Abstract
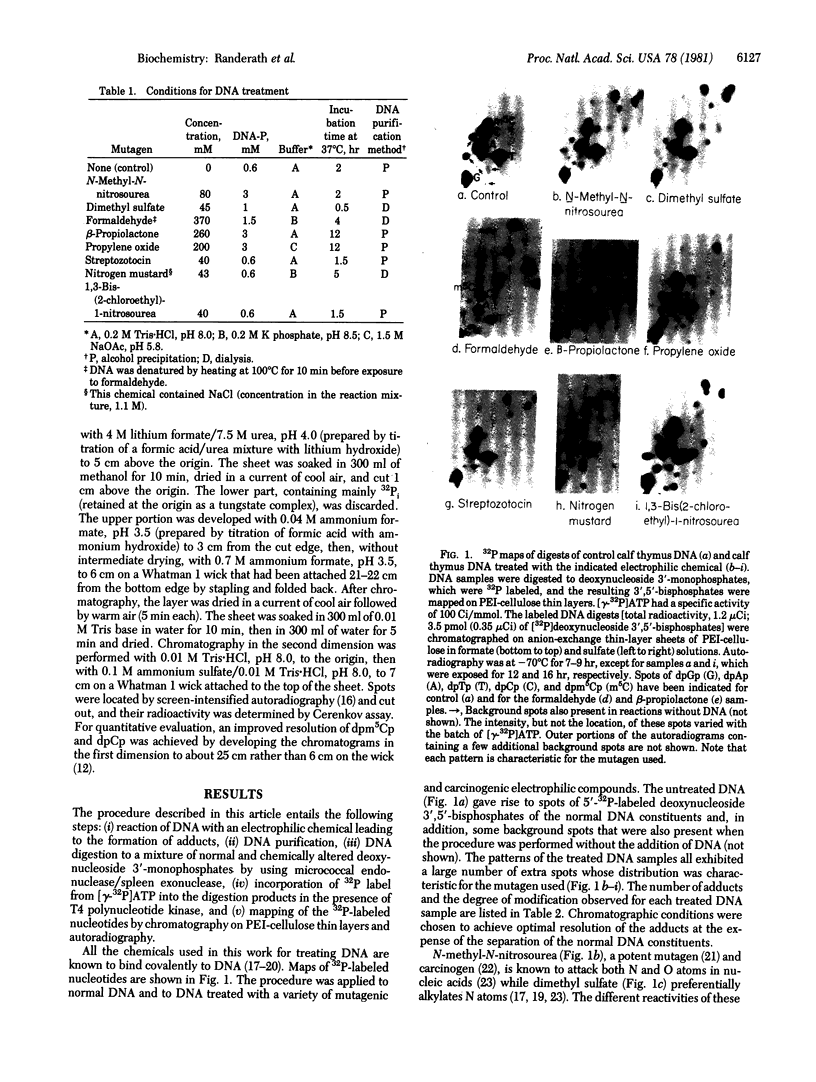
Covalent adducts formed by the reaction of DNA with chemical carcinogens and mutagens may be detected by a 32P-labeling test. DNA preparations exposed to chemicals known to bind covalently to DNA [N-methyl-N-nitrosourea, dimethyl sulfate, formaldehyde, beta-propiolactone, propylene oxide, streptozotocin, nitrogen mustard, and 1,3-bis(2-chloroethyl)-1-nitrosourea] were digested to a mixture of deoxynucleoside 3'-monophosphates by incubation with micrococcal endonuclease (EC 3.1.31.1) and spleen exonuclease (EC 3.1.16.1). The digests were treated with [gamma-32P]ATP and T4 polynucleotide kinase (ATP:5'-dephosphopolynucleotide 5'-phosphotransferase, EC 2.7.1.78) to convert the monophosphates to 5'-32P-labeled deoxynucleoside 3',5'-bis-phosphates. These compounds were then separated on polyethyleneimine-cellulose thin layers in ammonium formate and ammonium sulfate solutions. Autoradiograms of the chromatograms obtained by this high-resolution procedure showed the presence of nucleotides derived from chemically altered, as well as normal, DNA constituents. Maps from DNA exposed to any of the chemicals used exhibited a spot pattern typical for the particular chemical. This method detected a single adduct in 10(5) DNA nucleotides without requiring that the compound under investigation be radioactive and thus provides a useful test to screen chemicals for their capacity to damage DNA by covalent binding.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames B. N. Identifying environmental chemicals causing mutations and cancer. Science. 1979 May 11;204(4393):587–593. doi: 10.1126/science.373122. [DOI] [PubMed] [Google Scholar]
- Ames B. N., Mccann J., Yamasaki E. Methods for detecting carcinogens and mutagens with the Salmonella/mammalian-microsome mutagenicity test. Mutat Res. 1975 Dec;31(6):347–364. doi: 10.1016/0165-1161(75)90046-1. [DOI] [PubMed] [Google Scholar]
- Auerbach C., Moutschen-Dahmen M., Moutschen J. Genetic and cytogenetical effects of formaldehyde and related compounds. Mutat Res. 1977;39(3-4):317–361. doi: 10.1016/0165-1110(77)90011-2. [DOI] [PubMed] [Google Scholar]
- FRAENKEL-CONRAT H. Reaction of nucleic acid with formaldehyde. Biochim Biophys Acta. 1954 Oct;15(2):307–309. doi: 10.1016/0006-3002(54)90083-9. [DOI] [PubMed] [Google Scholar]
- Feldman M. Y. Reactions of nucleic acids and nucleoproteins with formaldehyde. Prog Nucleic Acid Res Mol Biol. 1973;13:1–49. doi: 10.1016/s0079-6603(08)60099-9. [DOI] [PubMed] [Google Scholar]
- Hollstein M., McCann J., Angelosanto F. A., Nichols W. W. Short-term tests for carcinogens and mutagens. Mutat Res. 1979 Sep;65(3):133–226. doi: 10.1016/0165-1110(79)90014-9. [DOI] [PubMed] [Google Scholar]
- Lawley P. D., Jarman M. Alkylation by propylene oxide of deoxyribonucleic acid, adenine, guanosine and deoxyguanylic acid. Biochem J. 1972 Feb;126(4):893–900. doi: 10.1042/bj1260893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewin S. Reaction of salmon sperm deoxyribonucleic acid with formaldehyde. Arch Biochem Biophys. 1966 Mar;113(3):584–602. doi: 10.1016/0003-9861(66)90236-0. [DOI] [PubMed] [Google Scholar]
