Abstract
We describe a new temperature and electric field dual-stimulus responsive nanoparticle system for programmed drug delivery. Nanoparticles of a conducting polymer (polypyrrole) are loaded with therapeutic pharmaceuticals and are subcutaneously localized in vivo with the assistance of a temperature-sensitive hydrogel (PLGA-PEG-PLGA). We have shown that drug release from the conductive nanoparticles is controlled by the application of a weak, external DC electric field. This approach represents a novel interactive drug delivery system that can show an externally tailored release profile with an excellent spatial, temporal, and dosage control.
Keywords: stimulus responsive materials, drug delivery, conductive nanoparticles, polypyrrole, controlled release
Stimuli-responsive or “smart” biomaterials are of great interest in the fields of biotechnology and biomedicine.1–5 Many different approaches have been undertaken to cause the response of such a system: stimuli-responsive materials which respond to heat,6,7 pH,1,8,9 light,10–12 enzymes,13–15 and magnetic field,16,17 have been used extensively by the biomedical community. Drug delivery systems based on stimulus responsive materials for controlled and long-term drug release under the action of an external stimulus offer the promise of new treatments for chronic diseases that require daily injections or precise doses of medication.
Although many materials that deliver drugs in response to ultrasound, light and magnetic signals have been developed, activating these materials typically requires the use of large or specialized equipment. Electrical signals, on the other hand, are easy to generate and control. Electric stimuli have been successfully utilized to trigger the release of molecules via conducting polymeric bulk materials or implantable electronic delivery devices.18–20 Abidian et al.21 prepared the poly(3,4-ethylenedioxythiophene) (PEDOT) coated poly(L-lactide) (PLLA) or poly(lactide-co-glycolide) (PLGA) nanofibers with dexamethasone (Dex) incorporated. After degradation of PLLA or PLGA, the resulted conducting polymer nanotubes provide precisely controlled release of Dex. Wadhwa et al.22 coated electrodes with polypyrrole (PPy)/Dex films, which allow electric-triggered release of Dex when applying a voltage. Recently, electrically actuatable pulsatile drug release using a polypyrrole-coated nanoporous membrane was reported.23 Another possibility is to combine light with an electric field, which has been demonstrated for Au nanoparticles.24
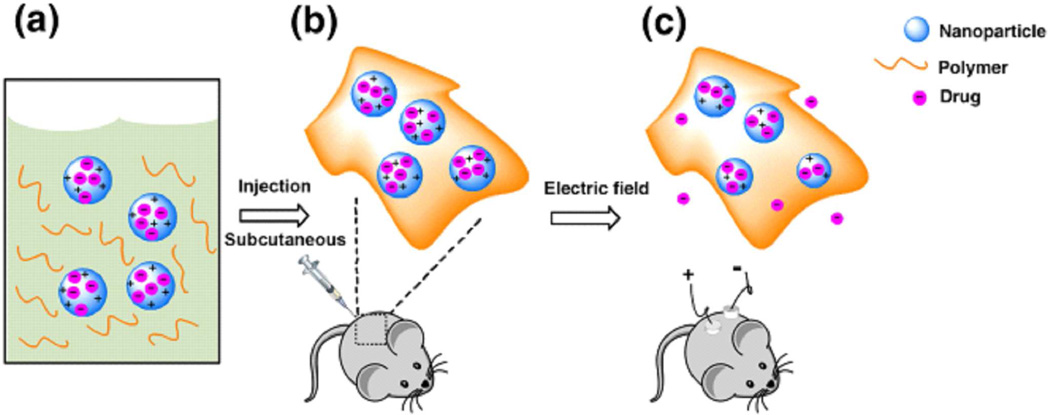
However, implantable electronic delivery devices often require invasive surgery. In order to bypass the limitations of traditional electric stimuli responsive drug delivery devices, we utilize emulsion polymerization techniques to encapsulate drug compounds in polypyrrole nanoparticles, and develop a new electric field and temperature responsive drug delivery system for triggered and localized release of cargos from these conductive nanoparticles. As shown in Scheme 1, the nanoparticles of conducting polymer loaded with a drug serve as a drug reservoir for electric field triggered release. They are suspended in a temperature-responsive hydrogel, which is a liquid at low temperature but becomes a gel at body temperature.25,26 This mixture can be subcutaneously localized by syringe injection at the place of interest. The application of a small external electric field releases the drug from the nanoparticles and allows the drug to diffuse through the hydrogel to the surroundings.
Scheme 1.
A general scheme for the application of this system. (a) The nanoparticle-polymer solution is (b) subcutaneously injected into a mouse; followed by (c) application of a DC electric field to induce release of the drug cargo inside the nanoparticles.
Nanoparticles have an increased surface area, allowing for a larger amount of drug loading and more sensitive release upon application of an applied electrical field. Our data strongly support our hypothesis that by incorporating conductive nanoparticles within a temperature-sensitive hydrogel we can develop a hybrid “smart” drug delivery system, where in vivo local release of drugs from conductive polymers is firstly successfully achieved in animals. To our knowledge such an in vivo approach has not been previously reported. In addition, the easy combination of conductive nanoparticles within a biodegradable temperature-sensitive hydrogel matrix is minimally invasive and promising for future potential clinical uses.
