Abstract
Many bacterial toxins covalently modify components of eukaryotic signalling pathways in a highly specific manner, and can be used as powerful tools to decipher the function of their molecular target(s). The Pasteurella multocida toxin (PMT) mediates its cellular effects through the activation of members of three of the four heterotrimeric G-protein families, Gq, G12 and Gi. PMT has been shown by others to lead to the deamidation of recombinant Gαi at Gln-205 to inhibit its intrinsic GTPase activity. We have investigated modification of native Gα subunits mediated by PMT in Swiss 3T3 cells using 2-D gel electrophoresis and antibody detection. An acidic change in the isoelectric point was observed for the Gα subunit of the Gq and Gi families following PMT treatment of Swiss 3T3 cells, which is consistent with the deamidation of these Gα subunits. Surprisingly, PMT also induced a similar modification of Gα11, a member of the Gq family of G-proteins that is not activated by PMT. Furthermore, an alkaline change in the isoelectric point of Gα13 was observed following PMT treatment of cells, suggesting differential modification of this Gα subunit by PMT. Gs was not affected by PMT treatment. Prolonged treatment with PMT led to a reduction in membrane-associated Gαi, but not Gαq. We also show that PMT inhibits the GTPase activity of Gq.
Introduction
Heterotrimeric G-proteins are a family of key signal transduction proteins that intercede between the many G-protein coupled receptors (GPCR) that the cell uses to interrogate its local environment and downstream signalling pathways that ultimately regulate fundamental cellular choices [1]. G-proteins are divided into 4 classes (Gq, G12, Gi and Gs) according to their constituent alpha subunit, which is a guanine nucleotide binding protein that can exist in an inactive GDP-bound or an active GTP-bound form [2]. Activation of a GPCR causes a conformational change in its cognate Gα subunit that triggers GDP to be exchanged for GTP. The activated state persists until GTP is hydrolysed to GDP by the intrinsic GTPase activity of the Gα subunit. G-proteins are also subject to reversible tyrosine phosphorylation and lipid modifications during their activation cycle, but the regulatory role of these events is not fully understood [3]. Each G-protein class activates a characteristic set of downstream targets. The Gs and Gi families activate or inhibit adenylate cyclase, respectively [4]. The Gq family activates phospholipase C (PLC) [5], while the G12 family is particularly linked to activation of the Rho GTPase [6].
Intracellularly-acting bacterial protein toxins enzymatically modify a limited and precise set of cellular proteins to modulate their function. The Pasteurella multocida toxin (PMT) activates multiple signalling pathways in cultured cells leading characteristically to a strong mitogenic response [7]. PMT has been shown to activate members of the Gq, G12 and Gi families [8]–[13]. PMT catalyses the deamidation of recombinant Gi at Gln-205 to inhibit its intrinsic GTPase activity [14]. We describe here the effects of PMT on all four classes of heterotrimeric G-proteins in Swiss 3T3 cells using two-dimensional (2-D) gel electrophoresis and other techniques.
Materials and Methods
Reagents
Cell culture reagents were obtained from Invitrogen. (γ-32P) GTP was obtained from PerkinElmer LAS. Anti-Gαq/11 (sc-392), anti-Gα11 (sc-394), anti-Gαs (sc-387), anti-Gα13 (sc-410) and anti-Gαi-2 (internal: sc-7276) antibodies were from Santa Cruz Biotechnology. Anti-Gαq (371752), anti-Gαi-1 (371720), anti-Gαi-1-2 (371723) and anti-Gαi-1-3 (371729: which is known to cross react with Gαi-1 and Gαi-2) antibodies were purchased from Calbiochem-Novabiochem. Phospho-FAK (Tyr397) was from New England Biolabs Ltd. All reagents used for 2-D gel electrophoresis were from GE HealthCare, unless otherwise stated. Recombinant PMT was purified essentially as described [15]. A recombinant His-tagged Gαq subunit (371765) was purchased from Calbiochem-Novabiochem. Recombinant His6-tagged human Gαi-1 was expressed and purified from E. coli containing pProEX-HTb, which was provided as a kind gift by Professor David Siderovski (Department of Pharmacology, University of North Carolina, USA) [16]. All other chemical reagents were of analytical grade and were obtained from Sigma-Aldrich, unless otherwise stated.
Cell culture
Swiss 3T3 cells, originally developed by Todaro and Green [17], and kindly provided by Theresa Higgins (Cancer Research UK, London, UK) were cultured as described [9]. Cells were grown to confluence and used when quiescent, before the addition of PMT or bombesin (Calbiochem-Novabiochem). The tyrosine kinase inhibitors Su6656 and St638 (Calbiochem-Novabiochem) were prepared in DMSO, diluted in DMEM containing 0.1% DMSO and added to cell cultures to give a final concentration of 100 µM 1 h prior to treatment with PMT.
Preparation of Swiss 3T3 membranes and cytoplasmic fractions
Swiss 3T3 cells were grown in 145 mm dishes, rinsed twice with ice cold PBS and scraped into 2 ml of PBS containing proteinase inhibitors (Complete™, Roche Diagnostics). Cells from 10 dishes were pooled, collected by centrifugation (200 g, 10 min, 4°C), and washed cell pastes were frozen at −70°C until required. The frozen cell pastes (∼5 mg) were thawed on ice and suspended in 5 ml of membrane buffer (10 mM Tris-HCl, 10 mM MgCl2, 0.1 mM EDTA, pH 7.4, containing proteinase inhibitors). The cells were ruptured by 25 passes through a 23-gauge needle, and the resulting homogenate was centrifuged at 800 g for 10 min to remove unbroken cells and nuclei. The supernatants were transferred to fresh tubes and centrifuged at 50,000 g for 10 min. The supernatant containing cytoplasmic proteins was transferred to a fresh tube, snap frozen in liquid nitrogen and stored at −70°C. The pellet was washed and suspended in 10 ml of membrane buffer. After a second centrifugation step the membrane pellet was suspended in membrane buffer to a protein concentration of 1 mg/ml and stored at −70°C.
SDS PAGE and urea gel electrophoresis
Membrane proteins were resolved by SDS PAGE on 12.8% acrylamide/0.06% bis acrylamide gels, or on these same gels containing 6M urea to separate the closely migrating Gα11 and Gαq subunits as described [18]. Proteins were transferred to PVDF membranes and immunoblotted as described below.
2-D gel electrophoresis
Swiss 3T3 membrane proteins were resolved by 2-D gel electrophoresis, as described [19]. The immunodetection of Gα subunits was performed by incubating the membrane overnight at 4°C with primary antibody at a dilution of 1∶1000, followed by incubation with horseradish peroxidase-coupled secondary antibody at a dilution of 1∶10000 (SouthernBiotech) for 1 h at room temperature. The membrane was incubated with ECL™ chemiluminescent substrate (GE HealthCare) and signals were detected using an automatic X-Ray film processor (Jungwon Precision Industries Co.).
Calcium microfluorimetry
Intracellular calcium was recorded as given previously [20]. Briefly, Swiss 3T3 cells were plated onto 19 mm glass cover slips and incubated in 5 µM Indo –AM (1 hour, 37°C, in the dark, Calbiochem). Cover slips were placed in a custom built chamber allowing gravity fed superfusion (10–12 ml/min) of a modified Krebs solution. Bombesin was applied by switching a multiway tap to a solution containing it and was removed by switching back to a bombesin free solution. The waste was removed by a peristaltic pump. Recordings were performed at room temperature by subtraction of background light and recording the emitted light from individual cells at 405 and 488 nm. The emission ratio (R) was converted to a calcium concentration after calibration (see reference 20] in which [Ca]i (nM) = 1028(R-0.86)/(12-R) and autofluorescence was less that 4%. .
