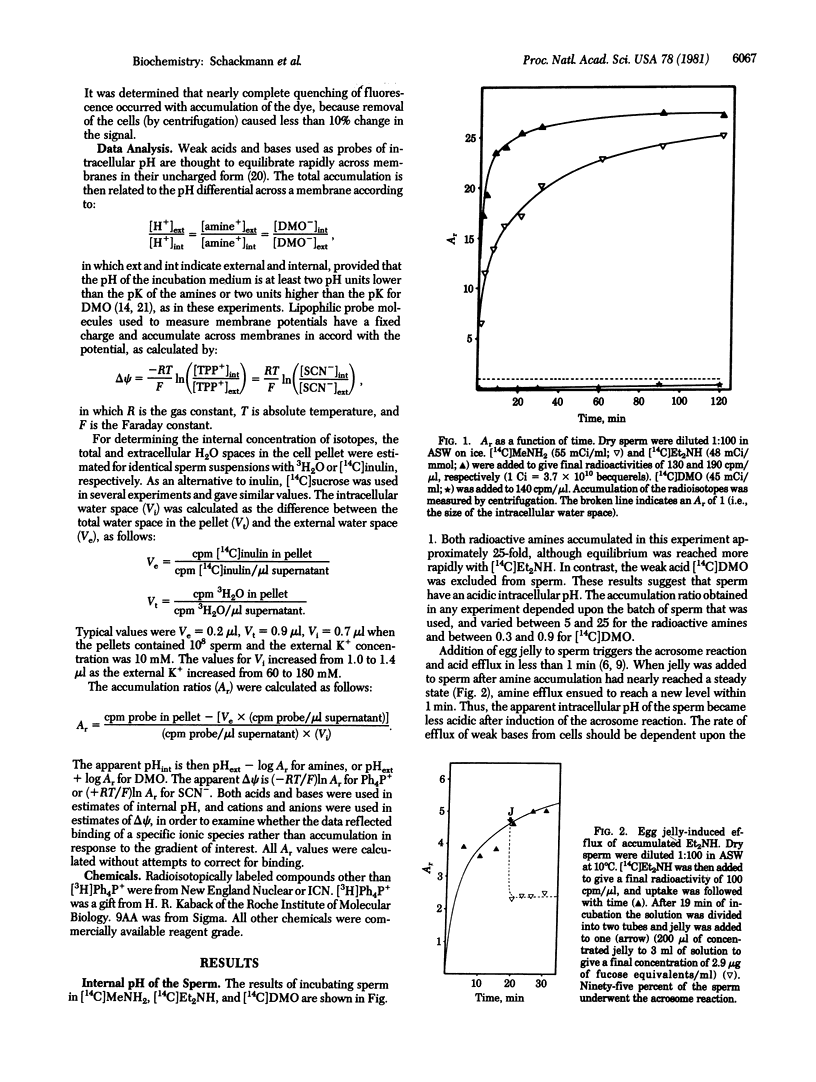
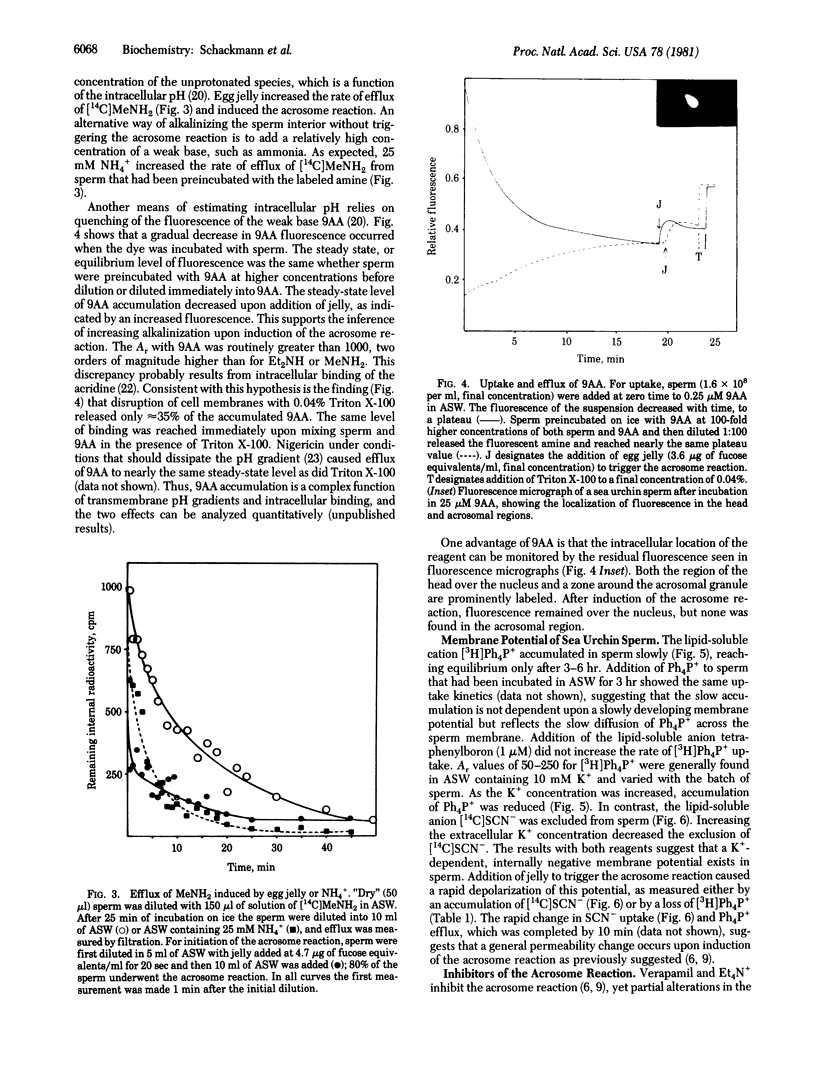
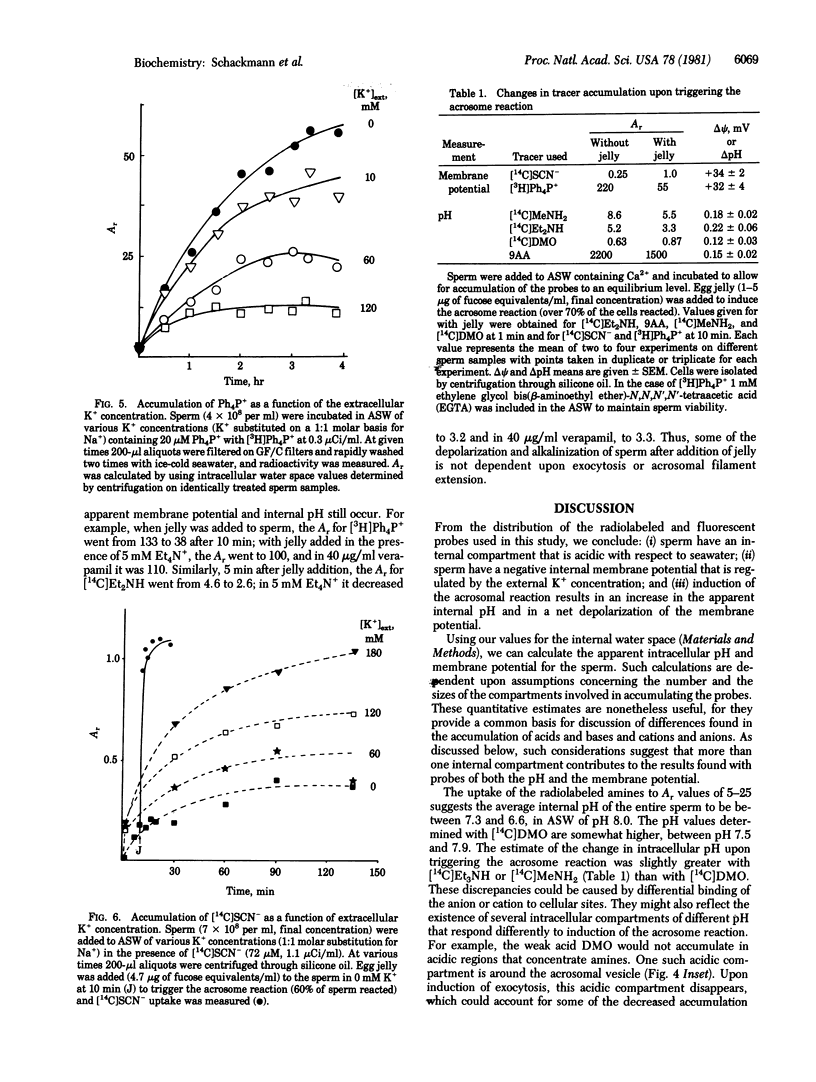
Abstract
The intracellular pH and membrane potential in sperm of the sea urchin Strongylocentrotus purpuratus were investigated by using fluorescent and radiolabeled probes. The weak bases [14C]methylamine, [14C]diethylamine, and 9-aminoacridine were concentrated within sperm 5-fold or greater. The weak acid [14C]dimethyloxazolidine-2,4-dione (DMO) was excluded from sperm. These data suggested that the apparent intracellular pH is acidic with respect to seawater (pH 8.0). Induction of the acrosome reaction caused efflux of the amines and uptake of DMO, consistent with an increase in apparent intracellular pH of 0.1-0.2 pH unit. The presence of an internally negative membrane potential was indicated by estimating the distribution of [3H]tetraphenylphosphonium (Ph4P+) and [14C]SCN-. From SCN- exclusion we estimated a value of about -30 mV for the nonmitochondrial membrane potential, whereas from Ph4P+ accumulation an apparent potential of -90 to -150 mV was demonstrated. The membrane potentials obtained with Ph4P+ and SCN- were dependent upon the external K+ concentration, with increasing K+ leading to depolarization. Induction of the acrosome reaction led to efflux of Ph4P+ and uptake of SCN- for an approximate depolarization of about 30 mV, primarily due to the collapse of the plasma membrane potential.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altendorf K., Hirata H., Harold F. M. Accumulation of lipid-soluble ions and of rubidium as indicators of the electrical potential in membrane vesicles of Escherichia coli. J Biol Chem. 1975 Feb 25;250(4):1405–1412. [PubMed] [Google Scholar]
- Boron W. F., Roos A. Comparison of microelectrode, DMO, and methylamine methods for measuring intracellular pH. Am J Physiol. 1976 Sep;231(3):799–809. doi: 10.1152/ajplegacy.1976.231.3.799. [DOI] [PubMed] [Google Scholar]