RESULTS AND DISCUSSION
Preparation of the Injectable Conductive Hydrogel
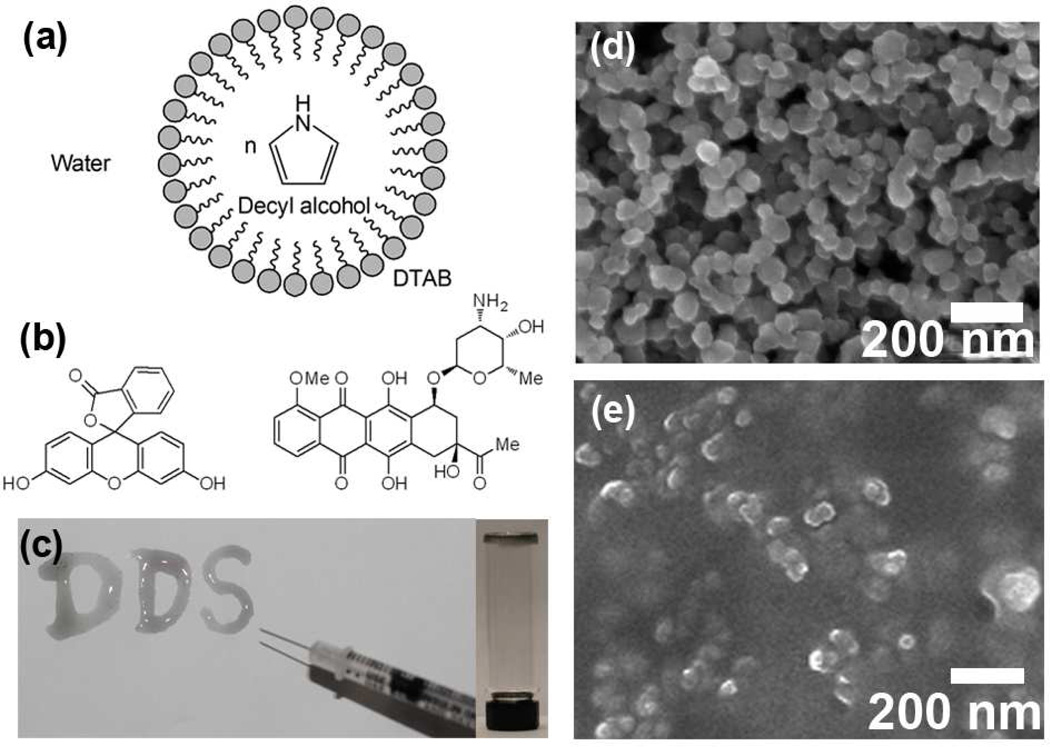
Figure 1a illustrates the emulsion polymerization of drug-encapsulated polypyrrole (PPy) nanoparticles. This method allows for uniform control of nanoparticle size. In a typical experiment, dodecyltrimethylammonium bromide (DTAB) was selected as a surfactant to form spherical micelles, while decyl alcohol was employed as a cosurfactant to stabilize the emulsion. After introducing the pyrrole monomer into the hydrophobic core of the DTAB/decyl alcohol micelles, ferric chloride as an oxidizing agent was added to initiate the chemical-oxidation polymerization. Two different compounds fluorescein and daunorubicin (Figure. 1b shows their chemical structures) were chosen to be loaded into the PPy nanoparticles during the synthesis. Due to the hydrophobicity of these drug compounds, they were localized in the hydrophobic cores of the micelles. After polymerization of pyrrole, polypyrrole nanoparticles were formed with the compounds being encapsulated. And a purification step was applied to remove the surfactants. Fluorescein, a fluorescent probe was used as a drug model for monitoring release. Daunorubicin is a chemotherapeutic agent of the anthracycline family. Both the fluorescein- and daunorubicin-encapsulated polypyrrole nanoparticles have a similar morphology with average diameters of 60 nm (Fig. 1d) determined by scanning electron microscopy (SEM), and ~150 nm determined by dynamic light scattering as some aggregates formed in aqueous solution.
Figure 1.
(a) Chemical synthesis of polypyrrole nanoparticles. (b) Chemical structures of fluorescein (left) and daunorubicin (right). (c) Photograph showing the sol-gel transition of the injectable conductive hydrogel. (DDS: drug delivery system) (d) SEM image of fluorescein-encapsulated polypyrrole nanoparticles. (e) SEM image of air-dried hydrogel containing polypyrrole nanoparticles.
The temperature-sensitive polymer poly[(D,L-lactic acid)-co-(glycolic acid)]-b-poly(ethylene oxide)-b-poly[(D,L-lactic acid)-co-(glycolic acid)] (PLGA-PEG-PLGA) which is biocompatible and biodegradable26 was selected to localize in vivo the PPy nanoparticles at the desired site. The aqueous solution of PLGA-PEG-PLGA exhibits a temperature-responsive sol-gel transition; the critical gelation temperature is dependent on the concentration of the polymer in solution. At lower temperatures the polymer solution is liquid; at higher temperatures (body temperature, 37 °C) the polymer forms a hydrogel. In this study, at 4 °C, 0.25 wt% of drug-encapsulated polypyrrole nanoparticles were dispersed in a PBS (pH 7.4) solution containing 25 wt% of PLGA-PEG-PLGA. At 4 °C, the temperature-sensitive polymer solution containing PPy nanoparticles could be easily injected through a syringe, while upon exposure at 37 °C the solution phase rapidly underwent transformation to a hydrogel. Figure 1c shows the solidified hydrogels containing PPy nanoparticles at the bottom of a glass bottle as well as on a paper after being injected from a syringe. The SEM image in Fig. 1e indicates the relatively uniform distribution of nanoparticles within the hydrogel. Polypyrrole is considered as biocompatible.27,28 In addition, for following the in vivo study, the PPy nanoparticle sizes were designed to be of 50–100 nm in size allowing for the facile passage and excretion through the circulatory system, after the temperature-sensitive hydrogel fully degrades in vivo.