Trypsin protection assay
The trypsin protection assay was adapted from Evanko et al. [21]. Briefly, membrane fractions (100 µg) were incubated with PMT, bombesin, GTPγS or GTP at the required concentrations at 37°C for times indicated. Membrane fractions were centrifuged at 18,000×g for 10 min at 4°C and the pellet was resuspended in 12.8 µl of solubilisation buffer (20 mM Tris-HCl, pH 7.5, 100 mM NaCl, 2 mM MgCl2, 0.1 mM EDTA, 1 mM dithiothreitol, 10% glycerol, 1% C12E10 (polyoxyethylene 10-lauryl ether), 0.1 mM phenylmethylsulfonyl fluoride), vortexed, incubated on ice for 20 min and centrifuged at 18,000×g for 10 min at 4°C. The supernatant was then transferred to a new microfuge tube, treated with 4 µl of trypsin mixture (100 µM GDP, 1.5 mg/ml trypsin in solubilisation buffer) for 30 min at 30°C. The trypsin activity was neutralised with 3 µl of soybean trypsin inhibitor (3 mg/ml). Trypsin-resistant fragments were resolved by SDS-PAGE, and detected by immunoblotting using antiserum against Gαq/11. The induction of trypsin protection by GTPγS and GTP alone or in the presence of bombesin or PMT were quantified relative to untrypsinised Gq using scanning densitometry (GeneTools, Syngene). Data were analysed using factorial analysis of variance (ANOVA) by Dr Ron Wilson (King's College London). Unactivated Gα subunits (GDP-bound) are highly susceptible to tryptic digestion; however tryptic cleavage is inhibited when G-proteins are activated (GTP-bound) as most cleavage sites are conformationally protected, and a product resulting from a small N-terminal cleavage can be visualised [22].
Measurement of high-affinity GTPase activity
Determination of GTPase activity was essentially as described [23]. High-affinity GTPase activity was determined by subtraction of Pi release in membranes incubated with 50 µM of GTP (low-affinity GTPase activity) from that with 0.5 µM GTP (total GTPase activity).
Results
PMT stimulates an acidic modification of Gαq and Gαi family proteins
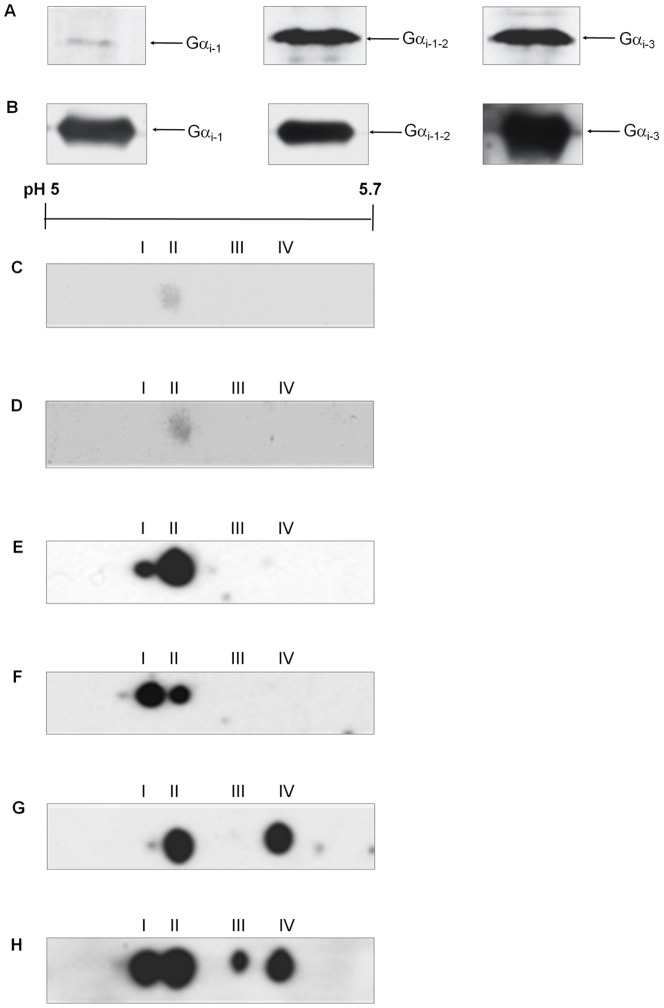
Gαq/11 antiserum detected both Gαq and Gα11 subunits at an apparent molecular mass of 42 kDa in membranes prepared from quiescent Swiss 3T3 cells. Separating these subunits on a urea gel showed that Gαq (which aberrantly runs slower in this system than G11 [24]) was more abundantly expressed than Gα11 in these cells (Fig. 1A). A similar relative abundance has been shown in rat neurons [25]. Four distinct Gαq/11 molecular isoforms, designated q-II, q-III, q-V and q-VI, were resolved by 2-D gel electrophoresis followed by immunoblotting with anti-Gq/11 antibody in membranes derived from untreated cells (Fig. 1B). These plus two additional isoforms, q-I and q-IV, were detected by 2D PAGE and Western blot analysis of membrane fractions derived from cells treated with PMT (150 pM) for 4 h (Fig. 1C; Table 1).
Figure 1. PMT induces the covalent modification of Gαq and Gα11.
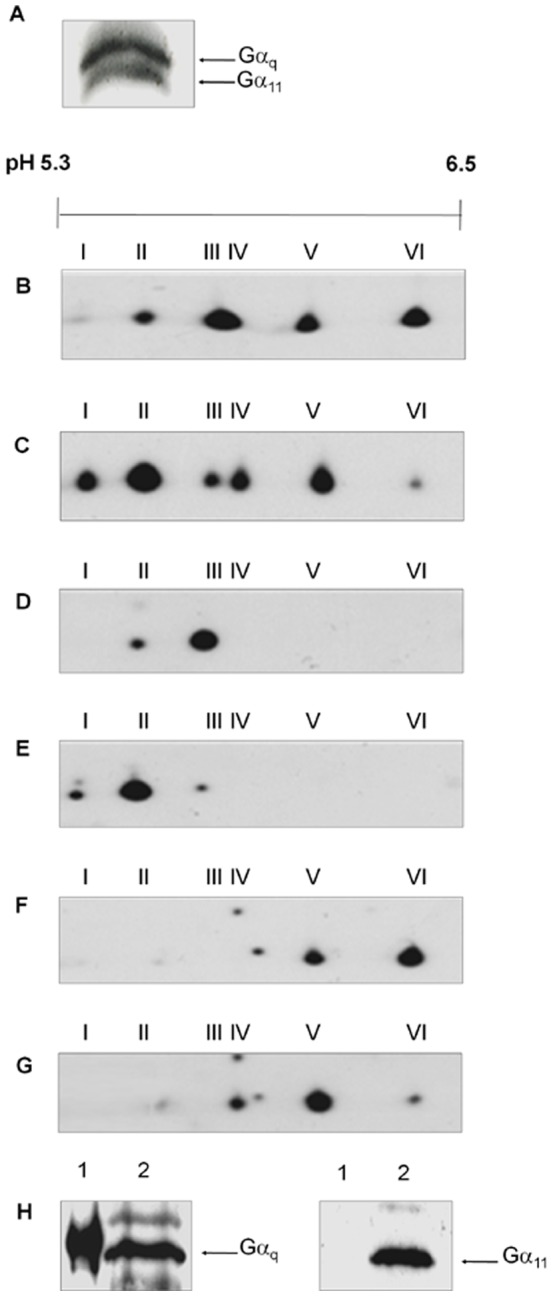
(A) Membrane proteins from Swiss 3T3 cells were separated by urea gel electrophoresis and Western blotted with anti-Gαq/11 antibody. The locations of Gαq and Gα11 are indicated by arrows. Membrane proteins from Swiss 3T3 cells (B, D, F) left untreated or (C, E, G) treated with 150 pM PMT for 4 h were separated by 2-D gel electrophoresis and Western blotted with (B, C)anti-Gαq/11, (D, E)anti-Gαq or (F, G) anti-Gα11 antibody. (H) Recombinant Gαq subunit (lane 1) and membrane proteins from Swiss 3T3 cells (lane 2) were separated by SDS PAGE and Western blotted with anti-Gαq (left panel) or -Gα11 (right panel) antibody. Samples from at least 3 independent experiments were resolved with similar results.
