Abstract
The standard treatment for CH-C, pegylated interferon-α and ribavirin (PEG-IFN + RBV), is associated with depression. Recent studies have proposed a new role for cytokines in the pathogenesis of depression. We aimed to assess differential gene expression related to depression in CH-C patients treated with PEG-IFN + RBV. We included 67 CH-C patients being treated with PEG-IFN+RBV. Of the entire study cohort, 22% had pre-existing depression, while another 37% developed new depression in course of the treatment. Pretreatment blood samples were collected into PAXgene™ RNA tubes, the RNAs extracted from peripheral blood mononuclear cells (PBMCs) were used for one step RT-PCR to profile 160 mRNAs. Differentially expressed genes were separated into up- and down-regulated genes according to presence or absence of depression at baseline (pre-existing depression) or following the initiation of treatment (treatment-related depression). The mRNA expression profile associated with any depression and with treatment-related depression included four and six genes, respectively. Our data demonstrate a significant down-regulation of TGF-β1 and the shift of Th1-Th2 cytokine balance in the depression associated with IFN-based treatment of HCV infection. We propose that TGF-β1 plays an important role in the imbalance of Th1/Th2 in patients with CH-C and depression. With further validation, TGF-β1 and other components of Th1/Th2 regulation pathway may provide a future marker for CH-C patients predisposed to depression.
Keywords: Depression, hepatitis C, interferon, ribavirin, TGFβ1, Th1/Th2 cytokines, treatment
Introduction
Chronic hepatitis C virus (HCV) is believed to affect approximately 170 million people worldwide extending across all economic and social groups (Armstrong et al. 2006). Since a large proportion of HCV-infected individuals are currently undiagnosed, the number of newly diagnosed patients with HCV and related liver disease is expected to grow. In fact, the proportion of chronic hepatitis C patients with cirrhosis is expected to reach 25% in 2010 and 45% in 2030 (Davis et al. 2010). The considerable burden of HCV on the health care system is further compounded by the fact that HCV-related cirrhosis is the most common indication for liver transplantation (Tan and Lok 2007).
The current standard treatment for HCV is a combination of pegylated interferon-α and ribavirin, administered over a 24- or 48-week course. Despite advances in treating HCV, even with the optimal delivery of the current interferon-based regimen for HCV, only about 50% of treated patients with genotype 1/4 successfully clear the virus (Mauss et al. 2011). The success of the current treatment is multi-factorial and depends on a combination of several host, viral, and treatment factors. Viral factors consist of HCV genotype, pretreatment viral load, and presence of viral quasi-species (Timm and Roggendorf 2007). Host factors include presence of co-morbidities such as obesity, cirrhosis, ethnic background, gender, and age (Bondini and Younossi 2006; Chen et al. 2007; Neumann-Haefelin et al. 2007; Sharma et al. 2007). Finally, treatment-related factors affecting response include adequate duration of treatment, patient adherence and, importantly, optimal management of PEG-IFN and RBV-related side effects (Mulhall and Younossi 2005; Sharma et al. 2007). Consequently, there are two main areas of focus to develop future treatment regimens for HCV. One of them focuses on new therapeutics that can potentially increase the rates for sustained virological response (SVR) by developing regimens that would include direct acting antiviral agents (DAA). The other, equally important area, is to optimize treatment regiments and reduce its side-effect profile.
Despite diligent efforts by clinicians and clinical investigators, successful management of treatment-associated side effects remains a substantial problem and contributes significantly to treatment discontinuation or dose reduction (10 and 35%, respectively) (Manns et al. 2001; Dan et al. 2006). Depression disorder is one of the least tangible, and one of the most difficult IFN-related side effects to quantify in the treatment of HCV. IFN-α-induced depression is markedly similar to major depressive disorder (MDD) and may be manifested as depressed mood, irritability, emotional lability, agitation, fatigue, apathy, anhedonia, anorexia, psychomotor retardation, sleep disturbance, sexual dysfunction, memory impairment, and diminished ability to concentrate (Valentine et al. 1998). Due to the variability of the symptoms and lack of unification in their measurements, studies on IFN-α-induced depression also produce variable results with incidence rates ranging from 16 to 45% in patients receiving treatment (Fattovich et al. 1996; Hauser et al. 2002; Dieperink et al. 2003).