- Catterall W. A., Ray R., Morrow C. S. Membrane potential dependent binding of scorpion toxin to action potential Na+ ionophore. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2682–2686. doi: 10.1073/pnas.73.8.2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christen R., Sardet C. Changes in permeability of sea urchin egg membrane to urea after fertilization or activation. J Physiol. 1980 Aug;305:1–11. doi: 10.1113/jphysiol.1980.sp013345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins F., Epel D. The role of calcium ions in the acrosome reaction of sea urchin sperm: regulation of exocytosis. Exp Cell Res. 1977 Apr;106(1):211–222. doi: 10.1016/0014-4827(77)90258-0. [DOI] [PubMed] [Google Scholar]
- DAN J., OHORI Y., KUSHIDA H. STUDIES ON THE ACROSOME. VII. FORMATION OF THE ACROSOMAL PROCESS IN SEA URCHIN SPERMATOZOA. J Ultrastruct Res. 1964 Dec;11:508–524. doi: 10.1016/s0022-5320(64)80079-4. [DOI] [PubMed] [Google Scholar]
- Date T., Goodman J. M., Wickner W. T. Procoat, the precursor of M13 coat protein, requires an electrochemical potential for membrane insertion. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4669–4673. doi: 10.1073/pnas.77.8.4669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decker G. L., Joseph D. B., Lennarz W. J. A study of factors involved in induction of the acrosomal reaction in sperm of the sea urchin, Arbacia punctulata. Dev Biol. 1976 Oct 1;53(1):115–125. doi: 10.1016/0012-1606(76)90213-x. [DOI] [PubMed] [Google Scholar]
- Deutsch C., Erecińska M., Werrlein R., Silver I. A. Cellular energy metabolism, trans-plasma and trans-mitochondrial membrane potentials, and pH gradients in mouse neuroblastoma. Proc Natl Acad Sci U S A. 1979 May;76(5):2175–2179. doi: 10.1073/pnas.76.5.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grainger J. L., Winkler M. M., Shen S. S., Steinhardt R. A. Intracellular pH controls protein synthesis rate in the sea urchine egg and early embryo. Dev Biol. 1979 Feb;68(2):396–406. doi: 10.1016/0012-1606(79)90213-6. [DOI] [PubMed] [Google Scholar]
- Hoek J. B., Nicholls D. G., Williamson J. R. Determination of the mitochondrial protonmotive force in isolated hepatocytes. J Biol Chem. 1980 Feb 25;255(4):1458–1464. [PubMed] [Google Scholar]