Table 1. Analysis of pI values of Gαq family isoforms after treatment with PMT.
| Control (pI) | PMT-treated (pI) | |||||
| Isoform | Gαq/11 | Gαq | Gα11 | Gαq/11 | Gαq | Gα11 |
| q-I | - | - | - | 5.39±0.04 | 5.42±0.02 | - |
| q-II | 5.45±0.04 | 5.49±0.02 | - | 5.49±0.01 | 5.51±0.02 | - |
| q-III | 5.61±0.05 | 5.59±0.05 | - | 5.60±0.08 | 5.60±0.03 | - |
| q-IV | - | - | - | 5.64±0.01 | - | 5.64±0.02 |
| q-V | 5.76±0.09 | - | 5.75±0.01 | 5.76±0.09 | - | 5.73±0.01 |
| q-VI | 5.89±0.1 | - | 5.85±0.01 | 5.83±0.02 | - | 5.82±0.05 |
The samples were as described in the legend to Figure 1 and the results are expressed as the mean ± standard error of the mean (n = 3).
Antiserum directed only against Gαq detected two isoforms with pI values corresponding to q-II and q-III (Fig. 1D; Table 1) in untreated cells. The Gαq antiserum detected an additional isoform with a pI value corresponding to q-I in PMT-treated cells (Fig. 1E; Table 1). Antiserum directed only against Gα11 detected two isoforms in untreated cells with pI values corresponding to the isoforms q-V and q-VI (Fig. 1F; Table 1) and one additional isoform with a pI value corresponding to q-IV in PMT-treated cells (Fig. 1G; Table 1).
We excluded the possibility that the Gα11 antibody could react with Gαq by testing the ability of the Gαq and Gα11 antibodies to react with a recombinant Gαq subunit. The Gαq but not the Gα11 antiserum could detect the Gαq subunit (Fig. 1H). The experimentally determined pI values for the Gαq/11 isoforms (Table 1) are similar to the predicted pI values of 5.48 and 5.70 for murine Gαq and Gα11, respectively [26], [27].
The expression of the Gαi-1, Gαi-2 and Gαi-3 subclasses, which have the widest tissue expression pattern of this family [28], was analysed in Swiss 3T3 cells using specific antisera. The Gαi-1-2 (directed against Gαi-1 and Gαi-2) and Gαi-1-3 antisera (directed against Gαi-1, Gαi-2 and Gαi-3) each detected an abundant protein band at 40 kDa in membranes from Swiss 3T3 cells. The antiserum specific for only Gαi-1 detected a weak band (Fig. 2A), although this antiserum could be shown to react strongly with a recombinant Gαi-1 subunit (Fig. 2B), demonstrating a low abundance of Gαi-1 in Swiss 3T3 cells.
Figure 2. PMT induces the covalent modification of Gαi.
(A) Membrane proteins from Swiss 3T3 cells were separated by SDS PAGE and Western blotted with anti-Gαi-1, anti-Gαi-1-2 or anti-Gαi-1-3 antibody, as indicated. (B) A recombinant Gαi-1 subunit was analysed by SDS PAGE and Western blotted with anti-Gαi-1, -Gαi-1-2 or -Gαi-1-3 antibody, as indicated. Membrane proteins from Swiss 3T3 cells (C, E, G) left untreated or (D, F, H) treated with 150 pM PMT for 4 h were separated by 2-D gel electrophoresis and Western blotted with (C, D) anti-Gαi-1, (E, F)anti-Gαi-1-2 or(G, H) anti-Gαi-1-3 antibody. Samples from at least 3 independent experiments were resolved with similar results.
Gαi-1 isoforms were present at low abundance in membranes prepared from either untreated or PMT-treated Swiss 3T3 cells as determined by 2-D gel electrophoresis followed by immunoblotting (Fig. 2C, D; Table 2). The Gαi-1-2 antiserum detected two Gαi isoforms in untreated and PMT-treated cells, designated i-I and i-II (Fig. 2E, F; Table 2), with a reproducible change in the relative abundance of the isoforms after PMT treatment. The Gαi-1-3 antiserum detected 3 Gαi isoforms in untreated cells, two of which appeared to correspond to i-I and i-II; the third isoform was designated i-IV (Fig. 2G; Table 2). The Gαi-1-3 antiserum also detected these and one additional isoform, i-III in PMT-treated cells (Fig. 2H; Table 2).
Table 2. Analysis of pI values of Gαi family isoforms after treatment with PMT.
| Control (pI) | PMT-treated (pI) | |||||
| Isoform | Gαi-1 | Gαi-1,2 | Gαi-1,2,3 | Gαi-1 | Gαi-1,2 | Gαi-1,2,3 |
| i-I | - | 5.11±0.01 | 5.09±0.03 | - | 5.10±0.01 | 5.07±0.02 |
| i-II | - | 5.22±0.03 | 5.18±0.03 | - | 5.17±0.01 | 5.17±0.02 |
| i-III | - | - | - | - | - | 5.34±0.02 |
| i-IV | - | - | 5.59±0.03 | - | - | 5.45±0.01 |
The samples were as described in the legend to Figure 2 and the results are expressed as the mean ± standard error of the mean (n = 3).
The predicted pI values of murine Gαi-1, Gαi-2 and Gαi-3 are 5.69, 5.28 and 5.50, respectively [29]. It seems probable that isoforms i-I and i-II detected by the Gαi-1-2 antiserum belong to the Gαi-2 subclass, as isoforms of the Gαi-1 subclass are expected to have a more basic pI, and Gαi-1 was not detected in Swiss 3T3 cells. Isoforms i-III and i-IV are therefore likely to belong to the Gαi-3 subclass. Orth et al. resolved Gαi-1 and Gαi-2 from mouse embryonic fibroblast cells by 2-D gel electrophoresis at an unspecified pI value and showed that PMT treatment of these cells caused an acidic pI shift consistent with deamidated recombinant Gαi-2 [14]. Our results suggest that PMT catalyses the acidic covalent modification of Gαi-2 and Gαi-3.
PMT induces an alkaline modification of Gα13
The two members of the Gα12 family, Gα12 and Gα13, are ubiquitously expressed [30]. Gα13 was detected in Swiss 3T3 membranes using antiserum against Gα13 (Fig. 3A). Three Gα13 isoforms, 13-I, 13-III and 13-IV, were identified in membranes from Swiss 3T3 cells (Fig. 3B). Two additional isoforms, 13-II and 13-V, were detected in membranes derived from PMT-treated cells (Fig. 3C; Table 3). The additional Gα13 isoforms seem to be the result of an alkaline pH shift, in contrast to the effect of PMT on Gαq/11 and Gαi isoforms. Under our experimental conditions, Gα12 could not be resolved by 2-D gel electrophoresis.
Figure 3. PMT induces the covalent modification of Gα13.
