Abstract
We investigated whether acute treatment with agonists affected subcellular distribution of κ opioid receptor (KOPR) in the dorsal horn of the rat lumbar spinal cord by using immunoelectron microscopy. Rats were injected intrathecally (i.t.) with U50,488H (100 nmole), dynorphin A (1-17) (15 nmole) or vehicle. The doses chosen have been shown to induce antinociception. Rats were perfused transcardially 30 min later and lumbar spinal cords were removed and processed for electron microscopic analysis. KOPR was stained with KT-2, a specific polyclonal antibody against the rat/mouse KOPR(371-380) peptide, followed by gold-labeled secondary antibody and silver intensification. The silver grains were present in axons, terminals, dendrites and somata and the association with plasma membranes were quantified in dendrites as KOPR-IR was most frequently observed in these profiles. In vehicle-treated rats, ~27% of KOPR-immunoreactivity was associated with plasma membranes. U50,488H, i.t., did not cause a significant change in the % of KOPR present on plasma membranes, whereas dynorphin A, i.t., significantly decreased cell surface KOPR to ~19%. In summary, these data indicate that U50,488H and dynorphin A differentially regulate the subcellular distribution of endogenous KOPR.
Keywords: internalization; dorsal horn; electron microscopy; dynorphin; U50,488H
Introduction
Opioid receptors, like other G-protein coupled receptors, can be regulated by ligands or associated proteins at different levels. When opioid receptors are exposed to agonists (minutes to hours), the receptors are phosphorylated by G-protein coupled receptor kinases followed by recruitment of arrestins and other adaptor proteins. The phosphorylated receptors are internalized via clathrin- and dynamin-dependent pathways resulting in a decrease in the number of cell surface receptors (Liu-Chen, 2004). This classical trafficking process, receptor internalization, is thought to contribute to receptor resensitization. It is thought to be one of the possible mechanisms underlying opioid receptor tolerance. In knock-in mice expressing a fusion protein of δ opioid receptor-enhanced green fluorescent protein (DOPR-EGFP), internalization of DOPR-EGFP by DOPR agonist correlated with desensitization of the animals to the subsequent drug treatment (Scherrer et al., 2006).
κ opioid receptor (KOPR) is one of three types of opioid receptors mediating many effects of endogenous and exogenous agonists, including analgesia, dysphoria, water diuresis, anti-pruritus and hypothermia [see (Liu-Chen, 2004) for references]. Robust internalization of the human KOPR has been shown in various cell lines (Li et al., 1999; Schulz et al., 2002). Thirty to 40% of the human KOPR stably expressed in Chinese Hamster Ovary (CHO) cells were internalized following treatment with 1 μM U50,488H for 30 min (Li et al., 1999). Interestingly, we have previously observed a species difference in U50,488H-promoted KOPR regulation in CHO cells (Li et al., 1999; Li et al., 2002; Zhang et al., 2002). The human KOPR, but not the rat KOPR, is phosphorylated, desensitized and internalized upon treatment with 1 μM U50,488H. Further characterization indicated that the C-tails of the KOPRs contributed to the difference. Another study showed that rat KOPR stably expressed in CHO cells was internalized by peptide agonists, such as dynorphin (0.1 μM), but not by non-peptide agonists, such as U50,488H (0.1 μM) (Jordan et al., 2000). However, McLaughlin et al. (2003b) reported that the rat KOPR-EGFP stably expressed in HEK293 cells internalized upon treatment with U50,488H at 10 μM, but not at 0.1 μM. The discrepancy may be due to the different cell lines or doses of U50,488H used in these studies. The mouse KOPR also did not undergo U50,488H-induced internalization although its activation by U50,488H promoted translocation of G-protein coupled receptor kinases (Schulz et al., 2002).