Release of Drugs in Solution
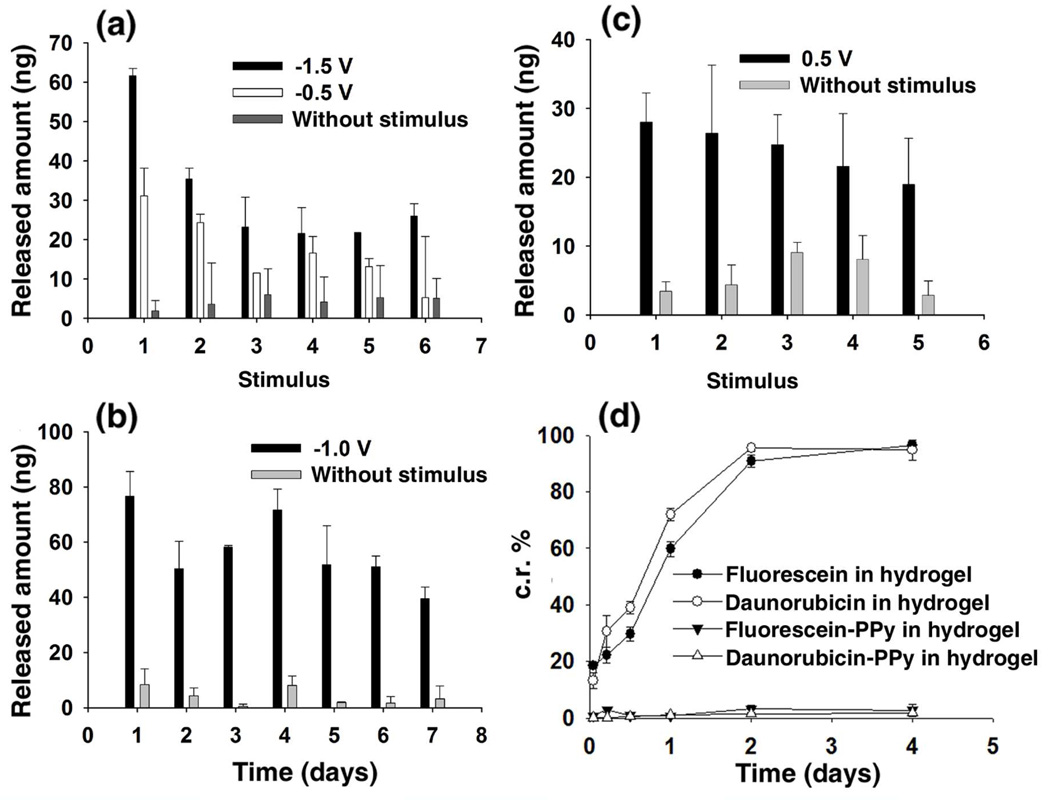
The triggered release capabilities of this system were firstly investigated in solution. In phosphate buffered saline (PBS, pH 7.2), a voltage of −0.5 V was applied between two platinum electrodes separated by a distance of 1 cm. The anode was coated with 100 mg of the hydrogel containing 0.25 wt% fluorescein-encapsulated PPy nanoparticles with a thickness around 0.1 cm. The resistivity of the swelled hydrogel and the PBS buffer was measured to be 5400 Ω•cm and 64 Ω•cm, respectively. Then, the electric field across the hydrogel was calculated to be approximately −4.5 V/cm. The electrical stimulus was applied for 10 seconds, which was repeated every five minutes, followed by measurements of the concentration of free fluorescein in the solution. Figure 2a shows that over a 30 minute period, fluorescein was released stepwise upon application of the electric field across the hydrogel. For each stimulus, ~20 ng of fluorescein was released. The voltage between the two electrodes was then set at −1.5 V (corresponding to an electric field across the hydrogel of −13.6 V/cm). At this higher voltage, as shown in Figure 2a, ~60 ng of fluorescein was released during the first stimulus; while upon each subsequent stimuli ~30 ng of fluorescein was released. The higher amount observed during the first stimulus may result from higher drug loading within the nanoparticles. Our interest in the practicability of this triggered release led us to perform a long-term release study over seven days. With the voltage between the two electrodes at −1.0 V (corresponding to an electric field across the hydrogel of −9.0 V/cm), the pulsed electric stimulus was applied to the conductive hydrogels for 20 s, once every 24 hr, followed by concentration measurements of free fluorescein in solution. Figure 2b shows that approximately 60 ng of fluorescein was released each day upon electric stimulus. As a control, no obvious release of fluorescein was detected without applying voltage. In the case of daunorubicin, an electric field across the hydrogel of 4.5 V/cm (the set voltage was 0.5 V) was applied for 10 seconds every 5 minutes. As shown in Fig. 2c, upon each stimulus, ~25 ng of daunorubicin was released into solution.
Figure 2.
Electric field induced release from the conductive hydrogel. (a) Released amount of fluorescein in PBS (pH 7.2) following an applied voltage (−0.5 or −1.5 V) duration of 10 s, repeated every five minutes. (b) Released amount of fluorescein in PBS (pH 7.2) following an applied voltage duration of 20 s, repeated every day. (c) Released amount of daunorubicin in PBS (pH 7.2) following an applied voltage (0.5 V) duration of 10 s, repeated every five minutes. (d) Cumulative release (c.r.) of drugs from hydrogel and from PPy nanoparticles in hydrogel without applying voltage.
By applying voltage until no obvious drug release could be detected, the loading percentage of fluorescein and daunorubicin in PPy nanoparticles was calculated to be around 3.6 wt% and 3.2 wt%. Comparing to sustained release of fluorescein and daunorubicin in hydrogel without encapsulating them in PPy nanoparticles (Fig. 2d shows most of the drugs in hydrogel were released in 4 days), no obvious release of encapsulated fluorescein or daunorubicin from PPy nanoparticles in hydrogel was detected without applying an electric field. This behavior indicates that encapsulation of drugs in PPy nanoparticles prevents the undesired release from the hydrogel. Only with an electric stimulus can drugs be released on command. This represents an important advantage of our delivery system over conventional sustained release of drugs from hydrogel. By comparing the above release studies, we have demonstrated that the released dose of the drug could be roughly controlled by either the strength of the electric field or the duration time of the electric field.