The most common risk factors for IFN-α-induced depression are related either to treatment regimen itself (i.e., higher dose and longer duration of medication) (Capuron and Ravaud 1999; Hauser et al. 2002; Dieperink et al. 2003; Capuron and Miller 2004) or to intrinsic factors predisposing patients to the development of DSM-IV symptom criteria for MDD. The most common risk factor of latter kind is pre-existing psychiatric problems or previously diagnosed MDD. Importantly, even subclinical depression and/or anxiety at the baseline, greatly increases the chances for the development of depression during treatment with IFN-α (Van Thiel et al. 1998; Capuron and Ravaud 1999; Fontana et al. 2002; Hauser et al. 2002; Dieperink et al. 2003; Capuron and Miller 2004). Information about “predisposition to depression” can help clinical practitioners to make some difficult decisions to more closely follow or “pre-treat” patients with a risk factor for depression with an antidepressant. This solution is imperfect, as at least 50% of patients do not suffer from any depressive symptoms during treatment, and are thereby being needlessly exposed to antidepressants. Therefore, a search for quantitatively measurable markers of IFN-α-induced depression could aid in identifying patients who would benefit from antidepressant pretreatment.
One approach is to screen for molecules connected to the pathological process of depression. IFN-α is a potent pro-inflammatory cytokine that acts to increase the serum concentrations of various other cytokines including interleukin IL-1, IL-6, tumor necrosis factor-α (TNF-α), IL-2, and IFN-α (Taylor and Grossberg 1998). Some studies have recently proposed a pivotal role for cytokine imbalance in the etiology of depression; in particular, the relevance of the Th1/Th2 cytokine imbalance in the brain during both psychological stress and with psychiatric disorders was discussed (Myint and Kim 2003). In this study, we examine the baseline expression of 153 cytokine response-related genes in patients undergoing HCV treatment and correlate our findings to treatment-induced depression symptoms.
Methods
Study cohort
This study cohort comprised of HCV-infected patients scheduled for treatment with PEG-IFN and RBV. Prior to treatment, clinical, demographic, and laboratory data, as well as blood samples were collected. The MDD was diagnosed according to DSM criteria at baseline and during treatment. Dose and duration of anti-HCV treatment were determined by genotype and “on-treatment” response pattern. From pretreatment blood samples, mRNA was extracted and relative gene expression levels were calculated as previously described (Garcia et al. 2005; Younossi et al. 2009).
Statistical analysis
Differentially expressed genes were separated into up and down-regulated gene lists according to the presence of depression at baseline (Pre-existing Depression) as well as to the development of depression during treatment (New Depression). Both gene lists were subjected to intensive literature searches to determine potential associations with depression. Using both data sets, predictive models for depression were designed. Clinical parameters were compared using the chi-square test, and gene expression levels were compared using the Mann–Whitney non-parametric test. Regression models for predicting any and new depression (with these parameters used as dependent variables) were generated by stepwise bi-directional selection. Only genes that demonstrated a trend to statistical significance (P ≤ 0.05 before multiple test correction) were used for stepwise selection. Bi-directional selection started with a full model that contained all the genes with significantly different expression levels (based on Mann–Whitney's outputs) and clinical parameters, and ended when no more improvement (estimated using coefficient of determination) of a depression-predicting model containing TGFβ1 gene was achieved with the addition or removal of any clinical or any other gene predictor The predictive performance that included sensitivity, specificity, positive, and negative predictive values and the area under the ROC-curve (AUC) was evaluated for the generated models using ten-fold cross-validation. All analyses were run using SAS 10.2 (SAS Institute, Cary, NC).
Results
Demographic and clinical data
The study included 67 CH-C patients [age 48.4 ± 6.7 years, 38.8% female, 16% African American, 60% Obese (BMI > 30), 51% Overweight (BMI > 27.5), 71% genotype 1, 21% cirrhosis, 12% DM, 76% with high pretreatment viral load, and 44% treatment naïve] treated with PEG-IFN+RBV. In this cohort, after a full course of treatment, 76% achieved EVR, 57% achieved cEVR, and 41% achieved SVR. Rates of SVR in genotype 1 were 35% and 55% in non-genotype 1 patients.
