Abstract
The Escherichia coli replication factor Y, in conjunction with other genetically undefined E. coli replication factors and the gene products of the E. coli dnaB, dnaC, and dnaG loci, is involved in de novo primer formation on the phi X174 (+) single-strand circular DNA template [(+) ss(c)DNA]. The participation of factor Y in this series of reactions is correlated with its phi X174 (+) ss(c)-specific DNA-dependent ATPase activity. Recently two factor Y effector DNA segments of the plasmid pBR322 have been identified in close proximity to the plasmid origin of DNA replication. We report here that insertion of these factor Y sites into the filamentous phage f1R229 (+) ss(c)DNA confers upon it the ability to be converted to RF DNA in vitro through a rifampicin-resistant dnaB, dnaC, and dnaG gene product-dependent pathway. Our data suggest that factor Y effector sites can function as origins of DNA replication.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arai K., Kornberg A. Unique primed start of phage phi X174 DNA replication and mobility of the primosome in a direction opposite chain synthesis. Proc Natl Acad Sci U S A. 1981 Jan;78(1):69–73. doi: 10.1073/pnas.78.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backman K., Betlach M., Boyer H. W., Yanofsky S. Genetic and physical studies on the replication of ColE1-type plasmids. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 1):69–76. doi: 10.1101/sqb.1979.043.01.012. [DOI] [PubMed] [Google Scholar]
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Benz E. W., Jr, Reinberg D., Vicuna R., Hurwitz J. Initiation of DNA replication by the dnaG protein. J Biol Chem. 1980 Feb 10;255(3):1096–1106. [PubMed] [Google Scholar]
- Boeke J. D. One and two codon insertion mutants of bacteriophage f1. Mol Gen Genet. 1981;181(3):288–291. doi: 10.1007/BF00425599. [DOI] [PubMed] [Google Scholar]
- Bouché J. P., Rowen L., Kornberg A. The RNA primer synthesized by primase to initiate phage G4 DNA replication. J Biol Chem. 1978 Feb 10;253(3):765–769. [PubMed] [Google Scholar]
- Bouché J. P., Zechel K., Kornberg A. dnaG gene product, a rifampicin-resistant RNA polymerase, initiates the conversion of a single-stranded coliphage DNA to its duplex replicative form. J Biol Chem. 1975 Aug 10;250(15):5995–6001. [PubMed] [Google Scholar]