Mechanism of Electric Field Triggered Release
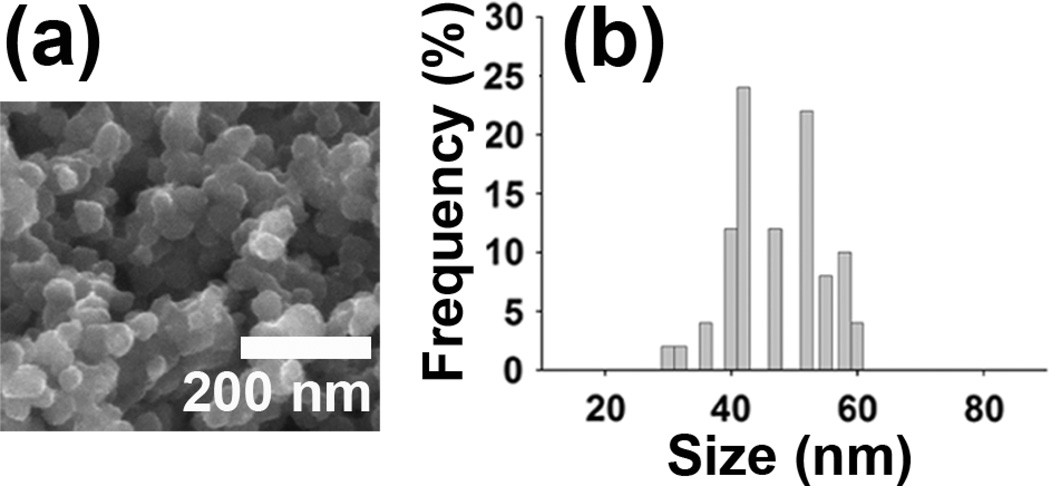
The electric field triggered release possibly involves a synergistic process of electrochemical reduction/oxidation and electric-field-driven movement of charged molecules. In our work, either negatively charged fluorescein or positively charged daunorubicin molecules were incorporated into PPy nanoparticles during the chemical synthesis. The release of molecules by electrochemical reduction/oxidation process is known for PPy bulk materials.20–22 Similar to that, in our study, upon reduction, fluorescein was released from the PPy nanoparticles, while daunorubicin was released upon oxidation. Release of the drug is directly related to the change of the overall net charge within the polymer nanoparticles upon reduction or oxidation, which is known to cause conformational change; as the charge density of the PPy nanoparticles changes the contraction of the nanoparticles and repulsion of noncovalently bonded drug molecules occurs. Upon reduction, the positive charge within the polypyrrole nanoparticles is reduced, expelling fluorescein molecules from the nanoparticles and causing net overall contraction of the nanoparticles. Upon oxidation, the positive charge within the polypyrrole nanoparticles is increased, which leads to repulsion of the positively charged daunorubicin molecules. After molecules are released from PPy nanoparticles by the electrochemical reduction/oxidation process, electric-field-driven migration plays an important role in the movement of charged entities toward the electrode bearing an opposite charge, which resulted in the escape of drugs from the hydrogel. The morphology of the fluoresceinencapsulated polypyrrole nanoparticles after release was shown by SEM images in Figure 3. For the release experiment, the anode was coated with 20 mg of the hydrogel containing 0.25 wt% fluorescein-encapsulated PPy nanoparticles. Then a voltage of −1.5 V between the two electrodes (corresponding to an electric field across the hydrogel of −13.6 V/cm) was applied for 60 seconds, which was repeated every 20 minutes. Compared to the uniform and spherical nanoparticles before release, after release most of the nanoparticles lost their uniform and spherical shapes and appeared more shrinked in size. By recording the sizes of nanoparticles from SEM images, the shrinkage of the nanoparticles was roughly calculated to be 17.2 % in diameter and thus 43.3 % in volume. Ab initio calculations29 show that a neutral polypyrrole chain in the ground state assumes a helical shape resulting from a novel bending mechanism, while upon oxidation the chain becomes planar, an effect attributed to enhanced inter-ring bonding.
Figure 3.
(a) SEM images of fluorescein-encapsulated polypyrrole nanoparticles after release. (b) histograms showing particle size distributions calculated from SEM images.
Biocompatibility of the Conductive Hydrogel
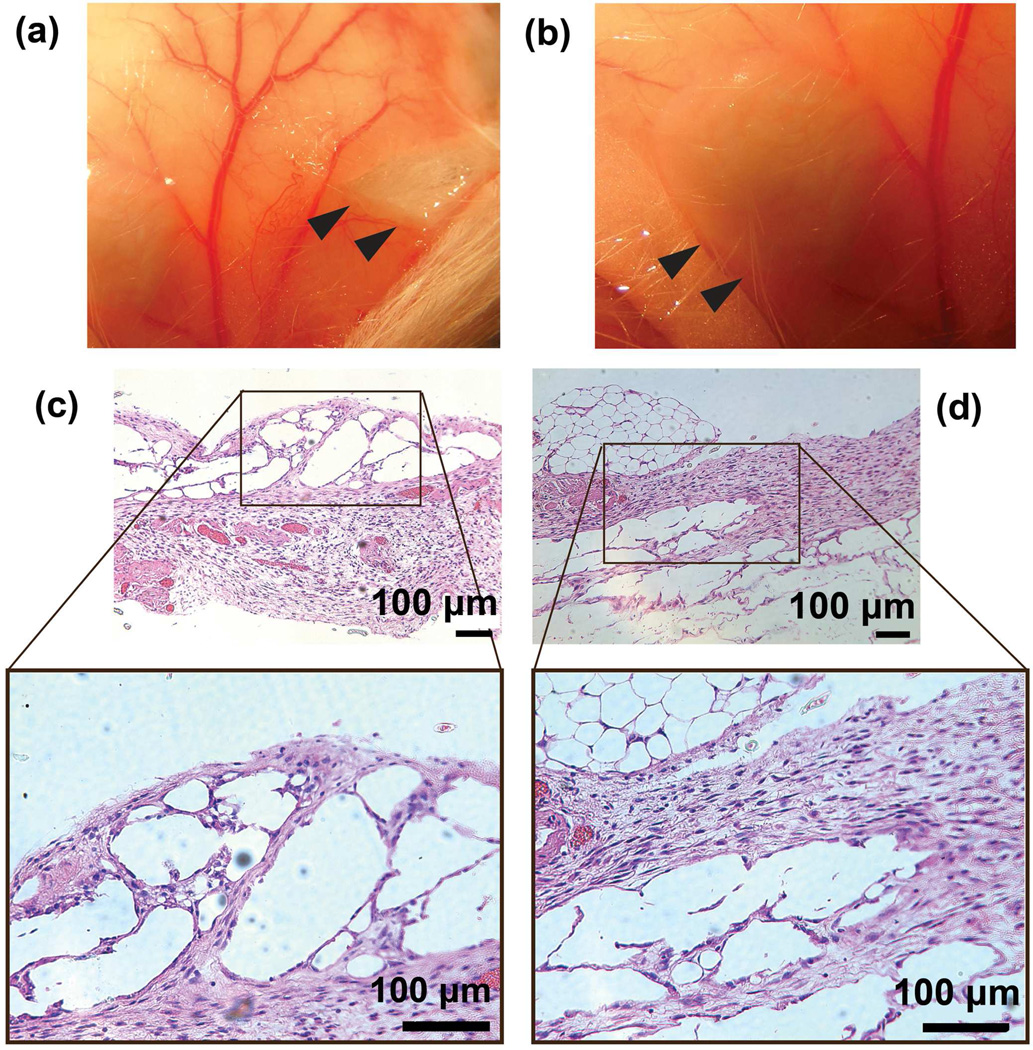