Pretreatment depression was seen in 22.4% of the patients. Within this group the prevalence for “Any Depression” (AD) (including those with pre-existing depression and those with new depression during treatment), was 55.22% (N = 67). The prevalence for “Treatment-related Depression” (TRD) was 36.54% (N = 52). The history of depression was evenly distributed across the treated cohort, regardless of their genotype, gender, pattern of response, as well as presence of cirrhosis, or obesity (Tables 1 and 2) and were not statistically correlated with any of these co-morbidities or demographics.
Table 1.
Differentially expressed genes in cohorts with “Any Depression” and “New Depression”, where down-regulation is indicated by the color red and up-regulation is indicated by the color blue. Gene abbreviations are as follows: PDGFA (platelet-derived growth factor alpha polypeptide), STAT4 (signal transducer and activator of transcription 4), TGFB1 (transforming growth factor β1), PF4 (platelet factor 4), EP300 (E1A binding protein p300), PRKRIR (repressor of interferon-inducible double-stranded RNA-dependent inhibitor protein-kinase), TRAF6 (TNF receptor-associated factor 6)
| Depression model | Prevalence (%) | Differentially expressed genes | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Any Depression | 55.22 | PDGFA | STAT4 | TGFB1 | PF4 | ||||
| P = 0.0054 | P = 0.0396 | P = 0.0152 | P = 0.0123 | ||||||
| New Depression | 36.54 | PDGFA | STAT4 | TGFB1 | EP300 | PRKRIR | TRAF6 | ||
| P = 0.0318 | P = 0.0082 | P = 0.0194 | P = 0.0275 | P = 0.0439 | P = 0.0142 | ||||
Table 2.
Distribution of the prevalence of “Any Depression” across group cohorts
| Cohorts | Number of subjects in cohort (% of total enrolled) | Incidence of depression in cohort (% of cohort) |
|---|---|---|
| Genotype I | 47 (70.1) | 28 (59.6) |
| Female | 26 (38.8) | 16 (61.5) |
| Re-treated | 37 (55.2) | 24 (64.9) |
| SVR | 28 (41.8) | 14 (50.0) |
| Cirrhosis | 13 (20.0) | 9 (69.2) |
| Obesity | 41 (61.2) | 25 (61.0) |
SVR, sustained virological response.
Gene expression data
The mRNA expression profile associated with Any Depression (AD) included four genes, three of them (PDGFA, PF4, and TGF-β1) were down-regulated (P-values: <0.0054, <0.0123 and <0.0152; respectively), while the STAT4 gene was up-regulated (P-value <0.0396) (Table 2). Gene expression profile associated with TRD included six genes three of them, PDGFA, EP300, and TGF-β1 were down-regulated (P-values: <0.0318, <0.0275 and <0.0194; respectively), while PRKRIR, TRAF6, and STAT4, genes were up-regulated (P-value <0.0439, <0.0142, <0.0082; respectively) (Table 3).
Table 3.
Distribution of the prevalence of “Treatment-related Depression” across group cohorts, where the TRD cohort excludes those (N = 15) having depression at the start of the study
| Cohorts | Number of subjects in cohort (% of total enrolled) | Incidence of depression in cohort (% of cohort) |
|---|---|---|
| Genotype 1 | 37 (71.2) | 16 (43.2) |
| Female | 16 (30.8) | 4 (25.0) |
| Re-treated | 28 (53.8) | 12 (42.9) |
| SVR | 26 (50.0) | 10 (38.5) |
| Cirrhosis | 9 (17.6) | 5 (55.6) |
| Obesity | 33 (63.5) | 16 (48.5) |
SVR, sustained virological response.
Using the gene expression profiles, predictive models for both classes of depression were calculated. The best model predicting treatment-related depression included expression levels of TRAF6 and TGF-β1 with a P-value of 0.001185, a sensitivity of 63.16% (38.4–83.7%), a specificity of 87.88% (71.8–96.6%), and an area under the curve (AUC) of 0.748 (0.608–0.858).
The predictive model for any depression relied solely on expression levels of TGF-β1 with a P-value of 0.01242, a sensitivity of 67.57% (50.2–82.0%), a specificity of 63.33% (43.9–80.1%), and an AUC of 0.642 (0.516–0.756).