All previous studies on KOPR internalization were carried out on cultured cells. Whether the KOPR undergoes internalization in vivo has not been investigated. In this study, we examined if agonist treatment changes subcellular distribution of endogenous KOPR in Sprague Dawley rats using immuno-electron microscopy. KT-2, a purified polyclonal antibody against a synthetic peptide corresponding to the amino acids 371-380 of the rat/mouse KOPR, was used to stain endogenous rat KOPR. Its specificity has been characterized previously (Drake et al., 1996). We focused on the dorsal horns (Laminae I and II) of lumbar spinal cords as this area is a relay center of sensory signals including pain from peripheral nerve endings. High levels of KOPR immunoreactivity and binding sites have been found in this region (Morris and Herz, 1987; Mansour et al., 1988; Besse et al., 1990; Arvidsson et al., 1995; Mansour et al., 1996; Harris et al., 2004), and activation of the KOPR here produces significant antinociception (Wood et al., 1981; Millan et al., 1989; Pelissier et al., 1990; Dawson-Basoa and Gintzler, 1996). Investigating in vivo trafficking of the KOPR following agonist stimulation may enhance our understanding of the adaptive changes of the receptor and the mechanisms of drug effects.
Materials and Methods
Animals and reagents
Male adult Sprague-Dawley rats (200-250g) were purchased from Ace Animals, Inc. (Boyertown, PA). The animal experiment procedures were reviewed and approved by the Institutional Animal Care and Use Committees at Temple University and Thomas Jefferson University. KT-2 is a polyclonal antibody raised in rabbits against a synthetic peptide corresponding to the KOPR (371-380) in the C-terminal domain of the rat/mouse KOPR. Dynorphin A (1-17) was purchased from Phoenix Pharmaceuticals (Belmont, CA). U50,488H was provided by the National Institute on Drug Abuse (Bethesda, MD). All the chemicals were purchased from Sigma-Aldrich, Corp. (St. Louis, MO), unless indicated otherwise. The sources of other reagents were indicated in the methods in which they were used.
Fluorescence immunocytochemistry
HEK293 cells were grown in Lab-Tek II 8-Well Slide Chambers (Nalge Nunc International, Rochester, NY). FLAG-tagged rat KOPR in pcDNA3 vector was transfected into the cells with Lipofectamine2000 (Invitrogen Corp., Carlsbad, California) according to the manufacturer’s instructions. Twenty four hours later, cells were fixed with 4% paraformaldehyde in 0.01 M phosphate-buffered saline (PBS, pH 7.4) for 15 min at room temperature (RT), washed three times with 0.01 M PBS and incubated with blocking solution (10% normal goat serum in PBS containing 0.1% Triton X-100) for 10 min. Cells were incubated with KT-2 (1:1,000) and monoclonal anti-FLAG antibody M2 (1:1,000) (Sigma-Aldrich Corp.) for 2 h at RT. After thorough washing with PBS, cells were incubated with both goat anti-rabbit IgG conjugated with Texas Red and goat anti-mouse IgG conjugated with AlexaFluor 488 (1:500) (Invitrogen Corp.) for 1 h at RT. Cells were rinsed several times and coverslipped with Citifluor (Ted Pella, Inc., Redding, CA). Coverslips were sealed with nail polish. Cells were examined under an Eclipse TE300 fluorescence microscope (Nikon Inc., Japan) and images were captured with a CCD camera. Annotations and minimal adjustment of brightness and contrast were made with Photoshop Elements (Adobe Systems Inc., San Jose, CA).
Intrathecal catheterization
Rats were deeply anesthetized with sodium pentobarbital (40 mg/kg). The atlanto-occipital membrane was exposed and incised. A saline-filled catheter (PE-10) was inserted through the incision and slowly placed down into the subarachnoid space around lumbar spinal cord (indicated by the distance from insertion site, ~7.5 cm). A knot was made on the outside end. The rats with intrathecal cannulation were housed individually and were allowed to recover at least 5 days before any experiment. Any subjects showing motor abnormalities were excluded from the study. The position of catheter was examined after the perfusion of the rats to ensure proper delivery of the drugs. Those that had catheter tips at the vertebral T-12 level were included in the study.
