Abstract
Abnormal tumor vasculature and endothelial cell anergy limit tumor/T-cell interactions. We have found that NGR-TNF, a tumor vasculature-homing derivative of TNF, selectively activates endothelial cells in neoplastic tissues and induces the release of chemokines that favor tumor infiltration by T cells, thereby enhancing the efficacy of active and adoptive immunotherapy.
Keywords: cell trafficking, endothelial cells, immunotherapy, T cells, tumor
One major hurdle that tumor-specific T cells must overcome to get in direct contact with their targets is crossing of the abnormal tumor vessel barrier and interstitium. Indeed, the tumor vasculature is disorganized, tortuous and leaker then normal vessels, and this may cause increased interstitial pressure, heterogeneous permeability and irregular blood flow. In addition, exposure to angiogenic factors [e.g., vascular endothelial growth factors (VEGF) and fibroblast growth factors (FGF)] causes down regulation of intracellular adhesion molecule-1/2 (ICAM-1/2), vascular cell adhesion molecule-1 (VCAM-1) and CD34 on endothelial cells (EC).3 Thus, leukocyte-vessel wall interactions are diminished in tumors, and effector T cells, regardless of being induced in vivo by vaccination or adoptively transferred, are impaired in their deployment at tumor sites where they get in direct contact with target tumor cells.
Tumor necrosis factor-α (TNF) is an inflammatory cytokine capable of inducing endothelial cell activation and of increasing vessel permeability. We have previously shown that targeted delivery of small amounts of TNF to tumor vessels is sufficient to increase their permeability, without causing toxic reactions.1 For example, this was achieved by coupling TNF with a peptide containing the Asn-Gly-Arg (NGR) motif, a ligand of an aminopeptidase N (CD13) isoform selectively expressed by EC in tumor vessels.2 Because of these properties this drug (originally developed by our group and called NGR-TNF) can increase the penetration of various chemotherapy agents in tumors.1 Thus, we hypothesized that pre-treatment of tumor-bearing subjects with NGR-TNF might favor lymphocyte infiltration in their neoplastic tissues.4
We have recently investigated this hypothesis in murine cancer models.5 We have found that administration of picogram doses of NGR-TNF to tumor-bearing mice induces, 2 h after injection, the upregulation VCAM-1 and ICAM-2 in EC, as well as the local release of several cytokines/chemokines involved in T cell activation and migration, including MCP-1/CCL-2, MCP-3/CCL-7, MIP-2, oncostatin-M and stem cell factor (SCF). This rapid and transient modification of the tumor microenvironment associates with tumor infiltration of fully activated endogenous or adoptively transferred cytotoxic T lymphocytes in transplantable models of melanoma and the transgenic adenocarcinoma of the mouse prostate (TRAMP) model of spontaneous prostate cancer (Fig. 1). Remarkably, NGR-TNF did not modify T cell distribution in the blood, spleen or kidney of tumor-bearing mice, highlighting the selective effects of NGR-TNF on tumor tissues.5
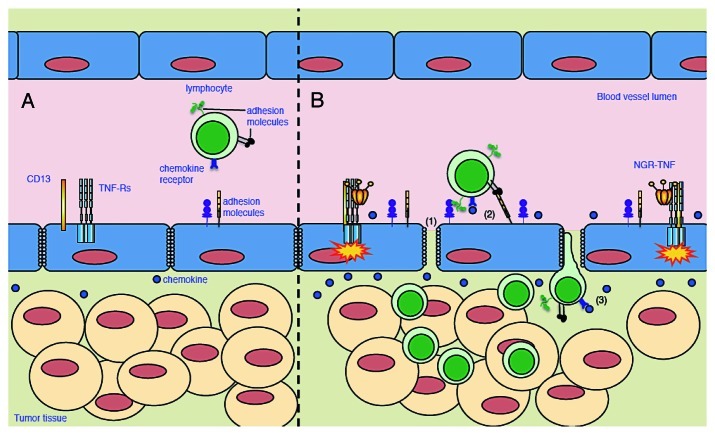
Figure 1.