Discussion
Patients with chronic hepatitis C undergoing PEG-IFN+RBV therapy are at an increased risk for developing depression or aggravating pre-existing depression. Several mechanisms for the development of IFN-related depression have been suggested, however, no solid evidence for a common molecular mechanism has yet been proffered. At the same time, markers capable of predicting depression in CH-C patients are highly desirable as active depression during HCV treatment may jeopardize desired therapeutic outcomes and patients' health-related quality of life (Dan et al. 2006; Younossi et al. 2007).
Previous studies in MDD, over the past decade, have increasingly shown the profound involvement of the deregulation of the immune system, including the cytokine network (Maes et al. 1990; Myint and Kim 2003; Schiepers et al. 2005). In particular, events causing activation of the immune system, with the resultant increase in pro-inflammatory cytokines, often coincides with the onset of depression (Maes 1995; Connor and Leonard 1998; Yirmiya 2000; Capuron and Dantzer 2003). In turn, the shift of the T-helper Th1/Th2 balance toward a Th1-type inflammatory response (Maes 1995) occurs in a large number of MDD cases (Myint and Kim 2003; Myint et al. 2005).
Of note, in our study, we observed a significant baseline up-regulation of STAT4 in HCV patients with both a history of depression and new treatment-related depression. This gene has been shown to be intimately involved in the signaling cascade necessary for the activation and consequent pro-inflammatory signal cascade of Th1 type cells (Saraiva et al. 2009).
A recent study on the effects of Th1/Th2 class cytokines on gene expression in cell culture found that Th2 class cytokines up-regulate the prepropeptide PDGF A chain (Lisak et al. 2007). Indeed, in our study, both the presence of “Any Depression” as well as “Treatment-related Depression” resulted in significantly lower expression of PDGFA. These studies point toward a decrease in the Th2 class cytokine signaling as the potential mechanism of depression for these patients. In addition, for HCV patients with “Any Depression”, the PF4 gene was significantly down-regulated. The PF4 encodes for the soluble protein CXCL4, which is directly involved in the up-regulation of Th2 class of cytokines (Romagnani et al. 2005; Mueller et al. 2008). Other genes of interest associated with TRD include TRAF6 and PRKRIR, both of which are involved in inflammatory diseases and in the inflammatory signaling cascade (Cejas et al. 2010; Kuenzel et al. 2010), and PDGFA which encodes the prepropeptide PDGF A chain. PDGFA is specifically up-regulated by Th2 class cytokines (Lisak et al. 2007). In our study, this gene was markedly suppressed, pointing to a decrease in the Th2 class cytokine signaling in HCV patients who develop depression. In fact, our data necessitate a closer examination of the pretreatment baseline levels of Th1 class and Th2 class cytokines in patients scheduled for IFN-α therapy, as interferon-induced depression may in fact involve a pre-existing imbalance in the host Th1/Th2 levels, rendering certain patients vulnerable to depression.
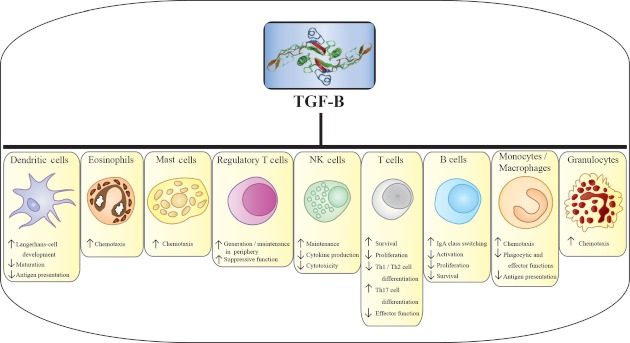
Our study also supports the potential role of TGF-β1 in IFN-related depression. TGF-β1 is mainly secreted by regulatory T cells such as type 1 regulatory T cells and T-helper type 3 cells (Th3) and is thought to be essential for the maintenance of immune homeostasis and for the suppression of autoimmunity (Groux et al. 1997; Taylor et al. 2006; Zhang et al. 2006). TGF-β1 is known to not only promote T-helper type 2 cell (Th2) differentiation (Barral-Netto et al. 1992) but also to exert a strong inhibitory effect on the production of pro-inflammatory cytokines such as interferons (IFNs), tumor necrosis factor (TNF-α), and IL-2 (Schmitt et al. 1994; Prud'homme and Piccirillo 2000) (Fig. 1). Recent studies indicate that, TGF-β1 plays a role in the development of depression by shifting the balance between the pro-inflammatory/anti-inflammatory cytokines seen in this disorder (Myint et al. 2005; Lee and Kim 2006). In fact, recent studies on MDD have shown that significantly lowered pretreatment TGF-β1 levels in the depressed patients increase following antidepressant therapy (Myint et al. 2005). The decreased baseline levels of TGF-β1 seen within our cohort of HCV patients who ultimately developed depression during treatment, may well follow the same etiology as seen in patients with MDD. Importantly, TGF-β1 has been extensively studied within the context of liver disease, particularly in relation to inflammation and fibrosis (Wynn and Barron 2010). However, little is known about its role within the context of PEG-IFN+RBV treatment of HCV and its associated side effects. The current study is the first to point to TGF-β1 as having a pivotal role in IFN-related depression.
