Abstract
We aimed to determine if the tumor microenvironment could be turned into a “self”-vaccine site. We show that provoking a local inflammatory response modulates endothelia to permit the infiltration of innate and adaptive effector cells which collaborate to eradicate the inflamed tumor and other tumor deposits, and provide long-term protection.
Keywords: B cells, immunotherapy, inflammation, innate immune cells, T cells, tumor endothelia
Most anti-cancer therapies aim to resolve all tumor deposits. Immunotherapeutic strategies take this further and hope to provide long-term protection against recurrence. Regardless of the therapeutic strategy used this is rarely achieved, particularly when faced with advanced and/or aggressive disease.
We hypothesized that effective anti-tumor immune responses require a similar profound and complex immune response to that seen in anti-pathogen responses. Responses to infection represent a coordinated local and systemic immune response. Activation of tissue-resident cells induces blood vessels to become amenable to the trafficking of large numbers of innate and adaptive effector cells into, and out of, the site of infection. Pathogen-associated antigens sourced from the infected site promote long-term effector/memory T cell immunity. Thus, we aimed to determine if the tumor microenvironment could also be turned into a potent ‘self’-vaccine site.
We used the lewis lung carcinoma and the AE17 mesothelioma models to address this hypothesis. The latter was established with the relevant human carcinogen, asbestos fibers, and inoculation of cloned tumor cells into mice results in progressing tumors confirmed by histopathology to be representative of human mesothelioma;1 this remains a rare feature of preclinical tumor models. Studies using tumor cells tagged with the ovalbumin protein, which acts as a cancer neo-antigen, were used to track tumor specific immune responses.
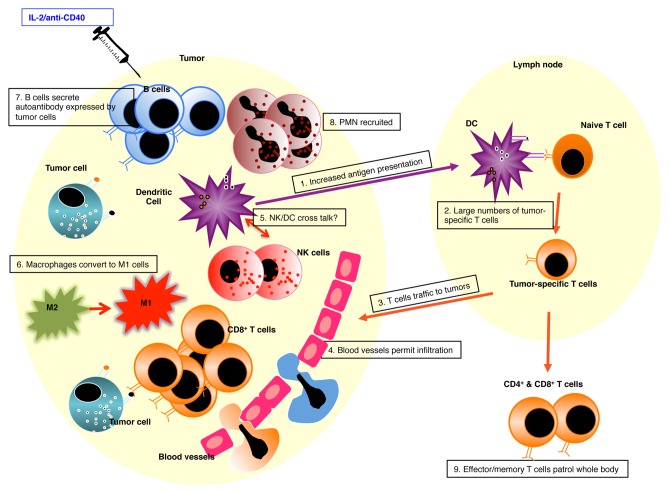
We have shown that developing tumors communicate with draining LNs (dLNs) leading to the generation of tumor-specific CD8+ cytotoxic T cells which traffic into the tumor microenvironment where they are ineffective.2-5 We have also shown that small established tumors with their own blood supply regress when challenged with single agent immunotherapies such as IL-21, IL-21,3 agonist anti-CD40 monoclonal antibody,6 or adoptive transfer of tumor-specific T cells.2 However, these single agent therapies fail at defined ‘cut-off’ tumor burdens. Thus, we used this model system to define the immune mechanisms required to mediate regression of larger tumors that are resistant to mono-immunotherapies.7 We found that injection of IL-2 combined with anti-CD40 antibody directly into the tumor bed resulted in permanent resolution of treated-site and untreated distal tumors. Systemic administration of IL-2/anti-CD40 antibody was not as effective and, unlike the intra-tumoral route, induced significant toxicities. Collaborating tumor-infiltrating CD8+ T cells and neutrophils were responsible for tumor regression, and tumor antigens were sourced from the tumor microenvironment. These data show that tumors can function as their own vaccine site if significant local acute inflammation is induced.
One concern is that the intra-tumoral approach may only induce a loco-regional T cell circuit involving dLNs and treated-site tumors. This was indeed the case for the IL-2 monotherapy which induced a potent CD4+ and CD8+ T cell response that was limited to the dLN and treated-site tumor.8 In contrast, anti-CD40 Ab treatment played an important role in expanding the systemic T cell response to non-dLNs, and distal untreated tumors which then regressed. When anti-CD40 Ab was combined with IL-2 tumor-specific T cells in dLNs were activated and transitioned into effector/memory migratory cells that patrolled the whole body accessing distal tumor deposits.
Anti-CD40 Ab affected specific B cell subsets, i.e., it obliterated marginal zone B cells and promoted follicular (FO) B-cell activity in tumors, dLNs and spleens.6 Adoptive transfer of CD40-activated B cells, or their immunoglobulin products, which recognized autoantigens on mesothelioma cells protected against tumor challenge. B-cell knockout mice confirmed that successful treatment required the presence of B cells. Thus, CD40-activated FO B cells can become an important component of an effective anti-tumor immune response.