Immunoelectron microscopy
All the animals following intrathecal cannulation were handled daily in the same way (including daily saline, i.t.) as on the experiment day for at least 5 days.for acclimation.
Five μl of dynorphin A (1-17) (15 nmole), U50,488H (100 nmole) or saline was administered over a 60 sec period to the lumbar subarachnoid space through the indwelling intrathecal catheter followed by a flush with 10 μl saline. Such an injection of dynorphin (15 nmole in 5 μl) did not cause acute hindlimb paralysis. The doses of dynorphin A and U50,488H used have been shown to induce antinociception in tail flick or electric foot shock test, respectively (Spampinato and Candeletti, 1985; Dawson-Basoa and Gintzler, 1996).
Thirty minutes after the injections, rats were deeply anesthetized with sodium pentobarbital (60 mg/kg) and were perfused transcardially with ice-cold 50 ml of 3.8% acrolein (Electron Microscopy Sciences, Fort Washington, PA) followed by 500 ml of 2% paraformaldehyde in 0.1 M phosphate buffer (PB, pH 7.4). Lumbar spinal cords were then removed. Coronal sections were cut with a Vibratome at 40 μm. Sections were immersed in 1% sodium borohydride in 0.1 M PB for 30 min to inactivate aldehydes, washed extensively in 0.1 M PB and incubated with 0.5% bovine serum albumin (BSA) in 0.1 M Tris-buffered saline (TBS, 150 mM NaCl, 25 mM Tris, pH 7.5) for 30 min to block nonspecific binding. Sections were incubated with KT-2 (1:100) in the antibody dilution buffer (0.1 M TBS with 0.1% BSA) on a rotating shaker overnight.
On the second day, sections were rinsed with 0.01 M PBS followed by incubation in 0.01 M PBS containing 0.2% gelatin and 0.8% BSA for 10 min. Sections were then incubated with goat anti-rabbit IgG conjugated with 1 nm gold particles (1:50, Amersham, Arlington Heights, IL) for 2 h at RT. Sections were rinsed with 0.01 M PBS containing 0.2% gelatin and 0.8% BSA again followed by 0.01 M PBS. Sections were incubated in 1.25% glutaraldehyde (Electron Microscopy Sciences) in 0.01 M PBS for 10 minutes, and washed with 0.01 M PBS and then with 0.2 M sodium citrate buffer (pH 7.4). A silver-enhancement kit (Amersham Corp.) was used to intensify gold particles.
Sections were washed with 0.1 M PB and incubated in 2% osmium tetroxide (Electron Microscopy Sciences) in 0.1 M PB for 1 h followed by washing with 0.1 M PB, dehydration, and flat embedded in EMbed 812 (Electron Microscopy Sciences). Thin sections of approximately 80 nm were cut from the outer surface of the tissue, with a diamond knife (Diatome, Fort Washington, PA) and a Leica ultramicrotome, and collected on copper mesh grids. Sections were examined with an electron microscope (FEI Comp., Hillsboro, Oregon). Annotations and minor adjustment of brightness and contrast were made with Photoshop Elements (Adobe Systems Inc.).
Data analysis
The classification of identified cellular elements was based on Peters et al. (1991). Profiles were classified as dendrites if they contained microtubules, mitochondria, but were largely void of other organelles. Another distinguishing feature of dendrites was that these profiles were apposed by axon terminals that contained numerous synaptic vesicles. An axon terminal was considered to be synaptic when it showed a junctional complex, a restricted zone of parallel membrane apposition with slight enlargement of the intercellular space and/or associated postsynaptic thickening. A cellular profile was defined as immunolabelled if it contained at least 2 silver grains. For quantification of KOPR internalization, tissue sections from 3 rats of each group (dynorphin A 1-17, U50,488H and saline) with optimal preservation of ultrastructural morphology were used.