- Kupersztoch-Portnoy Y. M., Lovett M. A., Helinski D. R. Strand and site specificity of the relaxation event for the relaxation complex of the antibiotic resistance plasmid R6K. Biochemistry. 1974 Dec 31;13(27):5484–5490. doi: 10.1021/bi00724a005. [DOI] [PubMed] [Google Scholar]
- Model P., Zinder N. D. In vitro synthesis of bacteriophage f1 proteins. J Mol Biol. 1974 Feb 25;83(2):231–251. doi: 10.1016/0022-2836(74)90389-1. [DOI] [PubMed] [Google Scholar]
- Nomura N., Ray D. S. Expression of a DNA strand initiation sequence of ColE1 plasmid in a single-stranded DNA phage. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6566–6570. doi: 10.1073/pnas.77.11.6566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothstein R. J., Lau L. F., Bahl C. P., Narang S. A., Wu R. Synthetic adaptors for cloning DNA. Methods Enzymol. 1979;68:98–109. doi: 10.1016/0076-6879(79)68009-6. [DOI] [PubMed] [Google Scholar]
- Rowen L., Kornberg A. Primase, the dnaG protein of Escherichia coli. An enzyme which starts DNA chains. J Biol Chem. 1978 Feb 10;253(3):758–764. [PubMed] [Google Scholar]
- Sakakibara Y. Discontinuous replication of colicin E1 plasmid DNA in a cell extract containing thermolabile DNA ligase. J Mol Biol. 1978 Sep 15;124(2):373–389. doi: 10.1016/0022-2836(78)90305-4. [DOI] [PubMed] [Google Scholar]
- Schekman R., Weiner J. H., Weiner A., Kornberg A. Ten proteins required for conversion of phiX174 single-stranded DNA to duplex form in vitro. Resolution and reconstitution. J Biol Chem. 1975 Aug 10;250(15):5859–5865. [PubMed] [Google Scholar]
- Shlomai J., Kornberg A. An Escherichia coli replication protein that recognizes a unique sequence within a hairpin region in phi X174 DNA. Proc Natl Acad Sci U S A. 1980 Feb;77(2):799–803. doi: 10.1073/pnas.77.2.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staudenbauer W. L., Scherzinger E., Lanka E. Replication of the colicin E1 plasmid in extracts of Escherichia coli: uncoupling of leading strand from lagging strand synthesis. Mol Gen Genet. 1979;177(1):113–120. doi: 10.1007/BF00267260. [DOI] [PubMed] [Google Scholar]