To confirm the biocompatability of the conductive hydrogel in mice, the solution containing PLGA-PEG-PLGA and 1 wt% of PPy nanoparticles were subcutaneously injected at dorsal sites of FVB adult mice (Figs. 4a and 4b). Once injected, the solution solidifies into hydrogel immediately at body temperature. Histological observation of hydrogel have been carried out after H&E staining and represented in Figs. 4c and 4d. The initial thermo-responsive hydrogel has no infiltrated cells. Various types of cells are observed to be in the hydrogel at 7 and 14 days after injection (Figs. 4c and 4d). The implanated hydrogel containing high concentrations of PPy NPs (>5 wt%) were observed to be encapsulated by fibrous tissue and covered with a regenerated thick pleura-like cell membrane after 2 weeks. The hydrogel containing an optimal concentration of PPy NPs (1 wt%) did not exhibit any fibrous tissue encapsulation as shown in Figs. 4a and 4b. H&E staining showed that the skin layers were structurally clear and no infiltration by neutrophilic granulocytes and lymphocytes were found at days 7 and 14 (Figs. 4c and 4d). Histologically, no obvious differences were observed between the experimental group and the control group. This result is consistent with previous reports on biocompatibility of PPy nanoparticles27,28,30,31 and PLGA-PEG-PLGA hydrogel26 in vivo.
Figure 4.
(a) and (b) are the photographs of in situ formed conductive hydrogels containing 1 wt% of PPy NPs after a subcutaneous injection in FVB mice. The hydrogel formed subcutaneously in the mouse and showed a spherical to ovoid shape after 1 week (a) and 2 weeks (b) healing. The gel was removed from the mouse on week 1 and 2. Black arrows indicated the implants. (c) and (d) are H&E stained images of the conductive hydrogels after subcutaneous implantation in an FVB mouse at 1 week (c) and 2 weeks (d). H&E stain cells could be observed in the hydrogel area.
Electric Field Triggered Release in vivo
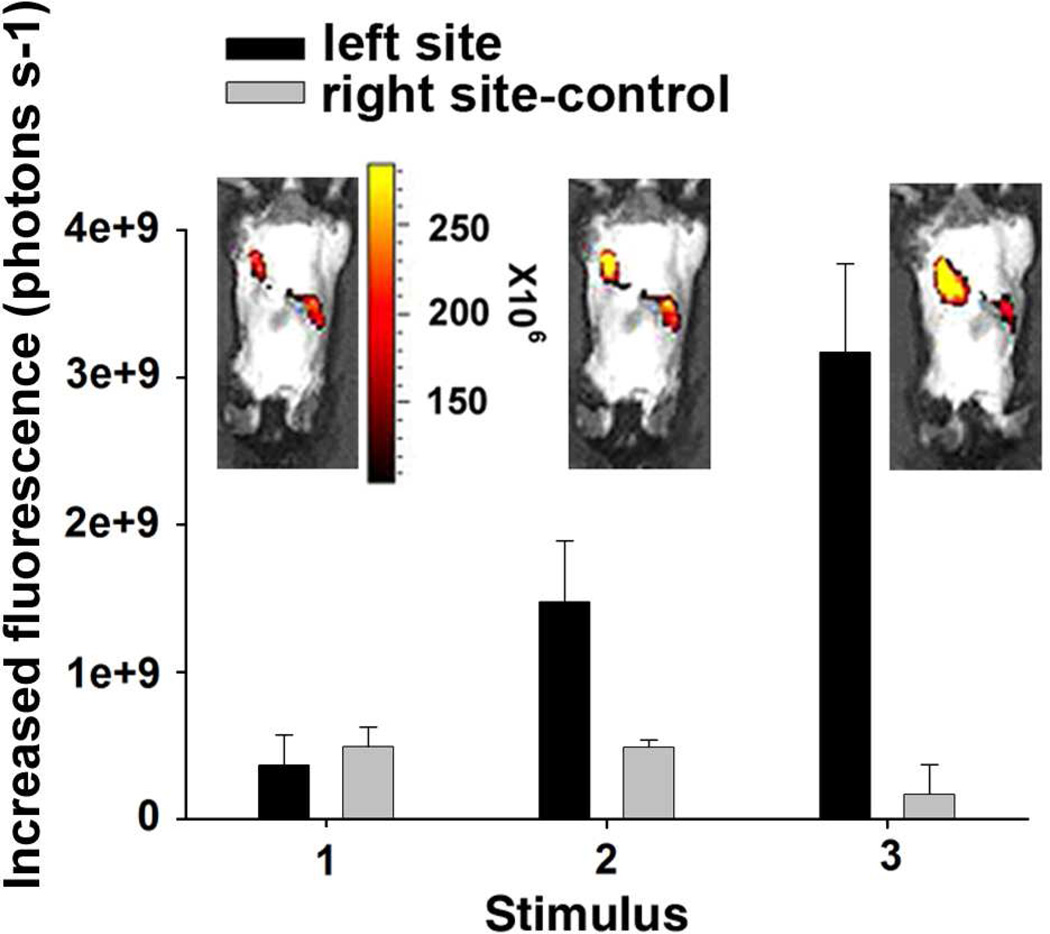
For in vivo release studies, 200 µL of fluorescein-encapsulated polypyrrole nanoparticles (1 wt%) dispersed in PBS (pH 7.2) (25 wt% PLGA-PEG-PLGA) was injected at two distinct dorsal sites of FVB adult mice. An electric field of −1.5 V/cm was applied for 40 s onto one of injection sites (left site in Fig. 5) during each stimulus, while the other injection site (right site in Fig. 5) was set as a control without applying voltage. The triggered release of fluorescein was monitored by in vivo fluorescent imaging, and the increased fluorescence in the region of interest was quantified (Fig. 5). After each stimulus, the release of fluorescein was observed, while as a comparison no obvious release of fluorescein was detected without applying electric field. A doubling of the increase in the fluorescence signal occurred for the second stimulus. We suggest that this increase is caused by release of fluorescent molecules in the hydrogel combined with new release of fluorescent molecules from the PPy nanoparticles. Rapid monitoring of the fluorescence arising from the released molecules provides a unique platform to optimize and develop the electric field responsive drug release.