- McCann J., Ames B. N. Detection of carcinogens as mutagens in the Salmonella/microsome test: assay of 300 chemicals: discussion. Proc Natl Acad Sci U S A. 1976 Mar;73(3):950–954. doi: 10.1073/pnas.73.3.950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCann J., Choi E., Yamasaki E., Ames B. N. Detection of carcinogens as mutagens in the Salmonella/microsome test: assay of 300 chemicals. Proc Natl Acad Sci U S A. 1975 Dec;72(12):5135–5139. doi: 10.1073/pnas.72.12.5135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller E. C. Some current perspectives on chemical carcinogenesis in humans and experimental animals: Presidential Address. Cancer Res. 1978 Jun;38(6):1479–1496. [PubMed] [Google Scholar]
- Miller J. A. Carcinogenesis by chemicals: an overview--G. H. A. Clowes memorial lecture. Cancer Res. 1970 Mar;30(3):559–576. [PubMed] [Google Scholar]
- Pegg A. E. Formation and metabolism of alkylated nucleosides: possible role in carcinogenesis by nitroso compounds and alkylating agents. Adv Cancer Res. 1977;25:195–269. doi: 10.1016/s0065-230x(08)60635-1. [DOI] [PubMed] [Google Scholar]
- Randerath E., Yu C. T., Randerath K. Base analysis of ribopolynucleotides by chemical tritium labeling: a methodological study with model nucleosides and purified tRNA species. Anal Biochem. 1972 Jul;48(1):172–198. doi: 10.1016/0003-2697(72)90181-9. [DOI] [PubMed] [Google Scholar]
- Randerath K., Gupta R. C., Randerath E. 3H and 32P derivative methods for base composition and sequence analysis of RNA. Methods Enzymol. 1980;65(1):638–680. doi: 10.1016/s0076-6879(80)65065-4. [DOI] [PubMed] [Google Scholar]
- Richardson C. C. Phosphorylation of nucleic acid by an enzyme from T4 bacteriophage-infected Escherichia coli. Proc Natl Acad Sci U S A. 1965 Jul;54(1):158–165. doi: 10.1073/pnas.54.1.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinkus S. J., Legator M. S. Chemical characterization of 465 known or suspected carcinogens and their correlation with mutagenic activity in the Salmonella typhimurium system. Cancer Res. 1979 Sep;39(9):3289–3318. [PubMed] [Google Scholar]
- Silberklang M., Gillum A. M., RajBhandary U. L. Use of in vitro 32P labeling in the sequence analysis of nonradioactive tRNAs. Methods Enzymol. 1979;59:58–109. doi: 10.1016/0076-6879(79)59072-7. [DOI] [PubMed] [Google Scholar]
- Singer B. N-nitroso alkylating agents: formation and persistence of alkyl derivatives in mammalian nucleic acids as contributing factors in carcinogenesis. J Natl Cancer Inst. 1979 Jun;62(6):1329–1339. [PubMed] [Google Scholar]
- Swann P. F., Magee P. N. Nitrosamine-induced carcinogenesis. The alklylation of nucleic acids of the rat by N-methyl-N-nitrosourea, dimethylnitrosamine, dimethyl sulphate and methyl methanesulphonate. Biochem J. 1968 Nov;110(1):39–47. doi: 10.1042/bj1100039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanstrom R., Shank P. R. X-Ray Intensifying Screens Greatly Enhance the Detection by Autoradiography of the Radioactive Isotopes 32P and 125I. Anal Biochem. 1978 May;86(1):184–192. doi: 10.1016/0003-2697(78)90333-0. [DOI] [PubMed] [Google Scholar]
- WHEELER G. P., SKIPPER H. E. Studies with mustards. III. In vivo fixation of C14 from nitrogen mustard-C14H3 in nucleic acid fractions of animal tissues. Arch Biochem Biophys. 1957 Dec;72(2):465–475. doi: 10.1016/0003-9861(57)90222-9. [DOI] [PubMed] [Google Scholar]