Figure 1.
Transforming growth factor-b (TGF-β) and its effects on a large group of secreted cytokines, with a wide range of functional properties.
Importantly, worldwide efforts in genome-wide profiling of the polymorphisms associated with MDD and antidepressant treatment outcomes produced only a handful of the candidate genes. Moreover, even in the largest of these studies, the genome-wide significance was not achieved (see Laje and McMahon 2011; Major Depressive Disorder Working Group of the Psychiatric GWAS Consortium 2012, for recent reviews). This observation most probably means that the genetic research in MDD shall proceed by studying smaller, but clearly defined groups of the patients rather than by sheer increase in overall power of the study. Thus, we believe that our approach to the dissection of IFN-α-induced depression may be worthwhile to replicate for other homogenous groups of MDD patients.
In conclusion, our data demonstrate a significant down-regulation of TGF-β1 and dysregulation of Th1-Th2 cytokine balance in the depression associated with IFN-based treatment of HCV infection. We propose that TGF-β1 may play a role in the imbalance of the Th1/Th2 cytokine ratio in patients with CH-C and depression. With further validation, TGF-β1 and other components of Th1/Th2 regulation pathway may provide a quantitative marker for HCV patients predisposed to treatment-related depression.
Acknowledgments
This study was supported by the Liver Outcomes Research Fund of the Center for Liver Diseases at Inova Fairfax Hospital, Inova Health System, Falls Church, Virginia. All the gene expression experiments were performed at Celera, Alameda, California.
Conflicts of Interest
The authors declare that they have no competing interests.
Authors' contributions: ABar and ZY designed the study and edited the manuscript. AA and IY collected the samples. MS performed statistical analysis. ABir performed gene expression analysis and drafted a manuscript. All authors read and approved the final manuscript.
Authors' information: ABar is an Associate Professor at the School of Systems Biology, College of Science, George Mason University (SSB COS GMU). ABir is Research Assistant Professor at SSB COS GMU. AA is a Research Associates and IZ is a Research Volunteer at Betty and Guy Beatty Center for Integrated Research, Inova Health System. ZY is a Chairman, Department of Medicine, Inova Fairfax Hospital and Vice President for Research, Inova Health System.