Dendrites with similar diameters and shapes were sampled from treatment and control groups, and from at least 10 grids per animal. Only cross-sectioned dendrites entirely surrounded by plasma membrane were counted. The mean diameters of the sampled dendrites were not statistically different among groups as analyzed by ANOVA (Fig. 4) (GraphPad Software Inc., San Diego, CA). The measure of internalization was the ratio of surface silver grains to total silver grains in a dendrite. Irregularly shaped silver grains were not included in the data analysis. Silver grains were identified as plasmalemmal if they were in contact with the plasma membrane, and cytoplasmic if they were not. The silver grains from each group of animals, counted by an observer who was unaware of the experimental group, were categorized as being either on the cell surface or in the cytoplasm. Statistical analyses were performed with Fisher’s exact test using the Prism 3.0 software (GraphPad Software Inc.) with significance defined as P<0.05.
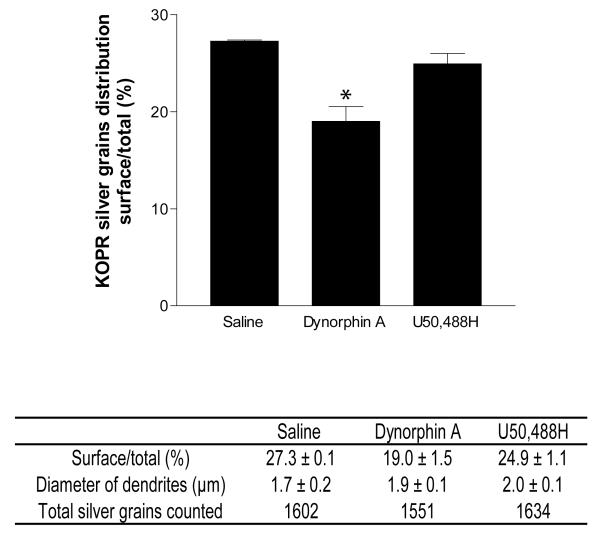
Fig. 4. Quantification of association of KOPR-IR with the plasma membrane in dorsal horn dendrites of rat lumbar spinal cords.
The diameters of the sampled dendrites and the number of total silver grains in each group were shown. Bars represented the mean percentage of KOPR-silver grains associated with the plasma membrane in rats intrathecally injected with dynorphin A (15 nmole), U50,488H (100 nmole) or saline. Values are mean ± SEM of data obtained from 3 rats per group, at least 150 dendrite profiles per rat.*, p<0.05, compared with saline group by Fisher’s exact test using the silver grain numbers categorized into on the surface or in the cytoplasm.
Results
Characterization of KOPR antibody
KT-2 is a rabbit polyclonal antibody raised against the KOPR (371-380) peptide in the C-terminal domain of the rat/mouse KOPR. It is widely used in immunohistochemistry for KOPR and its specificity has been characterized using different approaches (Drake et al., 1996). KT-2 has been shown to recognize the internalized KOPR efficiently in cells (McLaughlin et al., 2003b). We further examined whether KT-2 could recognize surface KOPR as efficiently as the antibody against the FLAG epitope, which was fused at the N-terminus of the receptor and thus is extracellular.