- Sutcliffe J. G. Complete nucleotide sequence of the Escherichia coli plasmid pBR322. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 1):77–90. doi: 10.1101/sqb.1979.043.01.013. [DOI] [PubMed] [Google Scholar]
- Twigg A. J., Sherratt D. Trans-complementable copy-number mutants of plasmid ColE1. Nature. 1980 Jan 10;283(5743):216–218. doi: 10.1038/283216a0. [DOI] [PubMed] [Google Scholar]
- Vicuna R., Hurwitz J., Wallace S., Girard M. Selective inhibition of in vitro DNA synthesis dependent on phiX174 compared with fd DNA. I. Protein requirements for selective inhibition. J Biol Chem. 1977 Apr 25;252(8):2524–2533. [PubMed] [Google Scholar]
- Vicuna R., Ikeda J. E., Hurwitz J. Selective inhibition of phiX RFII compared with fd RFII DNA synthesis in vitro. II. Resolution of discrimination reaction into multiple steps. J Biol Chem. 1977 Apr 25;252(8):2534–2544. [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren G. J., Twigg A. J., Sherratt D. J. ColE1 plasmid mobility and relaxation complex. Nature. 1978 Jul 20;274(5668):259–261. doi: 10.1038/274259a0. [DOI] [PubMed] [Google Scholar]
- Wickner S., Hurwitz J. Association of phiX174 DNA-dependent ATPase activity with an Escherichia coli protein, replication factor Y, required for in vitro synthesis of phiX174 DNA. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3342–3346. doi: 10.1073/pnas.72.9.3342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner S., Hurwitz J. Conversion of phiX174 viral DNA to double-stranded form by purified Escherichia coli proteins. Proc Natl Acad Sci U S A. 1974 Oct;71(10):4120–4124. doi: 10.1073/pnas.71.10.4120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner W., Brutlag D., Schekman R., Kornberg A. RNA synthesis initiates in vitro conversion of M13 DNA to its replicative form. Proc Natl Acad Sci U S A. 1972 Apr;69(4):965–969. doi: 10.1073/pnas.69.4.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
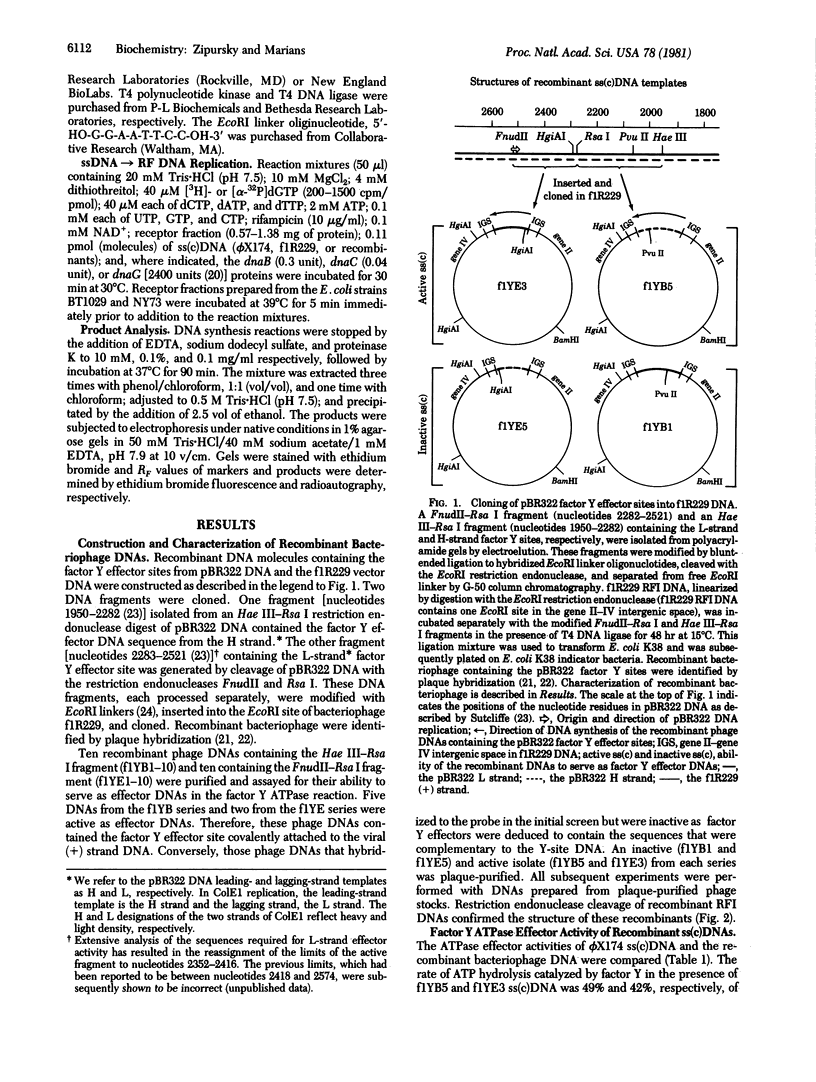
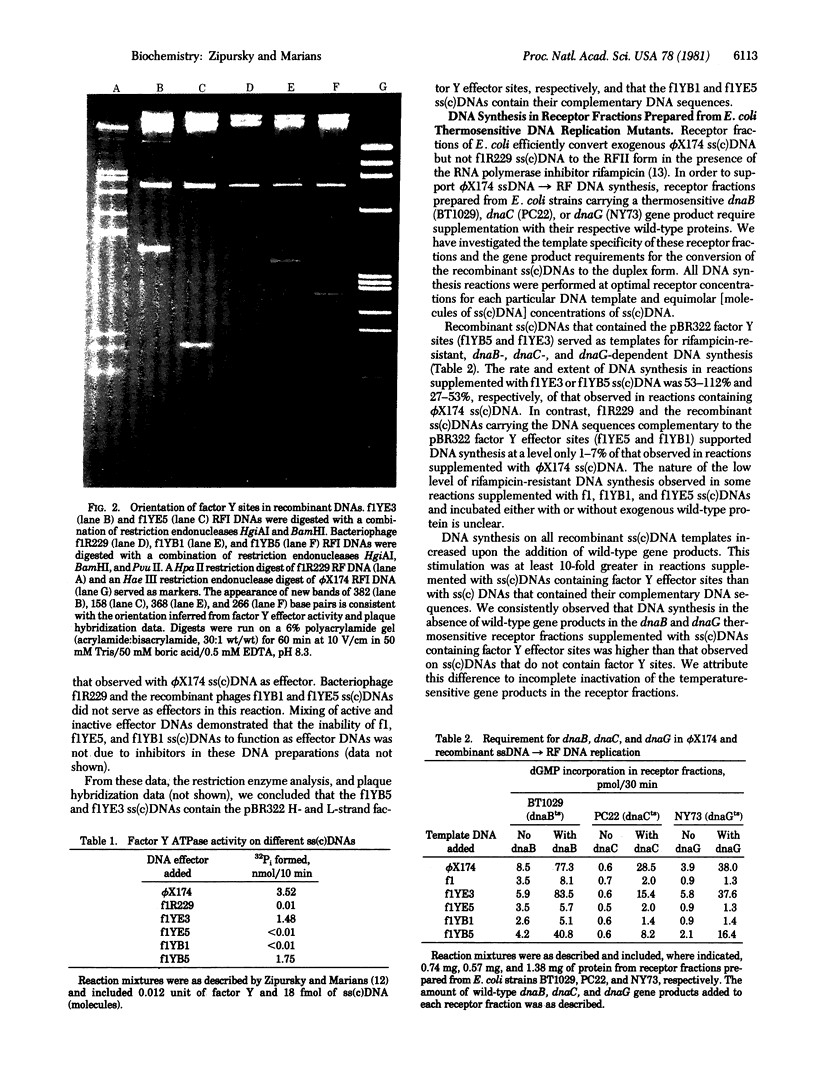
- Zipursky S. L., Marians K. J. Identification of two Escherichia coli factor Y effector sites near the origins of replication of the plasmids (ColE1 and pBR322. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6521–6525. doi: 10.1073/pnas.77.11.6521. [DOI] [PMC free article] [PubMed] [Google Scholar]