Abstract
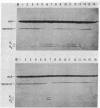
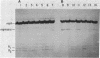
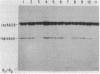
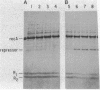
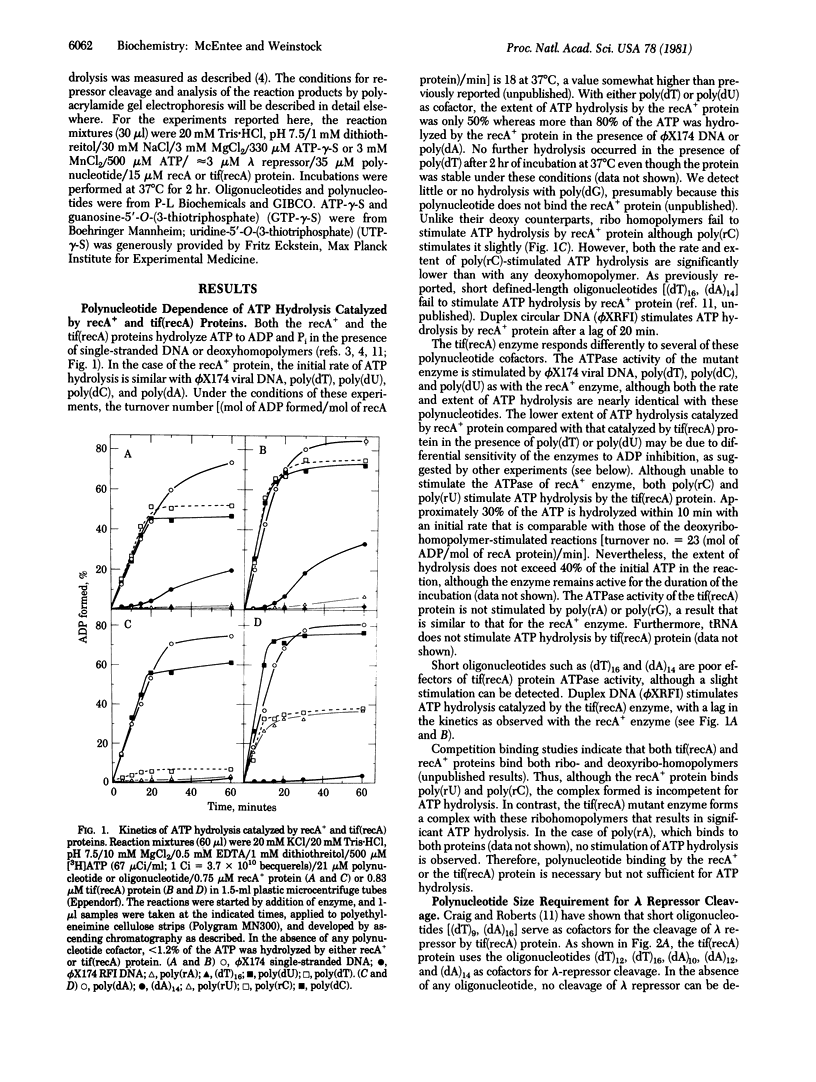
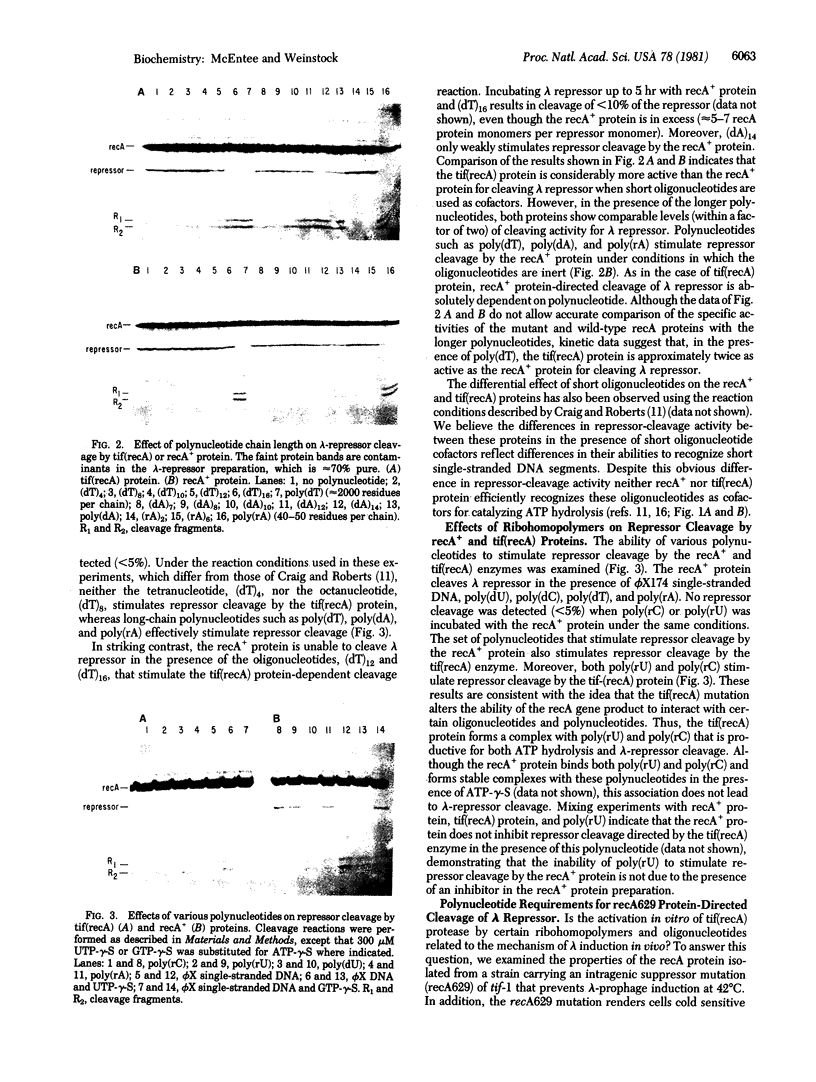
The requirements for polynucleotide-dependent hydrolysis of ATP and for proteolytic cleavage of phage lambda repressor have been examined for both the wild-type (recA+ protein) and the tif-1 mutant form [tif(recA) protein] of the recA gene product. The recA+ and tif(recA) proteins catalyze both reactions in the presence of long single-stranded DNAs or certain deoxyhomopolymers. However, short oligonucleotides [(dT)12, (dA)14] stimulate neither the protease nor the ATPase activities of the recA+ protein. In contrast, these short oligonucleotides activate tif(recA) protein to cleave lambda repressor without stimulating its ATPase activity. Moreover, both the ATPase and protease activities of the tif(recA) protein are stimulated by poly(rU) and poly(rC) whereas the recA+ protein does not respond to these ribopolymers. We have purified the recA protein from a strain in which the tif mutation is intragenically suppressed. This mutant protein (recA629) is inactive in the presence of (dT)12, (dA)14, poly(rU), and poly(rC) for lambda repressor cleavage and ATP hydrolysis. These results argue that the tif-1 mutation (or mutations) alters the DNA binding site of the recA protein. We suggest that in vivo the tif(recA) protein is activated for cleaving repressors of SOS genes by complex formation with short single-stranded regions or gaps that normally occur near the growing fork of replicating chromosomes and are too short for activating the recA+ enzyme. This mechanism can account for the expression of SOS functions in the absence of DNA damage in tif mutant strains.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cox M. M., McEntee K., Lehman I. R. A simple and rapid procedure for the large scale purification of the recA protein of Escherichia coli. J Biol Chem. 1981 May 10;256(9):4676–4678. [PubMed] [Google Scholar]
- Craig N. L., Roberts J. W. E. coli recA protein-directed cleavage of phage lambda repressor requires polynucleotide. Nature. 1980 Jan 3;283(5742):26–30. doi: 10.1038/283026a0. [DOI] [PubMed] [Google Scholar]
- D'Ari R., George J., Huisman O. Suppression of tif-mediated induction of SOS functions in Escherichia coli by an altered dnaB protein. J Bacteriol. 1979 Nov;140(2):381–387. doi: 10.1128/jb.140.2.381-387.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irbe R. M., Morin L. M., Oishi M. Prophage (phi 80) induction in Escherichia coli K-12 by specific deoxyoligonucleotides. Proc Natl Acad Sci U S A. 1981 Jan;78(1):138–142. doi: 10.1073/pnas.78.1.