Abstract
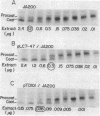
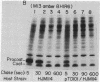
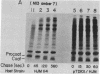
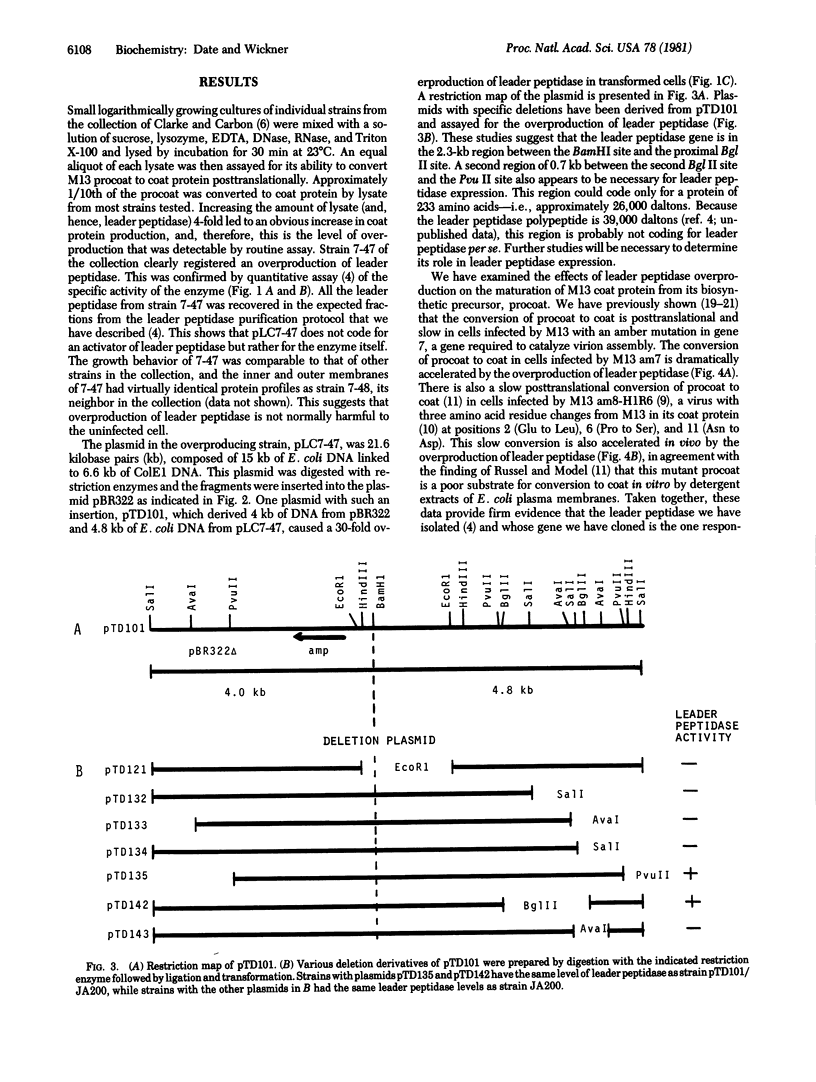
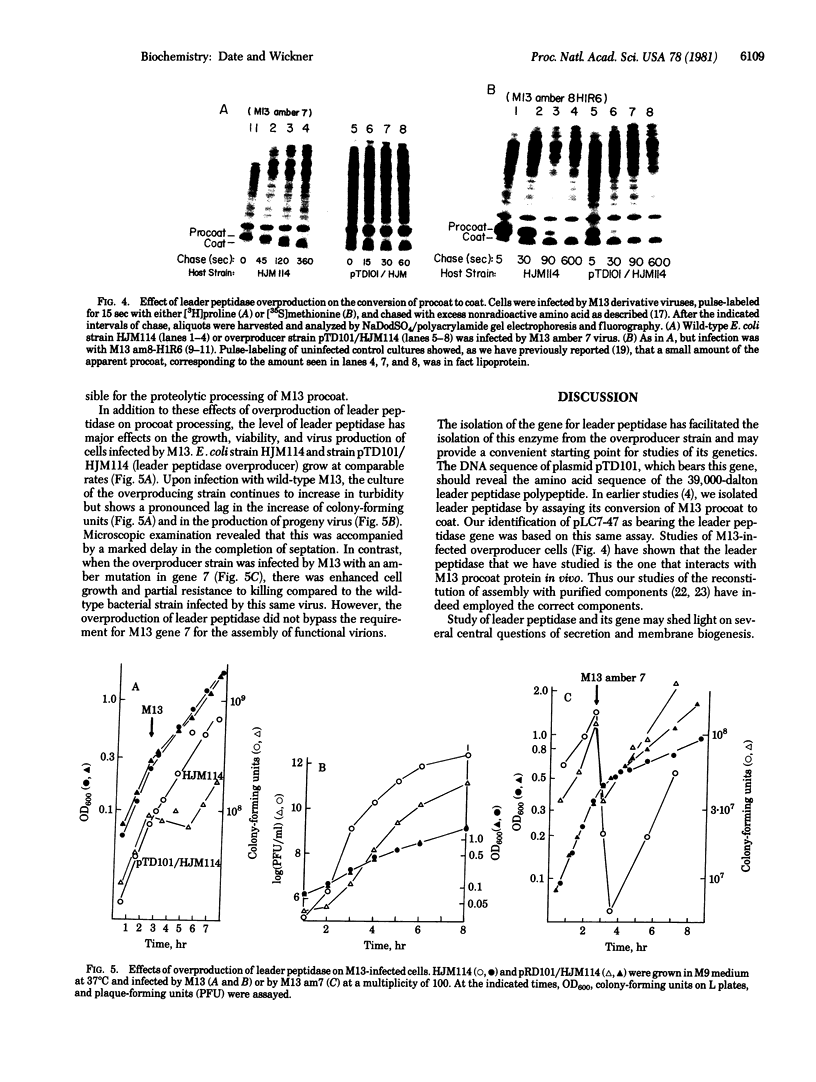
The only covalent modifications known to accompany protein insertion into membranes or protein secretion are glycosylation and the proteolytic removal of an NH2-terminal leader (signal) sequence. This latter reaction is catalyzed by leader peptidase, a constitutive, membrane-bound proteinase. We now report the identification of a plasmid-bearing strain of Escherichia coli that overproduces leader peptidase 4- to 6-fold. This strain grows normally and shows an unaltered polypeptide composition of inner ad outer membranes. The leader peptidase gene has been subcloned and transferred from this plasmid to the multicopy plasmid pBR322, yielding a new plasmid (pTD101). Strains transformed by pTD101 have a 30-fold increase in leader peptidase. We have studied the effect of leader peptidase overproduction on the insertion of newlymade M13 phage coat protein into the plasma membrane of infected cells. The overproducer strain, when infected by M13 phage, shows a dramatic acceleration in the conversion of procoat (a cytoplasmic precursor form) to coat (an integral, transmembrane protein). Thus the leader peptidase that converts M13 procoat to coat in vitro can catalyze this reaction in vivo as well.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boeke J. D., Russel M., Model P. Processing of filamentous phage pre-coat protein. Effect of sequence variations near the signal peptidase cleavage site. J Mol Biol. 1980 Dec 5;144(2):103–116. doi: 10.1016/0022-2836(80)90027-3. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Clarke L., Carbon J. A colony bank containing synthetic Col El hybrid plasmids representative of the entire E. coli genome. Cell. 1976 Sep;9(1):91–99. doi: 10.1016/0092-8674(76)90055-6. [DOI] [PubMed] [Google Scholar]
- Collins C. J., Jackson D. A., DeVries A. J. Biochemical construction of specific chimeric plasmids from ColE1 DNA and unfractionated Escherichia coli DNA. Proc Natl Acad Sci U S A. 1976 Nov;73(11):3838–3842. doi: 10.1073/pnas.73.11.3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Date T., Goodman J. M., Wickner W. T. Procoat, the precursor of M13 coat protein, requires an electrochemical potential for membrane insertion. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4669–4673. doi: 10.1073/pnas.77.8.4669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Date T., Zwizinski C., Ludmerer S., Wickner W. Mechanisms of membrane assembly: effects of energy poisons on the conversion of soluble M13 coliphage procoat to membrane-bound coat protein. Proc Natl Acad Sci U S A. 1980 Feb;77(2):827–831. doi: 10.1073/pnas.77.2.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K., Date T., Wickner W. Synthesis, assembly into the cytoplasmic membrane, and proteolytic processing of the precursor of coliphage M13 coat protein. J Biol Chem. 1980 Mar 10;255(5):2123–2130. [PubMed] [Google Scholar]