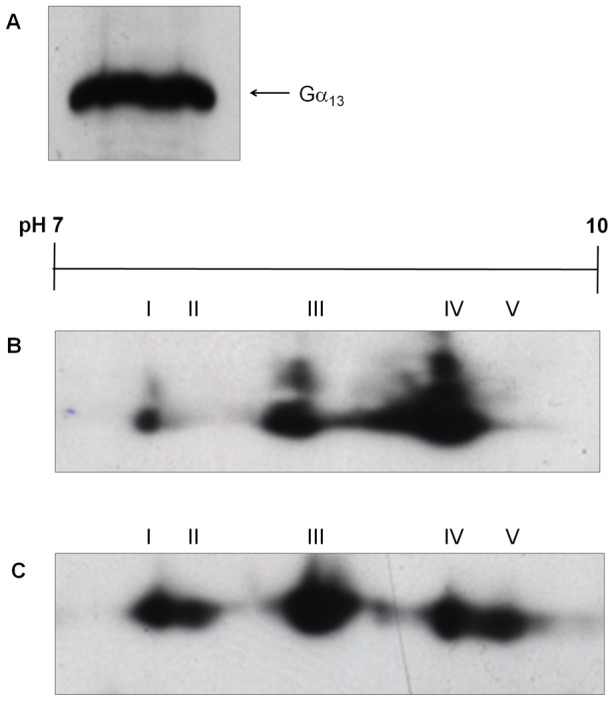
(A) Membrane proteins from Swiss 3T3 cells were separated by SDS PAGE and Western blotted with anti-Gα13 antibody. The location of Gα13 is indicated. Membrane proteins from Swiss 3T3 cells left (B) untreated or (C) treated with 150 pM PMT for 4 h were separated by 2-D gel electrophoresis and Western blotted with anti-Gα13 antibody. Samples from at least 3 independent experiments were resolved with similar results.
Table 3. Analysis of pI values of Gα13 isoforms after treatment with PMT.
| Control (pI) | PMT-treated (pI) | |
| Isoform | Gα13 | Gα13 |
| 13-I | 8.15±0.02 | 8.15±0.07 |
| 13-II | - | 8.24±0 |
| 13-III | 8.54±0.02 | 8.54±0.02 |
| 13-IV | 8.90±0.05 | 8.90±0.01 |
| 13-V | - | 9.04±0.01 |
The samples were as described in the legend to Figure 4 and the results are expressed as the mean ± standard error of the mean (n = 3).
PMT does not induce any modification of Gαs
The alpha subunits of the ubiquitously expressed Gs family can be expressed as four distinct forms as a result of alternative mRNA splicing [31]. Swiss 3T3 cells were shown to express both large (55 kDa) and small (52 kDa) forms of Gαs, with Gαs-large being more abundantly expressed than Gαs-small (Fig. S1A). Six isoforms of Gαs-large (s-I to s-VI) and two isoforms of Gαs-small (s-VII and s-VIII) were resolved in membranes derived from Swiss 3T3 cells by 2-D gel electrophoresis, followed by immunoblotting with the Gαs/olf antiserum (Fig. S1B; Table S1). The Gαs-large isoforms were detected at a more acidic pI than the Gαs-small isoforms, which concurs with previous findings [19]. PMT showed no discernable effect on the pI or molecular mass of the Gαs subunits (Fig. S1C; Table S1).
PMT stimulates the stable covalent modification of G-proteins
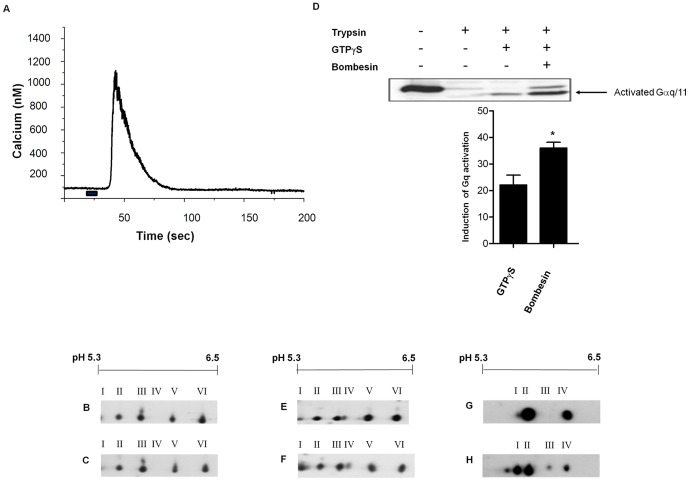
It was important to establish whether the additional isoforms detected in PMT-treated cells arose as a consequence of normal activation induced by PMT or if they were directly PMT-modified. Cells were challenged with the neuropeptide bombesin, which acts through a Gq-coupled receptor to stimulate phospholipase C (PLC) activation culminating in the release of Ca2+ from intracellular stores [32]. Bombesin at a concentration of 30 nM effectively stimulated Ca2+ release from cells (Fig. 4A), but no additional Gαq/11 isoforms were detected by 2D PAGE and Western blot analysis of a membrane fraction derived from cells exposed to bombesin (Fig. 4B, C). This suggested that Gαq-coupled receptor activation did not stimulate the stable covalent modification of Gαq.
Figure 4. Sodium vanadate treatment mimics PMT effects on Gαq and Gαi.
(A) Indo-1 AM labelled Swiss 3T3 cells were treated with 30 nM bombesin for 10 s (marked with solid bar beneath trace) and intracellular Ca2+ release was measured. Membrane proteins from Swiss 3T3 cells that were either (B) untreated or (C) treated with 30 nM bombesin for 1 min were separated by 2-D gel electrophoresis and Western blotted with anti-Gαq/11 antibody. (D) Swiss 3T3 membrane proteins were incubated in the presence or absence of 30 nM bombesin for 20 min with or without 0.05 nM GTPγS. The proteins were then analysed for trypsin protection as described under Materials and Methods, and activated Gαq/11 was separated by SDS PAGE and Western blotted with anti-Gαq/11 antibody. Quantification of activated Gαq/11 (lower panel) was determined by densitometric scanning and these data were analysed using factorial analysis of variance (ANOVA). The induction of activation shown is relative to the density of the band without GTPγS or bombesin. Bombesin significantly enhanced GTPγS binding to Gαq/11 (* p = 0.002). Membrane proteins from Swiss 3T3 cells were incubated with (E) 1 mM sodium vanadate for 20 min at 37°C or (F) 1 mM sodium vanadate and 30 nM bombesin for 20 min at 37°C, proteins were separated by 2-D gel electrophoresis and Western blotted with anti-Gαq/11 antibody. Membrane proteins from Swiss 3T3 cells were incubated (G) without or (H) with 1 mM sodium vanadate for 20 min at 37°C, proteins were separated by 2-D gel electrophoresis and Western blotted with anti-Gαi-1-3 antibody. Samples from at least 3 independent membrane preparations were resolved with similar results.
We have previously demonstrated that PMT induced the phosphorylation of Gαq on Tyr369 [9]. We stimulated membrane fractions with bombesin in the presence of sodium vanadate, a potent tyrosine phosphatase inhibitor, in order to prevent the dephosphorylation of Gαq/11. Bombesin activation of Gαq/11 in the membrane fractions was confirmed by the trypsin protection assay. Bombesin significantly enhanced GTPγS binding to Gαq/11 (p = 0.002), by up to 50% in some cases, the most likely explanation being that its action accelerated the rate of nucleotide exchange (Fig. 4D). The additional isoforms detected in membranes stimulated with bombesin appeared to be identical to those found in PMT-treated cells. However, the additional Gαq/11 and Gαi isoforms were also found in membranes derived from unstimulated cells, that had been treated with sodium vanadate alone (Fig. 4E, F and H). These findings suggest that PMT modification of Gαq/11 and Gαi produces a similar pI shift as the tyrosine phosphorylation of these Gα subunits.
The appearance of the additional isoforms observed in PMT-treated cells could not be blocked by the competitive kinase inhibitors Su6656 or St638, although these inhibitors were effective at blocking pervanadate-induced phosphorylation of focal adhesion kinase (FAK) (Fig. S2). We have previously shown that a mutant PMT (PMTC1165S) can stimulate the tyrosine phosphorylation of Gq, although it does not activate Gq downstream signalling [9]. Treatment of Swiss 3T3 cells with PMTC1165S did not result in the covalent modification of Gαq or Gαi (Fig. S3). Moreover, tyrosine phosphorylation is a transient reversible modification that cannot be readily detected unless tyrosine phosphatases are inhibited.. The PMT-induced modification of Gα subunits was detected in the absence of sodium vanadate, indicating that the PMT-induced modification was covalent and stable.