Effects of NGR-TNF on tumor microenvironment and T cell infiltration. (A) Increased interstitial pressure, heterogeneous permeability and irregular blood flow, together with reduced expression of adhesion molecules on EC, limit lymphocyte penetration in tumors. (B) NGR-TNF, which selectively binds CD13 expressed in EC of neoangiogenic vessels and favors the interaction of TNF with TNF receptors (TNF-R), alters tumor vessel permeability by loosening VE-cadherin dependent adherence junctions (1), induces upregulation of adhesion molecules in EC (2), and elicits the release of pro-inflammatory cytokines and chemokines (3), thereby favoring the recruitment and extravasation of T lymphocytes.
While the measured effects of NGR-TNF were short lasting, its beneficial effects on TIL persisted for days and the combination of NGR-TNF and adoptive immunotherapy increased the overall survival of tumor bearing mice with no evidence of toxic reactions.
Finally, we observed that NGR-TNF could also increase the efficacy of active immunotherapy (vaccination) either alone or in combination with chemotherapy.5 One explanation for these synergies is that the temporary reduction of the endothelial-barrier function induced by NGR-TNF might favor the penetration of both drug and lymphocytes in tumors, thereby increasing the tumor debulking by chemotherapy and improving immunotherapy.4
Noteworthy, in all the experimental conditions tested a comparable dose of TNF was marginally or not active, supporting the hypothesis that targeted delivery of TNF to tumor vessels was crucial for the activity.
One limit of our study is that we have investigated the effects of NGR-TNF treatment only on T cell infiltration in tumors. Different leukocyte populations can exploit the transient modifications of the tumor microenvironment induced by NGR-TNF (i.e. EC activation and release of chemokines). Thus, other leukocyte populations may be attracted within the tumor mass after NGR-TNF treatment, which may contribute to the modification of the tumor microenvironment by making it more favorable for lymphocyte infiltration and effector functions.
Other strategies have been implemented to improve T cell infiltration in tumors, based on the use of angiogenesis inhibitors like anginex, endostatin and angiostatin,6 or anti-VEGF reagents like soluble chimeric VEGF receptor (VEGFR)7 and anti-VEGF8 or VEGFR antibodies.9 These drugs transiently normalize the tumor vasculature, pruning away immature and leaky vessels and remodeling the remaining vasculature. As a result, the enhanced oncotic pressure gradient together with decreased interstitial fluid pressure gradient facilitate the delivery of oxygen, nutrients and also of chemotherapeutic agents into the tumor microenvironment.3 Anginex, endostatin and angiostatin can also overcome EC anergy preventing VCAM-1 and ICAM-1 down regulation, therefore promoting leukocyte infiltration in tumors.6 The mechanism by which anti-VEGF reagents favor T cell infiltration in tumors7-9 has not yet been defined.
From a conceptual point of view these strategies are different from that proposed in our study, as they are based on the use of anti-angiogenic compounds that inhibit the formation of new blood vessels and promote vascular “normalization,”10 whereas NGR-TNF is an inflammatory-vascular targeting agent that induces vascular “activation”. Notably, these therapeutic approaches require markedly different doses of drugs and different schedules of administration, thus with potentially different toxic reactions. The observation that extremely low doses of NGR-TNF (picograms) are sufficient to induce local inflammation in murine tumors with no signs of systemic toxicity (the corresponding dose is well tolerated also in patients) makes NGR-TNF an attractive agent for the combination with immunotherapy. At this regard, it is remarkable that most of the effects induced by NGR-TNF on the vessels of murine tumors have been observed also in patients.1 This suggests that murine models of cancer are reliable predictors of the response to NGR-TNF in patients. Based on this assumption and on the data obtained with melanoma models reported herein, a phase I/II clinical trial based on the combination of NGR-TNF with active immunotherapy has been recently started in melanoma patients.