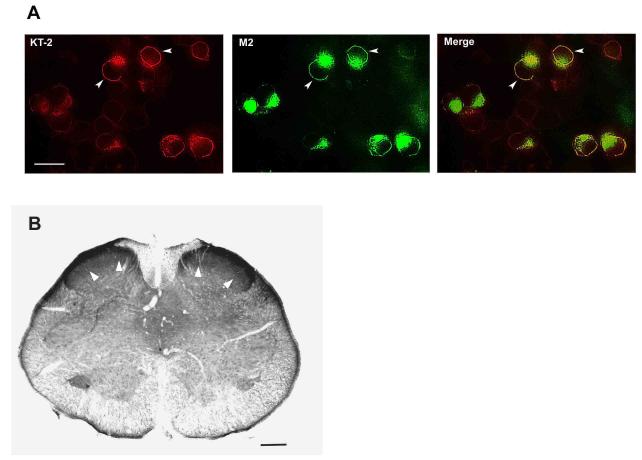
HEK293 cells were transiently transfected with pcDNA3-FLAG-rat KOPR and double labeled with the M2 monoclonal antibody against FLAG followed by AlexaFluor 488-conjugated goat anti-mouse IgG and KT-2 followed by Texas Red-conjugated goat anti-rabbit IgG. The two procedures displayed identical staining patterns with intense immunofluorescence on cell surface (Fig. 1A), indicating that there is no apparent inefficiency of recognizing cell surface KOPR by KT-2.
Fig. 1. Immunostaining of KOPR with KT-2 antibody on cells and tissue sections.
A) HEK cells were transiently transfected with FLAG-KOPR. Twenty four hours after transfection, cells were fixed and incubated with M2 monoclonal antibody and KT-2 together, followed by secondary antibodies AlexaFluor488-conjugated goat anti-mouse IgG (green) and TexasRed-conjugated goat anti-rabbit IgG (red), respectively. When the two images of transfected HEK cells taken from green and red channel were merged, most cells yielded yellowish color. Scale bar: 20 μm. B) Representative KOPR immunostaining with KT-2 antibody in rat spinal cord. Most dense immunoreactivity was found in the dorsal horn. Scale bar: 200 μm. White arrow heads: representative staining.
Immunohistochemistry was performed on rat lumbar spinal cords with KT-2. KOPR-immunoreactivity (IR) was most abundant in the superficial layers of dorsal horns (Fig. 1B), which was consistent with previous reports (Mansour et al., 1996; Harris and Drake, 2001).
Subcellular distribution of KOPR
Subcellular localization of KOPR was examined in laminae I and II of the rat lumbar spinal cord using immuno-electron microscopy. Silver grains representing KOPR-IR, were found both in presynaptic and postsynaptic sites. KOPR labeling was detected in axon terminals, axons, dendrites and somata.
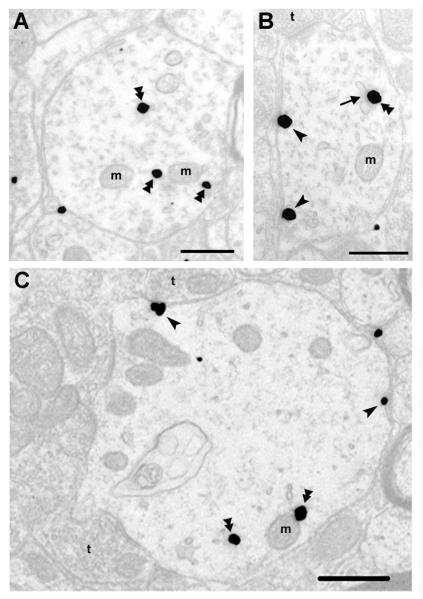
KOPR-IR was observed more frequently in dendrites than other neuronal compartments. Therefore, the analysis was focused on the distribution of KOPR in dendrites. Of dendrites examined (n = 155 ± 5 in each rat, 3 rats for the group), 27.3 % of KOPR-silver grains were associated with the plasma membranes (number of KOPR-silver grains on the cell surface/cytosol, 146 ± 13/388 ± 33)(Fig. 2 and Fig. 4). The silver grains were distributed along the intracellular face of plasma membranes, which was in accordance with the position of the epitope that KT-2 recognized, in the C-terminal domain of the KOPR. The rest of KOPR-silver grains were intracellular, associated with endosomes, mitochondria or dispersed in the cytoplasm without obvious association with any discernible organelles.
Fig. 2. Electron micrographs of KOPR labeling in lamina I and II of rat lumbar spinal cord.