Prolonged treatment of cells with PMT has differential effects on G-proteins
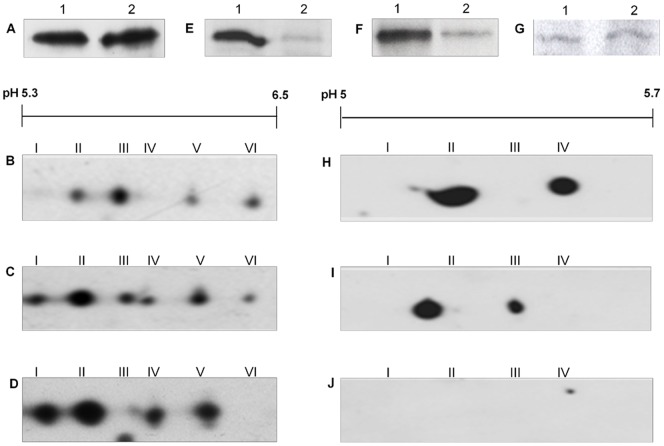
PMT treatment decreased the abundance of some of the pre-existing Gαq/11 and Gαi isoforms in membrane fractions. To explore if PMT caused G-protein removal from membranes, Swiss 3T3 cells were treated with PMT at a concentration of 1 nM for 16 h. This treatment did not cause loss of Gαq/11 from the membrane (Fig. 5A), but resulted in the complete loss of the most basic isoforms of Gαq and Gα11, q-III and q-VI, respectively (Fig. 5B, C, and D), while isoforms q-II and q-IV did not undergo an evident change in abundance. We speculate that the loss of detection of Gαq/11 isoforms q-III and q-VI is a result of the covalent modification of these isoforms to q-I and q-IV, respectively, induced by PMT.
Figure 5. Prolonged exposure of Swiss 3T3 cells to PMT causes the loss of Gαi but not Gq from cell membranes.
(A) Membrane proteins from Swiss 3T3 cells left untreated (lane 1) or treated with PMT at 1 nM for 16 h (lane 2) were separated by SDS PAGE and Western blotted with anti-Gαq/11 antibody. Membrane proteins from Swiss 3T3 cells (B) left untreated, or treated with 1 nM PMT for (C) 4 h or (D) 16 h were separated by 2-D gel electrophoresis and Western blotted with anti-Gαq/11 antibody. Samples from at least 3 independent experiments were resolved with similar results. Membrane proteins from Swiss 3T3 cells left untreated (lane 1) or treated with 1 nM PMT for 16 h (lane 2) were separated by SDS PAGE and Western blotted with (E) anti-Gαi-1-3 antibody or (F) an antibody recognising an internal epitope of Gαi-2. (G) Cytoplasmic proteins from Swiss 3T3 cells left untreated (lane 1) or treated with 1 nM PMT for 16 h (lane 2) were separated by SDS PAGE and Western blotted with anti-Gαi-1-3 antibody. Membrane proteins from Swiss 3T3 cells (H) left untreated, treated with 1 nM PMT for (I) 4 h or (J) 16 h were separated by 2-D PAGE and Western blotted with anti-Gαi-1-3 antibody. Samples from 3 independent experiments were resolved with similar results.
In contrast, prolonged treatment of Swiss 3T3 cells with PMT generally resulted in the almost complete loss of Gαi from membranes (Fig. 5E, F). It is unlikely that the failure to detect the Gαi isoforms reflects a modification that interferes with the Gαi-1-3 antigen recognition site, which is at the C-terminus of Gαi, as the loss of Gαi from membranes could also be demonstrated with an antiserum against an internal epitope of Gαi-2 (Fig. 5F). Cytoplasmic extracts of cells that had received prolonged treatment with PMT were probed with anti-Gαi-1-3 antibody, but no increase in Gαi subunits could be detected in these fractions (Fig. 5G). It appears that the sequential loss of Gαi from membranes proceeds by covalent modification of Gαi isoforms i-II and i-IV to produce isoforms i-I and i-III, respectively, followed over time by the loss of isoforms i-I and i-III from the membranes (Fig. 5H–J). In some cases only partial loss of Gαi isoforms was observed over this time period (data not shown).
PMT inhibits the GTPase activity of Gq
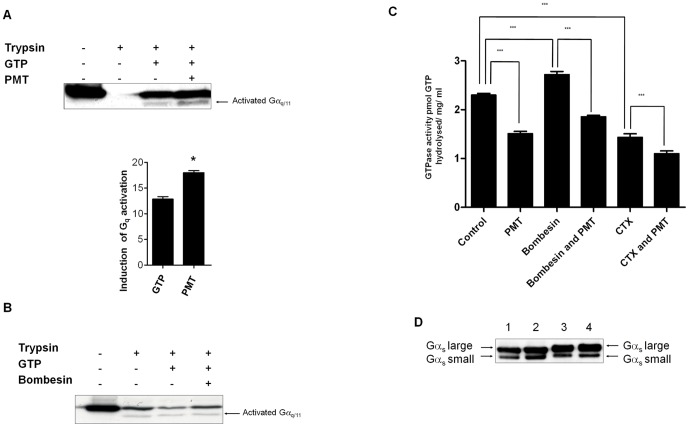
PMT did not significantly enhance GTPγS binding to Gαq/11 in contrast to bombesin (data not shown). Due to its enzymatic nature, PMT required a longer incubation time to promote GTP binding to Gαq compared to bombesin [9]. Therefore, it is likely that during the course of the incubation, Gαq was gradually saturated by GTPγS, thereby preventing the detection of PMT-enhanced GTPγS binding to Gαq above background levels. When GTP was used instead of GTPγS, PMT significantly enhanced GTP binding to Gαq as measured by trypsin protection (p = 0.03), by up to 30% (Fig. 6A), in contrast to bombesin (Fig. 6B). This finding suggested that PMT might inhibit the GTPase activity of Gαq, to prevent the hydrolysis of GTP to GDP.
Figure 6. PMT inhibits the GTPase activity of Gq.
(A) Membrane proteins were incubated in the presence or absence of 150 pM PMT for 1 h with 0.5 nM GTP and tested in a trypsin protection assay as described in Materials and Methods. Proteins were separated by SDS PAGE and Western blotted with anti-Gαq/11 antibody. Quantification of activated Gαq/11 (lower panel) was determined by densitometric scanning and the data were analysed using factorial analysis of variance (ANOVA). The induction of activation shown is relative to the density of the band without GTP or PMT. PMT significantly enhanced GTP binding to Gq (* p = 0.03). (B) Membrane proteins were incubated in the presence or absence of 30 nM bombesin 20 min with 0.5 nM GTP and tested in a trypsin protection assay as described in Materials and Methods. Proteins were separated by SDS PAGE and Western blotted with anti-Gαq/11 antibody. Samples from at least 3 independent membrane preparations were resolved with similar results. (C) Membranes derived from Swiss 3T3 cells that had either been treated or untreated with 150 pM PMT for 4 h or 100 ng cholera toxin, or both, were treated with or without 30 nM bombesin for 20 min in the presence of [γ-32P] GTP. All the experimental conditions were repeated three times, and all data are presented as mean ± standard deviation (SEM). The results for the groups were compared using single-factor analysis of variance (one-way ANOVA), followed by Newnan-Keuls test used to determine differences between groups. Significant changes are indicated by an asterisk (* P<0.05, *** P<0.001). (D) Membranes derived from Swiss 3T3 cells that had either been untreated (lane 1) or treated with 150 pM PMT for 4 h (lane 2), or 100 ng cholera toxin for 16 h (lane 3), or both PMT and cholera toxin (lane 4) were resolved by SDS PAGE followed by Western blotting with an anti-Gαs antibody. Samples from at least 3 independent membrane preparations were resolved with similar results.