Figure 5.
In vivo fluorescent images after applying an electric field of −1.5 V/cm to the implanted conductive hydrogels. The unit is photons per second. The unit of the scale on the right of the mouse image is photons per steradian per second. (1) Before applying voltage. (2) and (3) Apply voltage on the left injection site for 40 seconds; the right injection site is control without applying voltage.
One can envision clinical applications of this controlled release system for pain relief which needs a given dosage at desired periodical time by applying a weak external voltage from a small battery on the subcutaneously implanted hydrogel, and for anticancer therapy in which case the tumor cannot be easily removed by a surgery. One can also envision clinical application for the programmed drug delivery that is coupled to presence of weak electric fields in vivo. Specifically, tissues with naturally occurring electric fields can couple substance release and drug delivery to electrical activity within the tissue.
In cardiovascular tissue engineering intrinsic electrical activity of the pacemaker cells (sinoatrial node) can cause specific encapsulated substance to be released rhythmically with each pulse. Alternatively, transvenously inserted pacemakers can be programmed to generate electrical activity and thus programmed substance release to induce various desired responses such as stem cell homing, anti-apoptotic activity, or pro-angiogenic stimulus. Similarly, neuronal tissue with intrinsic weak electric fields can stimulate particular neurotransmitter release enclosed within the nanoparticles that is coupled with electrical activity within regions of the brain.
CONCLUSIONS
In summary, we have demonstrated that a dual-stimulus (temperature and electric field) responsive system containing nanoparticles made of the conducting polymer polypyrrole can be used to trigger sensitive dosage-controlled release of drugs. This approach is facile and minimally invasive for potential medical application. It represents a new electric field responsive drug delivery system that we suggest has excellent spatial and temporal control.
MATERIALS AND METHODS
Materials
Pyrrole (Py), dodecyltrimethylammonium bromide (DTAB), decyl alcohol, ferric chloride, fluorescein, and daunorubicin were purchased from Sigma-Aldrich. Poly[(D,L-lactic acid)-co-(glycolic acid)]-b-poly(ethylene oxide)-b-poly[(D,L-lactic acid)-co-(glycolic acid)] (PLGA-PEG-PLGA) ((molecular weight: 1500:1000:1500) was from Akina, Inc., West Lafayette, IN. Poly(styrenesulfonic acid), sodium salt (PSS-Na+) (molecular weight: 500000) was from Polysciences, Inc., Warrington, PA.
Synthesis of Polypyrrole Nanoparticles and Drug Encapsulation
DTAB and decyl alcohol were added to deionized water at concentrations of 50 mg/mL and 37.5 mg/mL, respectively at 4 °C. Then, pyrrole and fluorescein (or daunorubicin) were added to the emulsion to reach a concentration of 6.25 mg/mL and 0.6 mg/mL. For encapsulation of daunorubicin, 2 mg/mL of PSS−Na+ was added to the above emulsion to serve as counter-ions that incorporate positively charged daunorubicin in the nanoparticles. Then, ferric chloride aqueous solution (0.07 g/mL) was added to the above emulsion, followed by stirring at 4 °C for 2 h until completion. After the reaction, the product was precipitated out, washed with ethanol to remove DTAB and collected by centrifugation. We find that ~37.5% of fluorescein and ~33.3% of daunorubicin are incorporated into the PPY nanoparticles, based on the drug to pyrrole feed ratio in the emulsion polymerization.
Release Study in Solution
The platinum anode was firstly coated with the PLGA-PEG-PLGA polymer solution (100 mg of polymer) containing 0.25 wt% fluorescein (or daunorubicin)-encapsulated PPy nanoparticles, followed by solidification of the gel at room temperature. The PPy nanoparticels in the solidified gel was calculated to be 1 wt%. The coated platinum anode was then immersed in phosphate buffered saline (PBS, pH 7.2) together with a counter platinum electrode with a separated distance of 1 cm. For the release study in solution, specific voltages were applied between the two electrodes, followed by measurements of the concentration of released molecules by fluorescent assays.
Scanning Electron Microscopy (SEM) Measurement
SEM images were acquired using an FEI XL30 Sirion SEM with FEG source and EDX detector. Dry samples (dry PPy nanoparticles and airdried hydrogel containing PPy nanoparticles) on carbon sticky tapes were observed directly under SEM.