- Kiefer H., Blume A. J., Kaback H. R. Membrane potential changes during mitogenic stimulation of mouse spleen lymphocytes. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2200–2204. doi: 10.1073/pnas.77.4.2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtshtein D., Kaback H. R., Blume A. J. Use of a lipophilic cation for determination of membrane potential in neuroblastoma-glioma hybrid cell suspensions. Proc Natl Acad Sci U S A. 1979 Feb;76(2):650–654. doi: 10.1073/pnas.76.2.650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindemann C., Rikmenspoel R. Intracellular potentials in bull spermatozoa. J Physiol. 1971 Dec;219(1):127–138. doi: 10.1113/jphysiol.1971.sp009653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore R. D. Elevation of intracellular pH by insulin in frog skeletal muscle. Biochem Biophys Res Commun. 1979 Dec 14;91(3):900–904. doi: 10.1016/0006-291x(79)91964-8. [DOI] [PubMed] [Google Scholar]
- Nicholls D. G. The influence of respiration and ATP hydrolysis on the proton-electrochemical gradient across the inner membrane of rat-liver mitochondria as determined by ion distribution. Eur J Biochem. 1974 Dec 16;50(1):305–315. doi: 10.1111/j.1432-1033.1974.tb03899.x. [DOI] [PubMed] [Google Scholar]
- Ozawa S., Kimura N. Membrane potential changes caused by thyrotropin-releasing hormone in the clonal GH3 cell and their relationship to secretion of pituitary hormone. Proc Natl Acad Sci U S A. 1979 Nov;76(11):6017–6020. doi: 10.1073/pnas.76.11.6017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pressman B. C. Biological applications of ionophores. Annu Rev Biochem. 1976;45:501–530. doi: 10.1146/annurev.bi.45.070176.002441. [DOI] [PubMed] [Google Scholar]
- Rink T. J. Membrane potential of guinea-pig spermatozoa. J Reprod Fertil. 1977 Sep;51(1):155–157. doi: 10.1530/jrf.0.0510155. [DOI] [PubMed] [Google Scholar]
- Rottenberg H., Grunwald T., Avron M. Determination of pH in chloroplasts. I. Distribution of ( 14 C) methylamine. Eur J Biochem. 1972 Jan 31;25(1):54–63. doi: 10.1111/j.1432-1033.1972.tb01666.x. [DOI] [PubMed] [Google Scholar]
