Abstract
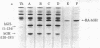
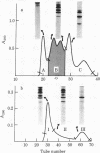
Thrombin has been shown to resynthesize the Arg134-Thr135 peptide bond between the 134- and 57-residue thrombin fragments of reduced and carbamidomethylated human somatotropin at pH 6.0 in 90% (vol/vol) glycerol. The maximal amount of synthesis was about 20% as estimated by sodium dodecyl sulfate gel electrophoresis and radioreceptor assay. The resynthesized polypeptide was isolated and shown to be indistinguishable from the reduced and carbamidomethylated hormone when tested by two receptor-binding assays, radioimmunoassay, and rat tibia test.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bewley T. A., Brovetto-Cruz J., Li C. H. Human pituitary growth hormone. Physicochemical investigations of the native and reduced-alkylated protein. Biochemistry. 1969 Dec;8(12):4701–4708. doi: 10.1021/bi00840a007. [DOI] [PubMed] [Google Scholar]
- GREENSPAN F. S., LI C. H. Bioassay of hypophyseal growth hormone; the tibia test. Endocrinology. 1949 Nov;45(5):455-63, illust. doi: 10.1210/endo-45-5-455. [DOI] [PubMed] [Google Scholar]
- Gráf L., Barát E., Borvendég J., Hermann I., Patthy A. Action of thrombin on ovine, bovine and human pituitary growth hormones. Eur J Biochem. 1976 May 1;64(2):333–340. doi: 10.1111/j.1432-1033.1976.tb10306.x. [DOI] [PubMed] [Google Scholar]
- Homandberg G. A., Laskowski M., Jr Enzymatic resynthesis of the hydrolyzed peptide bond(s) in ribonuclease S. Biochemistry. 1979 Feb 20;18(4):586–592. doi: 10.1021/bi00571a006. [DOI] [PubMed] [Google Scholar]
- Homandberg G. A., Mattis J. A., Laskowski M., Jr Synthesis of peptide bonds by proteinases. Addition of organic cosolvents shifts peptide bond equilibria toward synthesis. Biochemistry. 1978 Nov 28;17(24):5220–5227. doi: 10.1021/bi00617a023. [DOI] [PubMed] [Google Scholar]
- Komoriya A., Homandberg G. A., Chaiken I. M. Enzyme-catalyzed formation of semisynthetic staphylococcal nuclease using a new synthetic fragment, [48-glycine]synthetic-(6-49). Int J Pept Protein Res. 1980 Nov;16(5):433–439. doi: 10.1111/j.1399-3011.1980.tb02967.x. [DOI] [PubMed] [Google Scholar]
- LI C. H., LIU W. K., DIXON J. S. Human pituitary growth hormone. VI. Modified procedure of isolation and NH2-terminal amino acid sequence. Arch Biochem Biophys. 1962 Sep;Suppl 1:327–332. [PubMed] [Google Scholar]
- Lewis U. J., Singh R. N., Vanderlaan W. P., Tutwiler G. F. Enhancement of the hyperglycemic activity of human growth hormone by enzymic modification. Endocrinology. 1977 Nov;101(5):1587–1603. doi: 10.1210/endo-101-5-1587. [DOI] [PubMed] [Google Scholar]
- Li C. H., Blake J., Hayashida T. Human somatotropin: semisynthesis of the hormone by noncovalent interaction of the NH2-terminal fragment with synthetic analogs of the COOH-terminal fragment. Biochem Biophys Res Commun. 1978 May 15;82(1):217–222. doi: 10.1016/0006-291x(78)90598-3. [DOI] [PubMed] [Google Scholar]
- Li C. H., Blake J. Semisynthesis of human somatotropin analogs. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6124–6127. doi: 10.1073/pnas.76.12.6124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills J. B., Kostyo J. L., Reagan C. R., Wagner S. A., Moseley M. H., Wilhelmi A. E. Fragments of human growth hormone produced by digestion with thrombin: chemistry and biological properties. Endocrinology. 1980 Aug;107(2):391–399. doi: 10.1210/endo-107-2-391. [DOI] [PubMed] [Google Scholar]
- Reagan C. R., Mills J. B., Kostyo J. L., Wilhelmi A. E. Isolation and biological characterization of fragments of human growth hormone produced by digestion with plasmin. Endocrinology. 1975 Mar;96(3):625–636. doi: 10.1210/endo-96-3-625. [DOI] [PubMed] [Google Scholar]
- Sealock R. W., Laskowski M., Jr Enzymatic replacement of the arginyl by a lysyl residue in the reactive site of soybean trypsin inhibitor. Biochemistry. 1969 Sep;8(9):3703–3710. doi: 10.1021/bi00837a032. [DOI] [PubMed] [Google Scholar]
- Shiu R. P., Kelly P. A., Friesen H. G. Radioreceptor assay for prolactin and other lactogenic hormones. Science. 1973 Jun 1;180(4089):968–971. doi: 10.1126/science.180.4089.968. [DOI] [PubMed] [Google Scholar]
- Swank R. T., Munkres K. D. Molecular weight analysis of oligopeptides by electrophoresis in polyacrylamide gel with sodium dodecyl sulfate. Anal Biochem. 1971 Feb;39(2):462–477. doi: 10.1016/0003-2697(71)90436-2. [DOI] [PubMed] [Google Scholar]
- Tsushima T., Friesen H. G. Radioreceptor assay for growth hormone. J Clin Endocrinol Metab. 1973 Aug;37(2):334–337. doi: 10.1210/jcem-37-2-334. [DOI] [PubMed] [Google Scholar]
- Walz D. A., Seegers W. H., Reuterby J., McCoy L. E. Proteolytic specificity of thrombin. Thromb Res. 1974 May;4(5):713–717. doi: 10.1016/0049-3848(74)90226-6. [DOI] [PubMed] [Google Scholar]
- Wong T. M., Cheng C. H., Li C. H. Radioimmunoreactivity and receptor-binding activity of the recombined molecule obtained by complementation of two fibrinolysin fragments of ovine prolactin. Proc Natl Acad Sci U S A. 1981 Jan;78(1):88–90. doi: 10.1073/pnas.78.1.88. [DOI] [PMC free article] [PubMed] [Google Scholar]