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenyon C. J., Walker G. C. DNA-damaging agents stimulate gene expression at specific loci in Escherichia coli. Proc Natl Acad Sci U S A. 1980 May;77(5):2819–2823. doi: 10.1073/pnas.77.5.2819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little J. W., Edmiston S. H., Pacelli L. Z., Mount D. W. Cleavage of the Escherichia coli lexA protein by the recA protease. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3225–3229. doi: 10.1073/pnas.77.6.3225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEntee K. Protein X is the product of the recA gene of Escherichia coli. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5275–5279. doi: 10.1073/pnas.74.12.5275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEntee K., Weinstock G. M., Lehman I. R. Initiation of general recombination catalyzed in vitro by the recA protein of Escherichia coli. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2615–2619. doi: 10.1073/pnas.76.6.2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mount D. W. A mutant of Escherichia coli showing constitutive expression of the lysogenic induction and error-prone DNA repair pathways. Proc Natl Acad Sci U S A. 1977 Jan;74(1):300–304. doi: 10.1073/pnas.74.1.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa T., Wabiko H., Tsurimoto T., Horii T., Masukata H., Ogawa H. Characteristics of purified recA protein and the regulation of its synthesis in vivo. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 2):909–915. doi: 10.1101/sqb.1979.043.01.099. [DOI] [PubMed] [Google Scholar]
- Roberts J. W., Roberts C. W., Craig N. L. Escherichia coli recA gene product inactivates phage lambda repressor. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4714–4718. doi: 10.1073/pnas.75.10.4714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts J. W., Roberts C. W. Proteolytic cleavage of bacteriophage lambda repressor in induction. Proc Natl Acad Sci U S A. 1975 Jan;72(1):147–151. doi: 10.1073/pnas.72.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupp W. D., Howard-Flanders P. Discontinuities in the DNA synthesized in an excision-defective strain of Escherichia coli following ultraviolet irradiation. J Mol Biol. 1968 Jan 28;31(2):291–304. doi: 10.1016/0022-2836(68)90445-2. [DOI] [PubMed] [Google Scholar]
- Shibata T., DasGupta C., Cunningham R. P., Radding C. M. Purified Escherichia coli recA protein catalyzes homologous pairing of superhelical DNA and single-stranded fragments. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1638–1642. doi: 10.1073/pnas.76.4.1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstock G. M., McEntee K., Lehman I. R. ATP-dependent renaturation of DNA catalyzed by the recA protein of Escherichia coli. Proc Natl Acad Sci U S A. 1979 Jan;76(1):126–130. doi: 10.1073/pnas.76.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkin E. M. Ultraviolet mutagenesis and inducible DNA repair in Escherichia coli. Bacteriol Rev. 1976 Dec;40(4):869–907. doi: 10.1128/br.40.4.869-907.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]