In vivo Biocompatibility
For testing the biocompatibility of hydrogel containing PPy nanoparticles in vivo, 100 µL of PLGA-PEG-PLGA PBS (pH 7.4) solution (25 wt% of polymer) containing 0.25 wt% of PPy nanoparticles (sterilized under UV radiation for 2 hours) were subcutaneously injected by a syringe at dorsal sites of adult female FVB mice (10 weeks old) purchased from Charles River Laboratories (Wilmington, Mass). The PPy nanoparticles in the solidified gel was calculated to be 1 wt%. Histological examination was performed to observe the cell growth within the conductive hydrogel and to predict the biodegradation procedure of the conductive hydrogel. All mice were sacrificed and the implants were individually dissected and removed from the subcutaneous dorsum at 1 and 2 weeks after implantation. The specimens were immediately fixed in 4% paraformaldehyde, dehydrated, and embedded in paraffin blocks. The embedded specimens were sectioned (5 µm thick) along the longitudinal axis of the implant. Slides were stained by hematoxylin and eosin (H&E).
In vivo Release
For in vivo release, a patch of hair was removed from the dorsal side of FVB adult female mice (10 weeks old) with hair clippers; Nair depilatory cream (Church and Dwight) was applied for 60 s and then wiped and washed off. At 4 °C 0.25 wt% of fluorescein-encapsulated polypyrrole nanoparticles were dispersed in PBS (pH 7.2) containing 25 wt% of PLGA-PEG-PLGA. For each mouse, 200 µL of the above conductive hydrogel was subcutaneously injected at two separate dorsal sites. The PPy nanoparticles in the solidified gel was calculated to be 1 wt%. An electric field of −1.5 V/cm was applied onto the implanted gels for 40 s per each stimulus via two needle electrodes. Fluorescent imaging was performed using using an in vivo imaging system (Xenogen Corporation, Alameda, CA). For quantification, a region of interest (ROI) was manually selected based on the signal intensity. The area of ROI was kept constant and the intensity was recorded as average photons per second per square centimeter per steridian.
ACKNOWLEDGMENT
T.J.C. thanks the National Defense Science and Engineering Graduate Research Fellowship. This work was supported by the National Science Foundation (CBET-0827806), grants from Stanford School of Medicine, American Heart Association (11IRG5450017), and the NIBIB (1R21EB012155-01A1).
REFERENCES
- 1.Anderson D, Burdick J, Langer R. Smart Biomaterials. Science. 2004;305:1923–1924. doi: 10.1126/science.1099987. [DOI] [PubMed] [Google Scholar]
- 2.Stuart MAC, Huck WTS, Genzer J, Muller M, Ober C, Stamm M, Sukhorukov GB, Szleifer I, Tsukruk VV, Urban M, et al. Emerging Applications of Stimuli-Responsive Polymer Materials. Nat. Mater. 2010;9:101–113. doi: 10.1038/nmat2614. [DOI] [PubMed] [Google Scholar]
- 3.Guo X, Szoka FC. Chemical Approaches to Triggerable Lipid Vesicles for Drug and Gene Delivery. Acc. Chem. Res. 2003;36:335–341. doi: 10.1021/ar9703241. [DOI] [PubMed] [Google Scholar]
- 4.LaVan DA, McGuire T, Langer R. Small-Scale Systems for in vivo Drug Deliver. Nat. Biotechnol. 2003;21:1184–1191. doi: 10.1038/nbt876. [DOI] [PubMed] [Google Scholar]
- 5.Grayson ACR, Choi IS, Tyler BM, Wang PP, Brem H, Cima MJ, Langer R. Multi-Pulse Drug Delivery from a Resorbable Polymeric Microchip Device. Nat. Mater. 2003;2:767–772. doi: 10.1038/nmat998. [DOI] [PubMed] [Google Scholar]
- 6.Yavuz MS, Cheng Y, Chen J, Cobley CM, Zhang Q, Rycenga M, Xie J, Kim C, Song KH, Schwartz AG, et al. Gold Nanocages Covered by Smart Polymers for Controlled Release with Near-Infrared Light. Nat. Mater. 2009;8:935–939. doi: 10.1038/nmat2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choi SW, Zhang Y, Xia Y. A Temperature-Sensitive Drug Release System Based on Phase-Change Materials. Angew. Chem. Int. Ed. 2010;49:7904–7908. doi: 10.1002/anie.201004057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gillies ER, Jonsson TB, Frechet JMJ. Stimuli-Responsive Supramolecular Assemblies of Linear-Dendritic Copolymers. J. Am. Chem. Soc. 2004;126:11936–11943. doi: 10.1021/ja0463738. [DOI] [PubMed] [Google Scholar]
- 9.Kim KT, Cornelissen KJJLM, Nolte RJM, van Hest JCMA. Polymersome Nanoreactor with Controllable Permeability Induced by Stimuli-Responsive Block Copolymers. Adv. Mater. 2009;21:2787–2791. [Google Scholar]
- 10.Kostiainen MA, Kasyutich O, Cornelissen JJLM, Nolte RJM. Self-Assembly and Optically Triggered Disassembly of Hierarchical Dendron–Virus Complexes. Nat. Chem. 2010;2:394–399. doi: 10.1038/nchem.592. [DOI] [PubMed] [Google Scholar]