References
- Armstrong GL, Wasley A, Simard EP, McQuillan GM, Kuhnert WL, Alter MJ. The prevalence of hepatitis C virus infection in the United States, 1999 through 2002. Ann. Intern. Med. 2006;144:705–714. doi: 10.7326/0003-4819-144-10-200605160-00004. [DOI] [PubMed] [Google Scholar]
- Barral-Netto M, Barral A, Brownell CE, Skeiky YA, Ellingsworth LR, Twardzik DR, et al. Transforming growth factor-beta in leishmanial infection: a parasite escape mechanism. Science. 1992;257:545–548. doi: 10.1126/science.1636092. [DOI] [PubMed] [Google Scholar]
- Bondini S, Younossi ZM. Non-alcoholic fatty liver disease and hepatitis C infection. Minerva Gastroenterol. Dietol. 2006;52:135–143. [PubMed] [Google Scholar]
- Capuron L, Dantzer R. Cytokines and depression: the need for a new paradigm. Brain Behav. Immun. 2003;17(Suppl. 1):S119–S124. doi: 10.1016/s0889-1591(02)00078-8. [DOI] [PubMed] [Google Scholar]
- Capuron L, Miller AH. Cytokines and psychopathology: lessons from interferon-alpha. Biol. Psychiatry. 2004;56:819–824. doi: 10.1016/j.biopsych.2004.02.009. [DOI] [PubMed] [Google Scholar]
- Capuron L, Ravaud A. Prediction of the depressive effects of interferon alfa therapy by the patient's initial affective state. N. Engl. J. Med. 1999;340:1370. doi: 10.1056/NEJM199904293401716. [DOI] [PubMed] [Google Scholar]
- Cejas PJ, Walsh MC, Pearce EL, Han D, Harms GM, Artis D, et al. TRAF6 inhibits Th17 differentiation and TGF-beta-mediated suppression of IL-2. Blood. 2010;115:4750–4757. doi: 10.1182/blood-2009-09-242768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen TY, Hsieh YS, Wu TT, Yang SF, Wu CJ, Tsay GJ, et al. Impact of serum levels and gene polymorphism of cytokines on chronic hepatitis C infection. Transl. Res. 2007;150:116–121. doi: 10.1016/j.trsl.2007.01.007. [DOI] [PubMed] [Google Scholar]
- Connor TJ, Leonard BE. Depression, stress and immunological activation: the role of cytokines in depressive disorders. Life Sci. 1998;62:583–606. doi: 10.1016/s0024-3205(97)00990-9. [DOI] [PubMed] [Google Scholar]
- Dan AA, Martin LM, Crone C, Ong JP, Farmer DW, Wise T, et al. Depression, anemia and health-related quality of life in chronic hepatitis C. J. Hepatol. 2006;44:491–498. doi: 10.1016/j.jhep.2005.11.046. [DOI] [PubMed] [Google Scholar]
- Davis GL, Alter MJ, El-Serag H, Poynard T, Jennings LW. Aging of hepatitis C virus (HCV)-infected persons in the United States: a multiple cohort model of HCV prevalence and disease progression. Gastroenterology. 2010;138:513–521. doi: 10.1053/j.gastro.2009.09.067. [DOI] [PubMed] [Google Scholar]
- Dieperink E, Ho SB, Thuras P, Willenbring ML. A prospective study of neuropsychiatric symptoms associated with interferon-a-2b and ribavirin therapy for patients with chronic hepatitis C. Psychosomatics. 2003;44:104–112. doi: 10.1176/appi.psy.44.2.104. [DOI] [PubMed] [Google Scholar]
- Fattovich G, Giustina G, Favarato S, Ruol A. A survey of adverse events in 11241 patients with chronic viral hepatitis treated with alpha interferon. J. Hepatol. 1996;24:38–47. doi: 10.1016/s0168-8278(96)80184-x. [DOI] [PubMed] [Google Scholar]
- Fontana RJ, Schwartz SM, Gebremariam A, Lok AS, Moyer CA. Emotional distress during interferon-α-2B and ribavirin treatment of chronic hepatitis C. Psychosomatics. 2002;43:378–385. doi: 10.1176/appi.psy.43.5.378. [DOI] [PubMed] [Google Scholar]
- Garcia EP, Dowding LA, Stanton LW, Slepnev VI. Scalable transcriptional analysis routine–multiplexed quantitative real-time polymerase chain reaction platform for gene expression analysis and molecular diagnostics. J. Mol. Diagn. 2005;7:444–454. doi: 10.1016/S1525-1578(10)60575-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groux H, O'Garra A, Bigler M, Rouleau M, Antonenko S, de Vries JE, et al. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature. 1997;389:737–742. doi: 10.1038/39614. [DOI] [PubMed] [Google Scholar]
