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/20201
References
- 1.Nagaraj S, Gabrilovich DI. Myeloid-derived suppressor cells in human cancer. Cancer J. 2010;16:348–53. doi: 10.1097/PPO.0b013e3181eb3358. [DOI] [PubMed] [Google Scholar]
- 2.Ostrand-Rosenberg S, Sinha P. Myeloid-derived suppressor cells: linking inflammation and cancer. J Immunol. 2009;182:4499–506. doi: 10.4049/jimmunol.0802740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Condé C, Gloire G, Piette J. Enzymatic and non-enzymatic activities of SHIP-1 in signal transduction and cancer. Biochem Pharmacol. 2011;82:1320–34. doi: 10.1016/j.bcp.2011.05.031. [DOI] [PubMed] [Google Scholar]
- 4.Hamilton MJ, Ho VW, Kuroda E, Ruschmann J, Antignano F, Lam V, et al. Role of SHIP in cancer. Exp Hematol. 2011;39:2–13. doi: 10.1016/j.exphem.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 5.Ghansah T, Paraiso KH, Highfill S, Desponts C, May S, McIntosh JK, et al. Expansion of myeloid suppressor cells in SHIP-deficient mice represses allogeneic T cell responses. J Immunol. 2004;173:7324–30. doi: 10.4049/jimmunol.173.12.7324. [DOI] [PubMed] [Google Scholar]
- 6.Lakhanpal GK, Vecchiarelli-Federico LM, Li YJ, Cui JW, Bailey ML, Spaner DE, et al. The inositol phosphatase SHIP-1 is negatively regulated by Fli-1 and its loss accelerates leukemogenesis. Blood. 2010;116:428–36. doi: 10.1182/blood-2009-10-250217. [DOI] [PubMed] [Google Scholar]
- 7.Pilon-Thomas S, Nelson N, Vohra N, Jerald M, Pendleton L, Szekeres K, et al. Murine pancreatic adenocarcinoma dampens SHIP-1 expression and alters MDSC homeostasis and function. PLoS One. 2011;6:e27729. doi: 10.1371/journal.pone.0027729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O’Connell RM, Chaudhuri AA, Rao DS, Baltimore D. Inositol phosphatase SHIP1 is a primary target of miR-155. Proc Natl Acad Sci U S A. 2009;106:7113–8. doi: 10.1073/pnas.0902636106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alinikula J, Kohonen P, Nera KP, Lassila O. Concerted action of Helios and Ikaros controls the expression of the inositol 5-phosphatase SHIP. Eur J Immunol. 2010;40:2599–607. doi: 10.1002/eji.200940002. [DOI] [PubMed] [Google Scholar]
- 10.Kennah M, Yau TY, Nodwell M, Krystal G, Andersen RJ, Ong CJ, et al. Activation of SHIP via a small molecule agonist kills multiple myeloma cells. Exp Hematol. 2009;37:1274–83. doi: 10.1016/j.exphem.2009.08.001. [DOI] [PubMed] [Google Scholar]