- 11.Dvir T, Banghart MR, Timko BP, Langer R, Kohane DS. Photo-Targeted Nanoparticles. Nano Lett. 2010;10:250–254. doi: 10.1021/nl903411s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chakravarty P, Qian W, EI-Sayed MA, Prausnitz MR. Delivery of Molecules into Cells Using Carbon Nanoparticles Activated by Femtosecond Laser Pulses. Nat. Nanotechnol. 2010;5:607–611. doi: 10.1038/nnano.2010.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Azagarsamy MA, Sokkalingam P, Thayumanavan S. Enzyme-Triggered Disassembly of Dendrimer-Based Amphiphilic Nanocontainers. J. Am. Chem. Soc. 2009;131:14184–14185. doi: 10.1021/ja906162u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thornton PD, Heise A. Highly Specific Dual Enzyme-Mediated Payload Release from Peptide-Coated Silica Particles. J. Am. Chem. Soc. 2010;132:2024–2028. doi: 10.1021/ja9094439. [DOI] [PubMed] [Google Scholar]
- 15.Ge J, Lu D, Yang C, Liu Z. A Lipase-Responsive Vehicle Using Amphipathic Polymer Synthesized with the Lipase as Catalyst. Macromol. Rapid Commun. 2011;32:546–550. doi: 10.1002/marc.201000746. [DOI] [PubMed] [Google Scholar]
- 16.Namiki Y, Namiki T, Yoshida H, Ishii Y, Tsubota A, Koido S, Nariai K, Mitsunaga M, Yanagisawa S, Kashiwagi H, et al. A Novel Magnetic Crystal–Lipid Nanostructure for Magnetically Guided in vivo Gene Delivery. Nat. Nanotechnol. 2009;4:598–606. doi: 10.1038/nnano.2009.202. [DOI] [PubMed] [Google Scholar]
- 17.Dames P, Gleich B, Flemmer A, Hajek K, Seidl N, Wiekhorst F, Eberbeck D, Bittmann I, Bergemann C, Weyh T, et al. Targeted Delivery of Magnetic Aerosol Droplets to the Lung. Nat. Nanotechnol. 2007;2:495–499. doi: 10.1038/nnano.2007.217. [DOI] [PubMed] [Google Scholar]
- 18.Simon DT, Kurup S, Larsson KC, Hori R, Tybrandt K, Goiny M, Jager EW, Berggren M, Canlon B, Richter-Dahlfors A. Organic Electronics for Precise Delivery of Neurotransmitters to Modulate Mammalian Sensory Function. Nat. Mater. 2009;8:742–746. doi: 10.1038/nmat2494. [DOI] [PubMed] [Google Scholar]
- 19.George PM, LaVan DA, Burdick JA, Chen C-Y, Liang E, Langer R. Electrically Controlled Drug Delivery from Biotin-Doped Conductive Polypyrrole. Adv. Mater. 2006;18:577–581. [Google Scholar]
- 20.Svirskis D, Travas-Sejdic J, Rodgers A, Garg S. Electrochemically Controlled Drug Delivery Based on Intrinsically Conducting Polymers. J. Controlled Release. 2010;146:6–15. doi: 10.1016/j.jconrel.2010.03.023. [DOI] [PubMed] [Google Scholar]
- 21.Abidian MR, Kim DH, Martin DC. Conducting-Polymer Nanotubes for Controlled Drug Release. Adv. Mater. 2006;18:405–409. doi: 10.1002/adma.200501726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wadhwa R, Lagenaur CF, Cui XT. Electrochemically Controlled Release of Dexamethasone from Conducting Polymer Polypyrrole Coated Electrode. J. Controlled Release. 2006;110:531–541. doi: 10.1016/j.jconrel.2005.10.027. [DOI] [PubMed] [Google Scholar]
- 23.Jeon G, Yang SY, Byun J, Kim JK. Electrically Actuatable Smart Nanoporous Membrane for Pulsatile Drug Release. Nano Lett. 2011;11:1284–1288. doi: 10.1021/nl104329y. [DOI] [PubMed] [Google Scholar]
- 24.Balogh D, Tel-Vered R, Freeman R, Willner I. Photochemically and Electrochemically Triggered Au Nanoparticles "Sponges". J. Am. Chem. Soc. 2011;133:6533–6536. doi: 10.1021/ja2009899. [DOI] [PubMed] [Google Scholar]
- 25.Jeong B, Bae YH, Lee DS, Kim SW. Biodegradable Block Copolymers as Injectable Drug Delivery Systems. Nature. 2007;388:860–862. doi: 10.1038/42218. [DOI] [PubMed] [Google Scholar]
- 26.Yu L, Zhang Z, Zhang H, Ding J. Biodegradability and Biocompatibility of Thermoreversible Hydrogels Formed from Mixing a Sol and Ap of Block Copolymers in Water. Biomacromolecules. 2010;11:2169–2178. doi: 10.1021/bm100549q. [DOI] [PubMed] [Google Scholar]
- 27.George PM, Lyckman AW, LaVan DA, Hegde A, Leung Y, Avasare R, Testa C, Alexander PM, Langer R, Sur M. Fabrication and Biocompatibility of Polypyrrole Implants Suitable for Neural Prosthetics. Biomaterials. 2005;26:3511–3519. doi: 10.1016/j.biomaterials.2004.09.037. [DOI] [PubMed] [Google Scholar]
- 28.Ramanaviciene A, Kausaite A, Tautkus S, Ramanavicius A. Biocompatibility of Polypyrrole Particles: an in-vivo Study in Mice. J. Pharm. Pharmocol. 2007;59:311–315. doi: 10.1211/jpp.59.2.0017. [DOI] [PubMed] [Google Scholar]
- 29.Lin X, Li J, Smela E, Yip S. Polaron-Induced Conformation Change in Single Polypyrrole Chain: An Intrinsic Actuation Mechanism. Int. J. Quantum Chem. 2005;102:980–985. [Google Scholar]
- 30.Wang Z, Roberge C, Dao LH, Wan Y, Shi G, Rouabhia M, Guidoin R, Zhang Z. In vivo Evaluation of a Novel Electrically Conductive Polypyrrole/poly(D,L-lactide) Composite and Polypyrrole-Coated Poly(D,L-lactide-co-glycolide) Membranes. J. Biomed. Mater. Res., Part A. 2004;70A:28–38. doi: 10.1002/jbm.a.30047. [DOI] [PubMed] [Google Scholar]
- 31.Jiang X, Marois Y, Traoré A, Tessier D, Dao LH, Guidoin R, Zhang Z. Tissue Reaction to Polypyrrole-Coated Polyester Fabrics: an in vivo Study in Rats. Tissue Engineering. 2002;8:635–647. doi: 10.1089/107632702760240553. [DOI] [PubMed] [Google Scholar]