- Hauser P, Khosla J, Aurora H, Laurin J, Kling MA, Hill J, et al. A prospective study of the incidence and open-label treatment of interferon induced major depressive disorder in patients with hepatitis C. Mol. Psychiatry. 2002;7:942–947. doi: 10.1038/sj.mp.4001119. [DOI] [PubMed] [Google Scholar]
- Kuenzel S, Till A, Winkler M, Häsler R, Lipinski S, Jung S, et al. The nucleotide-binding oligomerization domain-like receptor NLRC5 is involved in IFN-dependent antiviral immune responses. J. Immunol. 2010;184:1990–2000. doi: 10.4049/jimmunol.0900557. [DOI] [PubMed] [Google Scholar]
- Laje G, McMahon FJ. Genome-wide association studies of antidepressant outcome: a brief review. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2011;35:1553–1557. doi: 10.1016/j.pnpbp.2010.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KM, Kim YK. The role of IL-12 and TGF-beta1 in the pathophysiology of major depressive disorder. Int. Immunopharmacol. 2006;6:1298–1304. doi: 10.1016/j.intimp.2006.03.015. [DOI] [PubMed] [Google Scholar]
- Lisak RP, Benjamins JA, Bealmear B, Nedelkoska L, Yao B, Land S, et al. Differential effects of Th1, monocyte/macrophage and Th2 cytokine mixtures on early gene expression for glial and neural-related molecules in central nervous system mixed glial cell cultures: neurotrophins, growth factors and structural proteins. J. Neuroinflammation. 2007;18:30. doi: 10.1186/1742-2094-4-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes M. Evidence for an immune response in major depression: a review and hypothesis. Prog. Neuropsychopharmacol. Biol. Psychiatry. 1995;19:11–38. doi: 10.1016/0278-5846(94)00101-m. [DOI] [PubMed] [Google Scholar]
- Maes M, Bosmans E, Suy E, Vandervorst C, De Jonckheere C, Raus J. Immune disturbances during major depression: upregulated expression of interleukin-2 receptors. Neuropsychobiology. 1990;24:115–120. doi: 10.1159/000119472. [DOI] [PubMed] [Google Scholar]
- Major Depressive Disorder Working Group of the Psychiatric GWAS Consortium. A mega-analysis of genome-wide association studies for major depressive disorder. Mol Psychiatry. 2012 doi: 10.1038/mp.2012.21. doi: 10.1038/mp.2012.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manns MP, McHutchison JG, Gordon SC, Rustgi VK, Shiffman M, Reindollar R, et al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet. 2001;358:958–965. doi: 10.1016/s0140-6736(01)06102-5. [DOI] [PubMed] [Google Scholar]
- Mauss S, Hueppe D, John C, Goelz J, Heyne R, Moeller B, et al. Estimating the likelihood of sustained virological response in chronic hepatitis C therapy. J. Viral Hepat. 2011;18:e81–e90. doi: 10.1111/j.1365-2893.2010.01372.x. doi: 10.1111/j.1365-2893.2010.01372.x. [DOI] [PubMed] [Google Scholar]
- Mueller A, Meiser A, McDonagh EM, Fox JM, Petit SJ, Xanthou G, et al. CXCL4-induced migration of activated T lymphocytes is mediated by the chemokine receptor CXCR3. J. Leukoc. Biol. 2008;83:875–882. doi: 10.1189/jlb.1006645. [DOI] [PubMed] [Google Scholar]
- Mulhall BP, Younossi Z. Impact of adherence on the outcome of antiviral therapy for chronic hepatitis C. J. Clin. Gastroenterol. 2005;39:S23–S27. doi: 10.1097/01.mcg.0000145538.43865.72. [DOI] [PubMed] [Google Scholar]
- Myint AM, Kim YK. Cytokine–serotonin interaction through IDO: a neurodegeneration hypothesis of depression. Med. Hypotheses. 2003;61:519–525. doi: 10.1016/s0306-9877(03)00207-x. [DOI] [PubMed] [Google Scholar]
- Myint AM, Leonard BE, Steinbusch HW, Kim YK. Th1, Th2, and Th3 cytokine alterations in major depression. J. Affect. Disord. 2005;88:167–173. doi: 10.1016/j.jad.2005.07.008. [DOI] [PubMed] [Google Scholar]
- Neumann-Haefelin C, Spangenberg HC, Blum HE, Thimme R. Host and viral factors contributing to CD8+ T cell failure in hepatitis C virus infection. World J. Gastroenterol. 2007;13:4839–4847. doi: 10.3748/wjg.v13.i36.4839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prud'homme GJ, Piccirillo CA. The inhibitory effects of transforming growth factor-beta-1 (TGF-beta1) in autoimmune diseases. J. Autoimmun. 2000;14:23–42. doi: 10.1006/jaut.1999.0339. [DOI] [PubMed] [Google Scholar]
