Abstract
In principle, T cells can recognize and kill cancer cells. However, tumors have the ability to escape T cell attack. By imaging the dynamic behavior of T cells in human lung tumor explants, we have recently established the importance of the extracellular matrix in limiting access of T cells to tumor cells.
Keywords: chemokines, extracellular matrix, human lung tumor, migration, stroma, T lymphocyte
T cells are key component of the anti-tumor immunity. To mount an effective immune response, T cells must achieve several distinct steps. First, T lymphocytes need to be fully activated by mature dendritic cells in the tumor-draining lymph node. Second, cancer-specific effector T cells must enter into the tumor after leaving the blood vessels. Finally, tumor-infiltrating lymphocytes (TIL) need to perform their function which ultimately leads to tumor regression. However, it is clearly recognized that tumors may escape T cell attack by variety of mechanisms. One of them could be the location of T cells within a tumor. Thus, in most human solid tumors, T cells are rarely in contact with cancer cells but greatly enriched in the stroma, a surrounding microenvironment composed of non cancer cells along with the extracellular matrix (ECM).1 Default in T cell infiltration into tumor islets might constitute a major obstacle for T cell-mediated anti-tumor activities.
In a recent study,2 we have used a live cell imaging approach to gain insight into the factors involved in T cell positioning and migration into and within human non-small cell lung tumors. In this system, purified T cells added acutely to human lung tumor slices behave exactly like TIL already present in the resected tumor: they migrate preferentially in the stroma, very rarely in the tumor islets.
What limits T cells from infiltrating the tumor cell regions? Reduced expression and/or inactivation of intratumoral chemokines have recently been put forward to explain the paucity of T cells in carcinoma regions.3,4 Our data in fresh human tumors fit with this notion, and we have shown that in a xenograft system, the infiltration of T cells in tumor islets was increased when cancer cells were engineered to express the chemokine CCL5.2
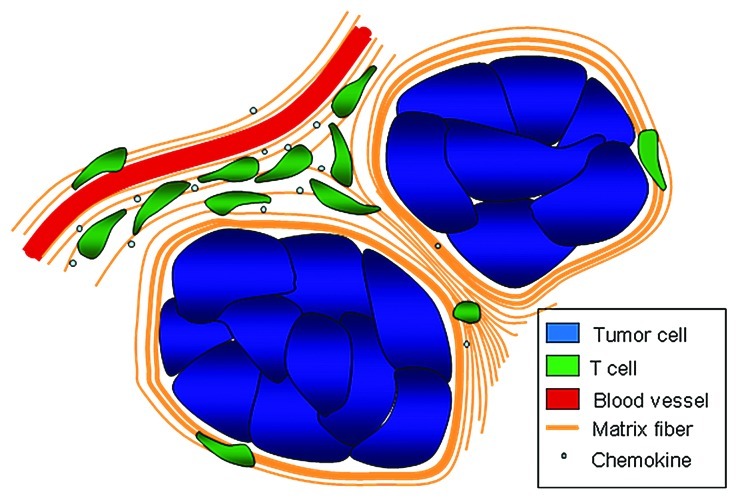
Apart from features that depend on tumor cells, the motile behavior of T cells and their capacity to infiltrate the tumor cell regions are also controlled by stromal components. By systematically imaging T lymphocytes in relation to other cells and structures, we have found that the stroma is composed of different territories in which T cell motility was either favored or restricted. Active T cell motility dependent on chemokines was observed in small but well delineated stromal regions. These favorable migration zones might optimize efficient immune surveillance and antitumor immunity. This is consistent with the previous description of lymphoid-like structures associated with a good outcome in human lung tumors.5 However, T cells outside these regions exhibited limited and constrained displacement. We have correlated the reduced ability of T cells to migrate with the density of the ECM. Hence, stromal regions enriched in fibers of collagen and fibronectin contain few T cells. Strikingly, dense and linear matrix fibers were observed surrounding tumor islets. Close examination of T cell movements in stromal regions immediately adjacent to cancer cells indicated that lymphocytes rarely migrate toward tumor cells but rather along fibers oriented parallel to the tumor-stroma boundary. Together, our recent work put forward an additional, so far unappreciated role for the stromal ECM that limits T cells from entering tumor islets (Fig. 1).
Figure 1. In human lung tumors, malignant epithelial areas are poorly accessible to T cells. A lack of T cell migration factors by cancer cells as well as the presence of dense matrix fibers surrounding the tumor islets prevents T cell infiltration. In contrast, T cells accumulate and migrate preferentially in defined stromal areas characterized by the presence of chemokines and a loose fiber network reminiscent of the microenvironment that exists in lymphoid organs.