Double arrowheads point to immunogold-silver labeling in the cytoplasm, while single arrowheads point to immunogold-silver labeling on the plasma membrane in dendrites from rats that received saline i.t.. t, axon terminal; m, mitochondria. A) KOPR-IR was detected in the cytoplasm without association with any discernible organelles in a dendritic profile; B) KOPR-IR was detected along the plasma membrane as well as associated with an endosome. The arrow points to an endosome with KOPR; C) Some intracellular KOPR-IR was associated with mitochondria. Scale bar = 0.5 μm.
Agonist effects on subcellular distribution of KOPR in dendrites
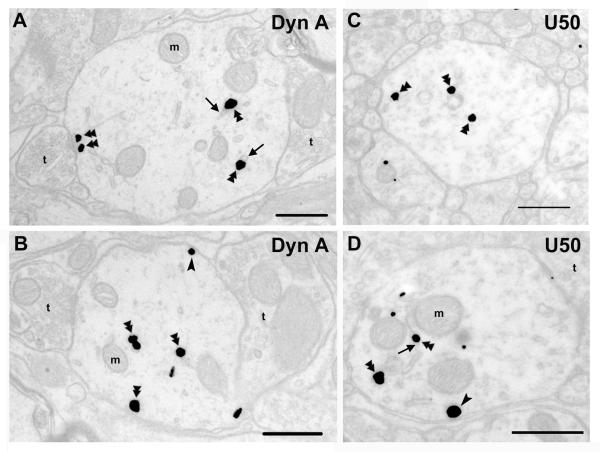
Each of the vehicle-, U50,488H- and dynorphin A(1-17)-treated groups consisted of three rats and for each rat, at least 150 dendritic profiles were examined. Dynorphin A i.t. significantly reduced the proportion of KOPR-silver grains associated with plasma membrane to 19.0 ± 1.5 % of total particles (number of KOPR-silver grains on the cell surface/cytosol, 99 ± 11/418 ± 7) (Figs. 3A, 3B and 4), compared with 27.3 ± 0.1 % in the saline control group. U50,488H i.t. did not significantly reduce the proportion of KOPR-silver grains on cell surface (24.9 ± 1.1 %; number of KOPR-silver grains on the cell surface/cytosol, 136 ± 7/409 ± 21) (Figs. 3C, 3D and 4).
Fig. 3. Subcellular distribution of KOPR-IR after acute agonist treatment.
Sections from rats that received an intrathecal injection of dynorphin A (1-17) (15 nmole) (A, B) or U50,488H (100 nmole) (C, D). The arrows point to the endosomes with KOPR. Double arrowheads point to immunogold-silver labeling in the cytoplasm, while single arrowheads point to immunogold-silver labeling on the plasma membrane. t, axon terminal; m, mitochondria. Scale bar = 0.5 μm.
Forced swim stress has been shown to activate the KOPR system (McLaughlin et al., 2003a). It is possible that stress associated with injection may release endogenous KOPR peptides and obscure the effects of exogenously administered agonists. To assess this possibility, we injected one group of 3 rats i.p. with norbinaltorphimine (norBNI, 10mg/kg), a long-acting selective KOPR antagonist, 90 min before i.t. injection of saline and perfusion. NorBNI, like other antagonists for opioid receptors, has pharmacological chaperone effects (Chen et al., 2006) which significantly increased the expression level of KOPR in CHO cells stably expressing KOPR after incubation for 4 h or longer. Therefore, a shorter time point was selected to minimize the chaperone effect of norBNI. Pretreatment of norBNI did not change the % of KOPR associated with plasma membranes in the dendrites in dorsal horns of rat lumbar spinal cords compared to the saline injected animals (data not shown).