- Romagnani P, Maggi L, Mazzinghi B, Cosmi L, Lasagni L, Liotta F, et al. CXCR3-mediated opposite effects of CXCL10 and CXCL4 on TH1 or TH2 cytokine production. J. Allergy Clin. Immunol. 2005;116:1372–1379. doi: 10.1016/j.jaci.2005.09.035. [DOI] [PubMed] [Google Scholar]
- Saraiva M, Christensen JR, Veldhoen M, Murphy TL, Murphy KM, O'Garra A. Interleukin-10 production by Th1 cells requires interleukin-12-induced STAT4 transcription factor and ERK MAP kinase activation by high antigen dose. Immunity. 2009;31:209–219. doi: 10.1016/j.immuni.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiepers OJ, Wichers MC, Maes M. Cytokines and major depression. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2005;29:201–217. doi: 10.1016/j.pnpbp.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Schmitt E, Hoehn P, Huels C, Goedert S, Palm N, Rüde E, et al. T helper type 1 development of naive CD4+ T cells requires the coordinate action of interleukin-12 and interferon-gamma and is inhibited by transforming growth factor-beta. Eur. J. Immunol. 1994;24:793–798. doi: 10.1002/eji.1830240403. [DOI] [PubMed] [Google Scholar]
- Sharma P, Marrero JA, Fontana RJ, Greenson JK, Conjeevaram H, Su GL, et al. Sustained virologic response to therapy of recurrent hepatitis C after liver transplantation is related to early virologic response and dose adherence. Liver Transpl. 2007;13:1100–1108. doi: 10.1002/lt.21121. [DOI] [PubMed] [Google Scholar]
- Tan J, Lok AS. Update on viral hepatitis: 2006. Curr. Opin. Gastroenterol. 2007;23:263–267. doi: 10.1097/MOG.0b013e328049ddc1. [DOI] [PubMed] [Google Scholar]
- Taylor JL, Grossberg SE. The effects of interferon-alpha on the production and action of other cytokines. Semin. Oncol. 1998;25:23–29. [PubMed] [Google Scholar]
- Taylor A, Verhagen J, Blaser K, Akdis M, Akdis CA. Mechanisms of immune suppression by interleukin-10 and transforming growth factor-beta: the role of T regulatory cells. Immunology. 2006;117:433–442. doi: 10.1111/j.1365-2567.2006.02321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timm J, Roggendorf M. Sequence diversity of hepatitis C virus: implications for immune control and therapy. World J. Gastroenterol. 2007;13:4808–4817. doi: 10.3748/wjg.v13.i36.4808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentine AD, Meyers CA, Kling MA, Richelson E, Hauser P. Mood and cognitive side effects of interferon-alpha therapy. Semin. Oncol. 1998;25:39–47. [PubMed] [Google Scholar]
- Van Thiel DH, Friedlander L, De Maria N, Molloy PJ, Kania RJ, Colantoni A. Treatment of chronic hepatitis C in individuals with pre-existing or confounding neuropsychiatric disease. Hepatogastroenterology. 1998;45:328–330. [PubMed] [Google Scholar]
- Wynn TA, Barron L. Macrophages: master regulators of inflammation and fibrosis. Semin. Liver Dis. 2010;30:245–257. doi: 10.1055/s-0030-1255354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yirmiya R. Depression in medical illness: the role of the immune system. West. J. Med. 2000;173:333–336. doi: 10.1136/ewjm.173.5.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Younossi Z, Kallman J, Kincaid J. The effects of HCV infection and management on health-related quality of life. Hepatology. 2007;45:806–816. doi: 10.1002/hep.21565. [DOI] [PubMed] [Google Scholar]
- Younossi ZM, Baranova A, Afendy A, Collantes R, Stepanova M, Manyam G, et al. Early gene expression profiles of patients with chronic hepatitis C treated with pegylated interferon-alfa and ribavirin. Hepatology. 2009;49:763–774. doi: 10.1002/hep.22729. [DOI] [PubMed] [Google Scholar]
- Zhang L, Yi H, Xia XP, Zhao Y. Transforming growth factor-beta: an important role in CD4+CD25+ regulatory T cells and immune tolerance. Autoimmunity. 2006;39:269–276. doi: 10.1080/08916930600753903. [DOI] [PubMed] [Google Scholar]