Bombesin stimulated the steady-state GTPase activity in Swiss 3T3 membrane preparations by up to 30%, whereas pre-treatment of cells with PMT at 150 pM for 4 h reduced the basal and bombesin-stimulated GTPase activity in membrane preparations (Fig. 6C). To further decrease the basal steady-state GTPase level, cells were pre-treated with cholera toxin, which ADP-ribosylates Gαs to inhibit its GTPase activity. Cholera toxin caused an increase in the molecular weight of both the long and short forms of Gαs, due to the addition of ADP ribose (Fig. 6D). Pre-treatment of cells with both cholera toxin and PMT further decreased the basal GTPase activity in membrane preparations, compared to cells pre-treated with PMT alone. Bombesin stimulated the steady state GTPase activity by up to 50% in cells pre-treated with cholera toxin, whereas the additional pre-treatment of cells with PMT reduced the bombesin-stimulated GTPase activity in membrane preparations, indicating that PMT inhibits the GTPase activity of Gαq but not Gs (Fig. 6C).
Discussion
PMT executes its cellular effects through the activation of the heterotrimeric G-proteins, Gq, G12 and Gi [8]–[13]. This has been shown to occur in recombinant Gi by PMT-induced deamidation of Gln-205 to glutamic acid, which inhibits its intrinsic GTPase activity [14]. The work we report here complements these studies by investigating covalent modifications of G-proteins in Swiss 3T3 cells treated with PMT. PMT treatment consistently led to the appearance of new isoforms at a lower pI for both Gαq and Gα11. PMT also stimulated the covalent modification of members of the Gi family. The Gα12 family proteins, unlike the other G-protein families, have predicted pI values within the alkaline pH range (>pH 8) and such proteins are difficult to resolve by 2-D gel electrophoresis [33]. Gα13, but not Gα12, subunits displayed a reproducible pattern and PMT treatment led to new Gα13 isoforms at slightly higher pI values. We found no evidence that PMT stimulates the covalent modification of Gαs, although the glutamine residue targeted by PMT is conserved in all G-proteins.
Stimulation of Gq-coupled receptors by bombesin only resulted in the detection of the additional Gαq/11 isoforms observed in PMT-treated cells when vanadate was present. The addition of sodium vanadate per se led to a similar pattern of isoforms to those observed in PMT-treated cells. However it is likely that these different treatments lead to different modifications. The modification of Gαq/11 and Gαi stimulated by PMT was detected without sodium vanadate, and is thus indicative of a stable covalent modification such as deamidation, whereas tyrosine phosphorylation is a transient covalent modification. We previously showed that a src family kinase mediates the phosphorylation of Gq in response to PMT [9]. However, pre-treatment of cells with a specific src kinase family inhibitor, SU6656, or a broad spectrum kinase inhibitor, St638, did not prevent PMT from stimulating the covalent modification of Gαq and Gαi, despite each kinase inhibitor being effective at blocking FAK phosphorylation. It is possible that the kinase inhibitors failed to completely block PMT-stimulated phosphorylation of G-proteins, due to their competitive nature and the enzymatic nature of PMT. However, this would suggest that deamidation by PMT results in the stable phosphorylation of these Gα subunits that is not reversed by the action of phosphatases, which is unlikely.
Deamidation and tyrosine phosphorylation of a Gα subunit would have a similar effect on the isoelectric point. The PMT-induced deamidation of in-vitro translated Gαq and recombinant Gαi-2 was reported to cause an acidic pI shift of 0.05 and 0.07, respectively [14]. This compares with the acidic pI shift of approximately ∼0.15 for both Gαq and Gαi that we have observed. There are various possible interpretations of this apparent discrepancy. First, pI shifts are known to be variable and depend on the overall pI of a protein and its local context [34], and thus Gαi expressed in E. coli may behave differently because of the absence of post-translational modifications. Alternatively, the PMT-induced modification in cells may differ from that observed following expression in E. coli.
PMT is reported not to activate G11, as PMT could not induce the activation of PLC in Gq-deficient cells [12], and further analysis using Gαq/Gα11 chimeras also confirmed that PMT did not lead to G11-linked stimulation of PLC [35].We were therefore surprised that PMT stimulated the covalent modification of Gα11. Gαq and Gα11 each contain Gln-209 that is functionally equivalent to Gln-205 in Gαi-2 and it would be unlikely that Gα11 could be deamidated and yet not activated by PMT, as the loss of the functional Gln would affect the GTPase activity of the G-protein. While this manuscript was in preparation, Kamitani et al. published evidence that an antibody against deamidated Gα subunits recognised Gα11 in PMT-treated mouse embryonic fibroblasts that were deficient in Gαq/11 but transfected to express Gα11 [36]. This result provides further evidence that G11 is also a substrate for PMT. In their experiments there was a small stimulation of PLC in cells expressing Gα11. All the other papers addressing this issue have used the same source of Gq/11-deficient MEF cells, whereas our work uses Swiss 3T3 cells. Further investigation of these puzzling and partially contradictory results is required.
PMT treatment of cells led to new Gα13 isoforms at slightly higher (0.09–0.15) pI values. The PMT catalytic triad has high structural similarity to eukaryotic transglutaminases [14], and it is possible that PMT can also function as a transglutaminase, in a similar manner to the cytotoxic necrotizing factor (CNF) which was originally considered to be a deamidase, but was later found to cause transglutamination in cells [37]. Transglutaminases catalyse the acyl transfer between the γ-carboxyamide of a peptide bound glutamine (acyl donor) to a primary amine (acyl acceptor). When water functions as an acyl acceptor the result is glutamine deamidation [38]. The choice between deamidation and transglutamination is influenced by the environment of the targeted glutamine residue [39], [40]. As transglutamination would impart a positive charge to produce an alkaline shift, it is possible that PMT preferentially transglutaminates Gα13 in cells.
The removal of G-proteins from the membrane is a regulatory phenomenon that can follow prolonged G-protein activation [41]. The ADP-ribosylation of Gs by cholera toxin leads to its downregulation, although ADP-ribosylation of Gαi by pertussis toxin does not result in its degradation [42]. We observed that prolonged treatment of cells with PMT caused the loss of Gαi, but not Gαq, from membranes prepared from Swiss 3T3 cells. Furthermore Gαi could not be detected in the cytoplasm following prolonged PMT treatment. Orth et al. had suggested that overnight treatment of Swiss 3T3 cells with 1 nM PMT uncoupled Gαi from its receptor, as the Gi-linked agonist lysophosphatidic acid could not stimulate GTPγS binding to Gαi in membranes derived from these cells [10]. The loss of Gi from the membrane that we observed over this time period would provide a more likely explanation for their observation. Furthermore, the site of the PMT-induced modification, Gln-205, is not thought to be linked to receptor interaction. A similar differential degradation has been observed with Rho proteins following modification by CNF [43].
We found that PMT could promote the binding of GTP to Gαq/11, whereas bombesin could not, which suggested that the action of PMT inhibits the GTPase activity of Gαq/11. PMT significantly inhibited the bombesin-mediated stimulation of steady-state GTPase activity in Swiss 3T3 membrane preparations. These results complement the demonstration that PMT inhibits the GTPase activity of E. coli-expressed Gαi [10], [14]. Furthermore, pre-treatment of cells with cholera toxin and PMT resulted in a greater inhibition of GTPase activity, supporting the view that PMT does not affect Gs.