Excessive accumulation of the ECM, a process termed desmoplasia, is a hallmark of cancer progression.6 The ECM possesses many functions essential for various biological processes. Therefore, deregulation of the ECM plays a causative role in cancer pathogenesis. Accordingly, a dense stroma correlates with adverse prognosis in several human carcinomas, including lung adenocarcinoma. Deregulated ECM has been shown to promote the epithelial cellular transformation. Moreover and in contrast to T cells, cancer cells utilize a dense and rigid stroma to migrate and metastasize.7 Of importance, the level and structural organization of collagen present within the tumor stroma can also influence response to therapy by regulating drug delivery. A fibrous stroma around tumor islets has been shown to act as a steric barrier for large molecular weight drugs.8 Besides this “wall-like” barrier, a dense stroma also contributes to high interstitial fluid pressure within the tumor. As a result, the penetration of anticancer drugs is compromised.9
Given the negative role played by a dense ECM, one important and timely question is now to determine if this matrix barrier can be ablated to increase accessibility of tumor cells to T cells and therapeutic molecules. Using collagenase, we have shown in a few tumor specimens that a reduction in collagen content enhances the number of T cells in contact with tumor cells.2 Although collagenase elicits multiple side effects which prevent its use in clinic, these observations give a rationale for therapeutic manipulation of the ECM in cancer treatment. Recent studies performed in mouse tumor models have identified several targets that hold promises in the control of matrix fiber formation. The first one is lysyl oxidase, an enzyme that cross-links collagen fibers and whose expression is frequently upregulated in many human tumors.7 Importantly, inhibition of lysyl oxidase reduces tissue fibrosis and tumor incidence in the Neu breast cancer model.7 The second is caveolin-1, a transmembrane protein expressed in tumor stromal fibroblasts that drives accumulation of dense matrix fibers and fosters tumor progression.10
In summary, abnormal ECM is known to promote cancer progression by various means. Our data showing an unfavorable effect of fibrous stroma on T cell migration, furnish an additional explanation for the tumor-promoting effect of a deregulated ECM.
Acknowledgments
We thank Nadège Bercovici and Alain Trautmann for critical reading of the manuscript.
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/20239
References
- 1.Ohtani H. Focus on TILs: prognostic significance of tumor infiltrating lymphocytes in human colorectal cancer. Cancer Immun. 2007;7:4. [PMC free article] [PubMed] [Google Scholar]
- 2.Salmon H, Franciszkiewicz K, Damotte D, Dieu-Nosjean MC, Validire P, Trautmann A, et al. Matrix architecture defines the preferential localization and migration of T cells into the stroma of human lung tumors. J Clin Invest. 2012;122:899–910. doi: 10.1172/JCI45817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pivarcsi A, Müller A, Hippe A, Rieker J, van Lierop A, Steinhoff M, et al. Tumor immune escape by the loss of homeostatic chemokine expression. Proc Natl Acad Sci U S A. 2007;104:19055–60. doi: 10.1073/pnas.0705673104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Molon B, Ugel S, Del Pozzo F, Soldani C, Zilio S, Avella D, et al. Chemokine nitration prevents intratumoral infiltration of antigen-specific T cells. J Exp Med. 2011;208:1949–62. doi: 10.1084/jem.20101956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dieu-Nosjean MC, Antoine M, Danel C, Heudes D, Wislez M, Poulot V, et al. Long-term survival for patients with non-small-cell lung cancer with intratumoral lymphoid structures. J Clin Oncol. 2008;26:4410–7. doi: 10.1200/JCO.2007.15.0284. [DOI] [PubMed] [Google Scholar]
- 6.Lu P, Weaver VM, Werb Z. The extracellular matrix: a dynamic niche in cancer progression. J Cell Biol. 2012;196:395–406. doi: 10.1083/jcb.201102147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Levental KR, Yu H, Kass L, Lakins JN, Egeblad M, Erler JT, et al. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell. 2009;139:891–906. doi: 10.1016/j.cell.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Diop-Frimpong B, Chauhan VP, Krane S, Boucher Y, Jain RK. Losartan inhibits collagen I synthesis and improves the distribution and efficacy of nanotherapeutics in tumors. Proc Natl Acad Sci U S A. 2011;108:2909–14. doi: 10.1073/pnas.1018892108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Provenzano PP, Cuevas C, Chang AE, Goel VK, Von Hoff DD, Hingorani SR. Enzymatic targeting of the stroma ablates physical barriers to treatment of pancreatic ductal adenocarcinoma. Cancer Cell. 2012;21:418–29. doi: 10.1016/j.ccr.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goetz JG, Minguet S, Navarro-Lérida I, Lazcano JJ, Samaniego R, Calvo E, et al. Biomechanical remodeling of the microenvironment by stromal caveolin-1 favors tumor invasion and metastasis. Cell. 2011;146:148–63. doi: 10.1016/j.cell.2011.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]