- Rottenberg H. The measurement of membrane potential and deltapH in cells, organelles, and vesicles. Methods Enzymol. 1979;55:547–569. doi: 10.1016/0076-6879(79)55066-6. [DOI] [PubMed] [Google Scholar]
- Schackmann R. W., Eddy E. M., Shapiro B. M. The acrosome reaction of Strongylocentrotus purpuratus sperm. Ion requirements and movements. Dev Biol. 1978 Aug;65(2):483–495. doi: 10.1016/0012-1606(78)90043-x. [DOI] [PubMed] [Google Scholar]
- Schackmann R. W., Shapiro B. M. A partial sequence of ionic changes associated with the acrosome reaction of Strongylocentrotus purpuratus. Dev Biol. 1981 Jan 15;81(1):145–154. doi: 10.1016/0012-1606(81)90357-2. [DOI] [PubMed] [Google Scholar]
- Schuldiner S., Kaback H. R. Membrane potential and active transport in membrane vesicles from Escherichia coli. Biochemistry. 1975 Dec 16;14(25):5451–5461. doi: 10.1021/bi00696a011. [DOI] [PubMed] [Google Scholar]
- Schuldiner S., Rottenberg H., Avron M. Determination of pH in chloroplasts. 2. Fluorescent amines as a probe for the determination of pH in chloroplasts. Eur J Biochem. 1972 Jan 31;25(1):64–70. doi: 10.1111/j.1432-1033.1972.tb01667.x. [DOI] [PubMed] [Google Scholar]
- Shapiro B. M., Eddy E. M. When sperm meets egg: biochemical mechanisms of gamete interaction. Int Rev Cytol. 1980;66:257–302. doi: 10.1016/s0074-7696(08)61976-2. [DOI] [PubMed] [Google Scholar]
- Shen S. S., Steinhardt R. A. Direct measurement of intracellular pH during metabolic derepression of the sea urchin egg. Nature. 1978 Mar 16;272(5650):253–254. doi: 10.1038/272253a0. [DOI] [PubMed] [Google Scholar]
- Slater E. C. Mechanism of oxidative phosphorylation. Annu Rev Biochem. 1977;46:1015–1026. doi: 10.1146/annurev.bi.46.070177.005055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilney L. G., Kiehart D. P., Sardet C., Tilney M. Polymerization of actin. IV. Role of Ca++ and H+ in the assembly of actin and in membrane fusion in the acrosomal reaction of echinoderm sperm. J Cell Biol. 1978 May;77(2):536–550. doi: 10.1083/jcb.77.2.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshioka T., Inoue H. High potassium effect on the mobility of sea urchin sperm. Nagoya J Med Sci. 1980 Mar;42(3-4):82–84. [PubMed] [Google Scholar]