Disclosure of Potential Conflicts of Interest
A. Corti is the inventor of a patent on NGR-TNF.
Acknowledgments
This study was supported by grants of the Italian Association for Cancer Research (AIRC, Milan) to M.B. and A.C., the Ministry of Health (Rome) to M.B., the Ministry of University and Research (FIRB; Rome) to M.B. and A.C., and Alleanza Contro il Cancro, Programma Straordinario di Ricerca Oncologica 2006, Programma 3 to M.B. and A.C. A. Calcinotto conducted this study in partial fulfillment of her Ph.D. at San Raffaele University.
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/20213
References
- 1.Corti A, Pastorino F, Curnis F, Arap W, Ponzoni M, Pasqualini R. Targeted drug delivery and penetration into solid tumors. Med Res Rev. 2011 doi: 10.1002/med.20238. In press. [DOI] [PubMed] [Google Scholar]
- 2.Corti A, Curnis F, Sacchi A, Borgna L, Magni F, Gasparri A. Enhancement of tumor necrosis factor alpha antitumor immunotherapeutic properties by targeted delivery to aminopeptidase N (CD13) Nat Biotechnol. 2000;18:1185–90. doi: 10.1038/81183. [DOI] [PubMed] [Google Scholar]
- 3.Chung AS, Lee J, Ferrara N. Targeting the tumour vasculature: insights from physiological angiogenesis. Nat Rev Cancer. 2010;10:505–14. doi: 10.1038/nrc2868. [DOI] [PubMed] [Google Scholar]
- 4.Bellone M, Mondino A, Corti A. Vascular targeting, chemotherapy and active immunotherapy: teaming up to attack cancer. Trends Immunol. 2008;29:235–41. doi: 10.1016/j.it.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 5.Calcinotto A, Grioni M, Jachetti E, Curnis F, Mondino A, Parmiani G, et al. Targeting TNF-α to Neoangiogenic Vessels Enhances Lymphocyte Infiltration in Tumors and Increases the Therapeutic Potential of Immunotherapy. J Immunol. 2012;188:2687–94. doi: 10.4049/jimmunol.1101877. [DOI] [PubMed] [Google Scholar]
- 6.Dirkx AE, oude Egbrink MG, Castermans K, van der Schaft DW, Thijssen VL, Dings RP, et al. Anti-angiogenesis therapy can overcome endothelial cell anergy and promote leukocyte-endothelium interactions and infiltration in tumors. FASEB J. 2006;20:621–30. doi: 10.1096/fj.05-4493com. [DOI] [PubMed] [Google Scholar]
- 7.Li B, Lalani AS, Harding TC, Luan B, Koprivnikar K, Huan Tu G, et al. Vascular endothelial growth factor blockade reduces intratumoral regulatory T cells and enhances the efficacy of a GM-CSF-secreting cancer immunotherapy. Clin Cancer Res. 2006;12:6808–16. doi: 10.1158/1078-0432.CCR-06-1558. [DOI] [PubMed] [Google Scholar]
- 8.Shrimali RK, Yu Z, Theoret MR, Chinnasamy D, Restifo NP, Rosenberg SA. Antiangiogenic agents can increase lymphocyte infiltration into tumor and enhance the effectiveness of adoptive immunotherapy of cancer. Cancer Res. 2010;70:6171–80. doi: 10.1158/0008-5472.CAN-10-0153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Manning EA, Ullman JG, Leatherman JM, Asquith JM, Hansen TR, Armstrong TD, et al. A vascular endothelial growth factor receptor-2 inhibitor enhances antitumor immunity through an immune-based mechanism. Clin Cancer Res. 2007;13:3951–9. doi: 10.1158/1078-0432.CCR-07-0374. [DOI] [PubMed] [Google Scholar]
- 10.Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005;307:58–62. doi: 10.1126/science.1104819. [DOI] [PubMed] [Google Scholar]