Importantly, the IL-2/anti-CD40 antibody combination induced life-long CD4+ and CD8+ T cell protective memory. Unexpectedly, we found that natural killer (NK) cells played a key role in the installation and maintenance of IL-2/CD40-driven systemic memory responses.9 These data were interesting as while NK cells infiltrate developing mesothelioma tumors, similar to T cells, they cannot function as effector cells without help. IL-2 promoted NK cell expansion in tumors and dLNs. Interestingly, NK cells were not key effector cells however, tumor cell re-challenged NK-deficient mice were unable to provide protection, implying insufficient memory. Our data show that NK cells infiltrate mesothelioma tumors which, after local IL-2 and/or anti-CD40 antibody treatment, provide help, likely to dendritic cells, for the acquisition of systemic immunity and long-term effector/memory responses.
The mesothelioma tumor microenvironment contains a significant number of tumor-associated macrophages that are likely to be immunosuppressive.3 We found that IL-2/anti-CD40 antibody treatment polarized macrophages toward M1 macrophages (manuscript submitted) which may contribute to the acute inflammation, improved T cell responses and tumor regression seen with this therapy.
Tumour endothelia can express CD40. We have shown in mesothelioma (our unpublished observations) and in insulinoma10 that intra-tumoral anti-CD40 antibody therapy activates CD40+ tumor endothelia to become permissive to immune cell infiltration. The mechanisms by which CD40-activated tumor vessels facilitate the accumulation of effector cells are as yet unknown, but they may be a critical step for tumor eradication.
Our data shows that effective anti-tumor immunity requires induction of a local inflammatory response similar to that seen when faced with a pathogen. Signaling through IL-2 and CD40 provoked profound intra-tumoral inflammation involving tumor endothelia, innate and adaptive immunity thereby skewing the tumor microenvironment from tumorigenic to immunogenic (summarized in Figure 1). This approach generated a whole of body, effector/memory CD4+ and CD8+ T cell response resulting in the resolution of untreated distal tumors and long-term protective memory. It is also clinically useful because only one tumor site has to be accessible for treatment.
Figure 1. Provoking Intra-tumoral inflammation turns tumors into a self vaccine site.
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/20238
References
- 1.Jackaman C, Bundell CS, Kinnear BF, Smith AM, Filion P, van Hagen D, et al. IL-2 intratumoral immunotherapy enhances CD8+ T cells that mediate destruction of tumor cells and tumor-associated vasculature: a novel mechanism for IL-2. J Immunol. 2003;171:5051–63. doi: 10.4049/jimmunol.171.10.5051. [DOI] [PubMed] [Google Scholar]
- 2.Nelson DJ, Mukherjee S, Bundell C, Fisher S, van Hagen D, Robinson BWS. Tumor progression despite efficient tumor antigen cross-presentation and effective “arming” of tumor antigen-specific CTL. J Immunol. 2001;166:5557–66. doi: 10.4049/jimmunol.166.9.5557. [DOI] [PubMed] [Google Scholar]
- 3.Jackaman C, Cornwall S, Lew AM, Zhan Y, Robinson BWS, Nelson DJ. Local effector failure in mesothelioma is not mediated by CD4+ CD25+ T-regulator cells. Eur Respir J. 2009;34:162–75. doi: 10.1183/09031936.00101008. [DOI] [PubMed] [Google Scholar]
- 4.Marzo AL, Lake RA, Lo D, Sherman L, McWilliam A, Nelson D, et al. Tumor antigens are constitutively presented in the draining lymph nodes. J Immunol. 1999;162:5838–45. [PubMed] [Google Scholar]
- 5.Robinson BWS, Lake RA, Nelson DJ, Scott BA, Marzo AL. Cross-presentation of tumour antigens: evaluation of threshold, duration, distribution and regulation. Immunol Cell Biol. 1999;77:552–8. doi: 10.1046/j.1440-1711.1999.00876.x. [DOI] [PubMed] [Google Scholar]
- 6.Jackaman C, Cornwall S, Graham PT, Nelson DJ. CD40-activated B cells contribute to mesothelioma tumor regression. Immunol Cell Biol. 2011;89:255–67. doi: 10.1038/icb.2010.88. [DOI] [PubMed] [Google Scholar]
- 7.Jackaman C, Lew AM, Zhan Y, Allan JE, Koloska B, Graham PT, et al. Deliberately provoking local inflammation drives tumors to become their own protective vaccine site. Int Immunol. 2008;20:1467–79. doi: 10.1093/intimm/dxn104. [DOI] [PubMed] [Google Scholar]
- 8.Jackaman C, Nelson DJ. Intratumoral interleukin-2/agonist CD40 antibody drives CD4(+)-independent resolution of treated-tumors and CD4 (+)-dependent systemic and memory responses. Cancer Immunol Immunother. 2012;61:549–60. doi: 10.1007/s00262-011-1120-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jackaman C, Lansley S, Allan JE, Robinson BWS, Nelson DJ. IL-2/CD40-driven NK cells install and maintain potency in the anti-mesothelioma effector/memory phase. Int Immunol. 2012;••• doi: 10.1093/intimm/dxs005. [DOI] [PubMed] [Google Scholar]
- 10.Hamzah J, Nelson D, Moldenhauer G, Arnold B, Hämmerling GJ, Ganss R. Vascular targeting of anti-CD40 antibodies and IL-2 into autochthonous tumors enhances immunotherapy in mice. J Clin Invest. 2008;118:1691–9. doi: 10.1172/JCI33201. [DOI] [PMC free article] [PubMed] [Google Scholar]