- Ito K., Mandel G., Wickner W. Soluble precursor of an integral membrane protein: synthesis of procoat protein in Escherichia coli infected with bacteriophage M13. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1199–1203. doi: 10.1073/pnas.76.3.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshland D., Botstein D. Secretion of beta-lactamase requires the carboxy end of the protein. Cell. 1980 Jul;20(3):749–760. doi: 10.1016/0092-8674(80)90321-9. [DOI] [PubMed] [Google Scholar]
- Mandel G., Wickner W. Translational and post-translational cleavage of M13 procoat protein: extracts of both the cytoplasmic and outer membranes of Escherichia coli contain leader peptidase activity. Proc Natl Acad Sci U S A. 1979 Jan;76(1):236–240. doi: 10.1073/pnas.76.1.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt D., Tzagoloff H., Beaudoin J. Conditional lethal mutants of the small filamentous coliphage M13. II. Two genes for coat proteins. Virology. 1969 Sep;39(1):42–53. doi: 10.1016/0042-6822(69)90346-8. [DOI] [PubMed] [Google Scholar]
- Russel M., Model P. A mutation downstream from the signal peptidase cleavage site affects cleavage but not membrane insertion of phage coat protein. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1717–1721. doi: 10.1073/pnas.78.3.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner W. Assembly of proteins into membranes. Science. 1980 Nov 21;210(4472):861–868. doi: 10.1126/science.7001628. [DOI] [PubMed] [Google Scholar]
- Wickner W., Killick T. Membrane-associated assembly of M13 phage in extracts of virus-infected Escherichia coli. Proc Natl Acad Sci U S A. 1977 Feb;74(2):505–509. doi: 10.1073/pnas.74.2.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwizinski C., Date T., Wickner W. Leader peptidase is found in both the inner and outer membranes of Escherichia coli. J Biol Chem. 1981 Apr 10;256(7):3593–3597. [PubMed] [Google Scholar]
- Zwizinski C., Wickner W. Purification and characterization of leader (signal) peptidase from Escherichia coli. J Biol Chem. 1980 Aug 25;255(16):7973–7977. [PubMed] [Google Scholar]
- de Vries F. A., Collins C. J., Jackson D. A. Joining of simian virus 40 DNA molecules at endonuclease R Eco Ri sites by polynucleotide ligase and analysis of the products by agarose gel electrophoresis. Biochim Biophys Acta. 1976 Jul 2;435(3):213–227. doi: 10.1016/0005-2787(76)90103-9. [DOI] [PubMed] [Google Scholar]