In conclusion, our results demonstrate that treatment of Swiss 3T3 cells with PMT induces the irreversible modification of G-proteins belonging to the Gi and Gq families resulting in an acidic pI shift, which is consistent with the observation that PMT catalyses deamidation of recombinantly expressed Gi causing a similar shift in pI. We found that PMT inhibits the intrinsic GTPase activity of Gq, which complements the finding that PMT-stimulated deamidation of Gαi-2 inhibits its GTPase activity. We showed that stimulation of cells with PMT results in the degradation of Gi which provides an explanation for the observation that PMT-treatment blocks Gi activation by a receptor agonist. The unexpected modification of Gα11 requires further investigation. We demonstrated that PMT treatment causes an alkaline pI shift in Gα13 and speculate that PMT might preferentially transglutaminate Gα13. Working with cells enables the PMT/G-protein interaction to be investigated in a more natural context than when working with recombinantly expressed proteins. However, the further interpretation of results is impeded by the near impossibility of purifying these low abundance proteins in a modified form from cell lines, and thus both in-vitro and in-vivo studies are required to unravel the complexity of the toxin/G-protein interactions.
Supporting Information
PMT does not induce the covalent modification of Gαs. (A) Membrane proteins from Swiss 3T3 cells were separated by SDS PAGE and Western blotted with anti-Gαs antibody. Membrane proteins from Swiss 3T3 cells left (B) untreated or (C) treated with 150 pM PMT for 4 h were separated by 2-D gel electrophoresis and Western blotted with anti-Gαs antibody. Samples from at least 3 independent experiments were resolved with similar results.
(TIF)
Kinase inhibitors do not block PMT induced modification of Gαq/11 or Gαi. Swiss 3T3 cells were either not treated (Lane 1) or pre-treated (Lane 2) for 1 h with (A) SU6656 or (B) St638, then stimulated with 0.5 nM pervanadate for 5 min. The cells were lysed in SDS-buffer and proteins were resolved by SDS PAGE followed by Western blotting with an anti-phospho-FAK antibody. Three independent experiments gave similar results. Swiss 3T3 cells were (C, D, G, H) not treated or pre-treated with either (E, I) SU6656 or (F, J) St638 and then either treated with (D, E, F, H, I, J) 150 pM PMT or (C, G) not treated with PMT. Samples were resolved from 3 independent experiments with similar results. Membrane proteins were separated by 2-D gel electrophoresis and Western blotted with (C–F) anti-Gαq/11 antibody or (G–J) anti-Gαi-1-3 antibody. Samples were resolved from 2 independent experiments with similar results.
(TIF)
Mutant PMT does not induces the covalent modifications of Gαq or Gαi. Membrane proteins from Swiss 3T3 cells (A, C) left untreated or (B, D) treated with 150 pM PMTC1165S for 4 h, separated by 2-D gel electrophoresis and Western blotted with either (A, B) anti-Gαq/11 or (C, D) anti-Gαi-1-3 antibodies. Samples from 3 independent experiments were resolved with similar results.
(TIF)
Analysis of pI values of Gs family isoforms after treatment with PMT. The samples were as described in the legend to Figure. S1 and the results are expressed as the mean ± standard error of the mean.
(DOC)
Acknowledgments
We thank Susmitha Rao (King's College London Dental Institute) for technical support with 2-D gel electrophoresis, and Dr Ron Wilson (King's College London Dental Institute) for help with statistical analysis.
Funding Statement
This work was supported by a studentship grant to RCB from the Biotechnology and Biological Sciences Research Council, UK. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Sprang SR (1997) G protein mechanisms: insights from structural analysis. Annu Rev Biochem 66: 639–678. [DOI] [PubMed] [Google Scholar]
- 2. Oldham WM, Hamm HE (2008) Heterotrimeric G protein activation by G-protein-coupled receptors. Nat Rev Mol Cell Biol 9: 60–71. [DOI] [PubMed] [Google Scholar]
- 3. Chen CA, Manning DR (2001) Regulation of G proteins by covalent modification. Oncogene 20: 1643–1652. [DOI] [PubMed] [Google Scholar]
- 4. Sunahara RK, Dessauer CW, Gilman AG (1996) Complexity and diversity of mammalian adenylyl cyclases. Annu Rev Pharmacol Toxicol 36: 461–480. [DOI] [PubMed] [Google Scholar]
- 5. Hubbard KB, Hepler JR (2006) Cell signalling diversity of the Gqα family of heterotrimeric G proteins. Cell Signal 18: 135–150. [DOI] [PubMed] [Google Scholar]
- 6. Suzuki N, Hajicek N, Kozasa T (2009) Regulation and physiological functions of G12/13-mediated signaling pathways. Neurosignals 17: 55–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rozengurt E, Higgins T, Chanter N, Lax AJ, Staddon JM (1990) Pasteurella multocida toxin: potent mitogen for cultured fibroblasts. Proc Natl Acad Sci U S A 87: 123–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Murphy AC, Rozengurt E (1992) Pasteurella multocida toxin selectively facilitates phosphatidylinositol 4,5-bisphosphate hydrolysis by bombesin, vasopressin, and endothelin. Requirement for a functional G protein. J Biol Chem 267: 25296–25303. [PubMed] [Google Scholar]
- 9. Baldwin MR, Pullinger GD, Lax AJ (2003) Pasteurella multocida toxin facilitates inositol phosphate formation by bombesin through tyrosine phosphorylation of Gαq . J Biol Chem 278: 32719–32725. [DOI] [PubMed] [Google Scholar]
- 10. Orth JHC, Fester I, Preuss I, Agnoletto L, Wilson BA, et al. (2008) Activation of Gαi and subsequent uncoupling of receptor-Gαi signaling by Pasteurella multocida toxin. J Biol Chem 283: 23288–23294. [DOI] [PubMed] [Google Scholar]
- 11. Orth JHC, Lang S, Taniguchi M, Aktories K (2005) Pasteurella multocida toxin-induced activation of RhoA is mediated via two families of Gα proteins, Gαq and Gα12/13 . J Biol Chem 280: 36701–36707. [DOI] [PubMed] [Google Scholar]
- 12. Zywietz A, Gohla A, Schmelz M, Schultz G, Offermanns S (2001) Pleiotropic effects of Pasteurella multocida toxin are mediated by Gq-dependent and -independent mechanisms - involvement of Gq but not G11 . J Biol Chem 276: 3840–3845. [DOI] [PubMed] [Google Scholar]
- 13. Mullan PB, Lax AJ (1996) Pasteurella multocida toxin is a mitogen for bone cells in primary culture. Infect Immun 64: 959–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Orth JHC, Preuss I, Fester I, Schlosser A, Wilson BA, et al. (2009) Pasteurella multocida toxin activation of heterotrimeric G proteins by deamidation. Proc Natl Acad Sci U S A 106: 7179–7184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ward PN, Miles AJ, Sumner IG, Thomas LH, Lax AJ (1998) Activity of the mitogenic Pasteurella multocida toxin requires an essential C-terminal residue. Infect Immun 66: 5636–5642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kimple RJ, De Vries L, Tronchère H, Behe CI, Morris RA, et al. (2001) RGS12 and RGS14 GoLoco motifs are Gαi interaction sites with guanine nucleotide dissociation inhibitor activity. J Biol Chem 276: 29275–29281. [DOI] [PubMed] [Google Scholar]
- 17. Todaro GJ, Green H (1963) Quantitative studies of the growth of mouse embryo cells in culture and their development into established lines. J Cell Biol 17: 299–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Svoboda P, Milligan G (1994) Agonist-induced transfer of the α subunits of the guanine-nucleotide-binding regulatory proteins Gq and G11 and of muscarinic m1 acetylcholine receptors from plasma membranes to a light-vesicular membrane fraction. Eur J Biochem 224: 455–462. [DOI] [PubMed] [Google Scholar]
- 19. Matoušek P, Novotný J, Svoboda P (2004) Resolution of Gsα and Gqα/G11α proteins in membrane domains by two-dimensional electrophoresis: the effect of long-term agonist stimulation. Physiol Res 53: 295–303. [PubMed] [Google Scholar]
- 20. Hayat S, Wigley CB, Robbins J (2003) Intracellular calcium handling in rat olfactory ensheathing cells and its role in axonal regeneration. Mol Cell Neurosci 22: 259–270. [DOI] [PubMed] [Google Scholar]
- 21. Evanko DS, Thiyagarajan MM, Wedegaertner PB (2000) Interaction with Gβγ is required for membrane targeting and palmitoylation of Gαs, and Gαq . J Biol Chem 275: 1327–1336. [DOI] [PubMed] [Google Scholar]
- 22. Fung BK-K, Nash CR (1983) Characterization of transducin from bovine retinal rod outer segments. II. Evidence for distinct binding sites and conformational changes revealed by limited proteolysis with trypsin. J Biol Chem 258: 10503–10510. [PubMed] [Google Scholar]
- 23. Cassel D, Selinger Z (1976) Catecholamine-stimulated GTPase activity in turkey erythrocyte membranes. Biochim Biophys Acta 452: 538–551. [DOI] [PubMed] [Google Scholar]
- 24. Mullaney I, Mitchell FM, McCallum JF, Buckley NJ, Milligan G (1993) The human muscarinic M1 acetylcholine receptor, when expressed in CHO cells, activates and downregulates both Gqα and G11α equally and non-selectively. FEBS Letts 324: 241–245. [DOI] [PubMed] [Google Scholar]
- 25. Caulfield MP, Jones S, Vallis Y, Buckley NJ, Kim G-D, et al. (1994) Muscarinic M-current inhibition via Gαq/11 and α-adrenoceptor inhibition of Ca2+ current via Gαo in rat sympathetic neurones. J Physiol (Lond) 477: 415–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wettschureck N (2009) G protein alpha q. UCSD-Nature Molecule Pages (doi:10.1038/mp.a000978.01). [Google Scholar]
- 27. Kurrasch DM, Huang J, Wilkie TM (2004) G protein alpha 11. UCSD-Nature Molecule Pages (doi:10.1038/mp.a000970.01). [Google Scholar]
- 28. Jones DT, Reed RR (1987) Molecular cloning of five GTP-binding protein cDNA species from rat olfactory neuroepithelium. J Biol Chem 262: 14241–14249. [PubMed] [Google Scholar]
- 29. Bajpayee NS, Jiang M (2010) G protein alpha i1. UCSD-Nature Molecule Pages (doi:10.1038/mp.a000974.01). [Google Scholar]
- 30. Strathmann MP, Simon MI (1991) Gα12 and Gα13 subunits define a fourth class of G protein α subunits. Proc Natl Acad Sci U S A 88: 5582–5586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kozasa T, Itoh H, Tsukamoto T, Kaziro Y (1988) Isolation and characterization of the human Gsα gene. Proc Natl Acad Sci U S A 85: 2081–2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Takuwa N, Takuwa Y, Bollag WE, Rasmussen H (1987) The effects of bombesin on polyphosphoinositide and calcium metabolism in Swiss 3T3 cells. J Biol Chem 262: 182–188. [PubMed] [Google Scholar]
- 33. Görg A, Obermaier C, Boguth G, Harder A, Scheibe B, et al. (2000) The current state of two-dimensional electrophoresis with immobilized pH gradients. Electrophoresis 21: 1037–1053. [DOI] [PubMed] [Google Scholar]
- 34. Zhu K, Zhao J, Lubman DM, Miller FR, Barder TJ (2005) Protein pI shifts due to posttranslational modifications in the separation and characterization of proteins. Anal Chem 77: 2745–2755. [DOI] [PubMed] [Google Scholar]
- 35. Orth JHC, Lang S, Aktories K (2004) Action of Pasteurella multocida toxin depends on the helical domain of Gαq . J Biol Chem 279: 34150–34155. [DOI] [PubMed] [Google Scholar]
- 36. Kamitani S, Ao S, Toshima H, Tachibana T, Hashimoto M, et al. (2011) Enzymatic actions of Pasteurella multocida toxin detected by monoclonal antibodies recognizing the deamidated α subunit of the heterotrimeric GTPase Gq . FEBS J 278: 2702–2712. [DOI] [PubMed] [Google Scholar]
- 37. Schmidt G, Selzer J, Lerm M, Aktories K (1998) The Rho-deamidating cytotoxic necrotizing factor 1 from Escherichia coli possesses transglutaminase activity: cysteine 866 and histidine 881 are essential for enzyme activity. J Biol Chem 273: 13669–13674. [DOI] [PubMed] [Google Scholar]
- 38. Folk JE, Chung SI (1973) Molecular and catalytic properties of transglutaminases. Adv Enzymol Relat Areas Mol Biol 38: 109–191. [DOI] [PubMed] [Google Scholar]
- 39. Stamnaes J, Fleckenstein B, Sollid LM (2008) The propensity for deamidation and transamidation of peptides by transglutaminase 2 is dependent on substrate affinity and reaction conditions. Biochim Biophys Acta 1784: 1804–1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Esposito C, Caputo I (2005) Mammalian transglutaminases: identification of substrates as a key to physiological function and physiopathological relevance. FEBS J 272: 615–631. [DOI] [PubMed] [Google Scholar]
- 41. Milligan G (1993) Agonist regulation of cellular G protein levels and distribution: mechanisms and functional implications. Trends Pharmacol Sci 14: 413–418. [DOI] [PubMed] [Google Scholar]
- 42. Milligan G, Unson CG, Wakelam MJO (1989) Cholera toxin treatment produces down-regulation of the alpha-subunit of the stimulatory guanine-nucleotide-binding protein (Gs). Biochem J 262: 643–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lerm M, Pop M, Fritz G, Aktories K, Schmidt G (2002) Proteasomal degradation of cytotoxic necrotizing factor 1-activated Rac. Infect Immun 70: 4053–4058. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PMT does not induce the covalent modification of Gαs. (A) Membrane proteins from Swiss 3T3 cells were separated by SDS PAGE and Western blotted with anti-Gαs antibody. Membrane proteins from Swiss 3T3 cells left (B) untreated or (C) treated with 150 pM PMT for 4 h were separated by 2-D gel electrophoresis and Western blotted with anti-Gαs antibody. Samples from at least 3 independent experiments were resolved with similar results.
(TIF)
Kinase inhibitors do not block PMT induced modification of Gαq/11 or Gαi. Swiss 3T3 cells were either not treated (Lane 1) or pre-treated (Lane 2) for 1 h with (A) SU6656 or (B) St638, then stimulated with 0.5 nM pervanadate for 5 min. The cells were lysed in SDS-buffer and proteins were resolved by SDS PAGE followed by Western blotting with an anti-phospho-FAK antibody. Three independent experiments gave similar results. Swiss 3T3 cells were (C, D, G, H) not treated or pre-treated with either (E, I) SU6656 or (F, J) St638 and then either treated with (D, E, F, H, I, J) 150 pM PMT or (C, G) not treated with PMT. Samples were resolved from 3 independent experiments with similar results. Membrane proteins were separated by 2-D gel electrophoresis and Western blotted with (C–F) anti-Gαq/11 antibody or (G–J) anti-Gαi-1-3 antibody. Samples were resolved from 2 independent experiments with similar results.
(TIF)
Mutant PMT does not induces the covalent modifications of Gαq or Gαi. Membrane proteins from Swiss 3T3 cells (A, C) left untreated or (B, D) treated with 150 pM PMTC1165S for 4 h, separated by 2-D gel electrophoresis and Western blotted with either (A, B) anti-Gαq/11 or (C, D) anti-Gαi-1-3 antibodies. Samples from 3 independent experiments were resolved with similar results.
(TIF)
Analysis of pI values of Gs family isoforms after treatment with PMT. The samples were as described in the legend to Figure. S1 and the results are expressed as the mean ± standard error of the mean.
(DOC)