Discussion
In this study, the effect of acute agonist treatment on subcellular distribution of the KOPR in dendrites of the rat dorsal horn was investigated. In vehicle treated animals, the majority of KOPR-IR was associated with dendrites where immunolabeling was located primarily intracellularly. While intrathecal injection of dynorphin A caused significant internalization of KOPR, U50,488H did not. To the best of our knowledge, this is the first report on internalization of the endogenous KOPR in vivo.
Differential effects of KOPR agonists on internalization of KOPR in vivo
U50,488H and dynorphin A are both full agonists for the KOPR. U50,488H is a synthetic chemical compound with high selectivity for KOPR, whereas dynorphin A is an endogenous peptide showing about 10-fold higher affinity to KOPR than to μ opioid receptor (MOPR). When injected i.t. at the doses used in this study, U50,488H was reported to induce maximal antinociceptive effects (Dawson-Basoa and Gintzler, 1996), whereas dynorphin A produced sub-maximal effects, without causing hindlimb paralysis. Thus, the lack of internalization by U50,488H was not due to an insufficient dose. This in vivo differential internalization of the rat KOPR by the two agonists is similar to the in vitro results of Jordan et al. (2000). That U50,488H did not cause internalization of the rat KOPR in vivo is similar to our in vitro observations (Li et al., 1999; Zhang et al., 2002).
Intrathecal injection of U50,488H displays antinociception effects via activation of the KOPR (Millan et al., 1989; Pelissier et al., 1990; Miaskowski et al., 1990). However, dynorphin A has more complicated in vivo effects. In addition to opioid receptor-mediated antinociception, i.t. injection of high doses of dynorphin A (20 nmole or higher) caused long-lasting paralysis of hindlimbs which can not be antagonized by naloxone (Przewlocki et al., 1983a; Herman and Goldstein, 1985). In addition, Vanderah et al. (1996) reported that dynorphin A i.t. at non-paralysis doses (15 nmole) produced long-lasting allodynia in rats in which the NMDA receptor, but not opioid receptors, was suggested to be involved. The effect of i.t. dynorphin A (15 nmole) on KOPR distribution in the current study is most likely directed against the KOPR. However, the possibility can not be ruled out that its activation of non-KOPR targets may indirectly affect trafficking of the KOPR.
Although the number of KOPR on cell surface was modest, only ~30% of cell surface KOPR was internalized by dynorphin A. One likely explanation is that this reflects the extent dynorphin A-promoted internalization of endogenous KOPR. Another possibility is that internalization of the KOPR is offset by fast recycling of endocytosed KOPR and/or targeting of intracellular KOPR to plasma membrane, similar to what Stroh et al. (2000) reported for sst5 somatostatin receptor, resulting in apparent lack of internalization. It will be interesting to study the kinetics of internalization and recycling of endogenous KOPR. A third possibility is that dynorphin A may cause translocation of KOPR from other segments of the dendrites into the regions analyzed and resulted in the lower ratio of surface KOPR to the total receptors. However, we think it is not likely since we did not observe a significant increase in the silver grains in dendrites of the dynorphin A-treated group.
Subcellular distribution of KOPR
The finding that abundant KOPR-IR was present in both pre- and post-synaptic sites is in accordance with the report that KOPR binding sites were decreased by 53% in the superficial layers of the dorsal horn of the rat cervical spinal cord after unilateral dorsal rhizotomy (Besse et al., 1990).
The majority of KOPR-IR was located intracellularly in dendrites in the dorsal horn of rat spinal cords. This finding was not likely to result from inefficient recognition of cell surface KOPR by KT-2 since the staining pattern of KT-2 and M2 was identical in HEK293 cells transiently expressing FLAG-tagged rat KOPR (see Fig. 1).
Our observation that endogenous KOPR has a significant intracellular pool is in accordance with several reports. Harris et al. (2004) demonstrated previously that ~55% of KOPR-IR was located intracellularly in the dendrites of rat spinal cords of both sexes. In axon terminals, ~55% and 70% of KOPR-IR were intracellular in male and female rats, respectively. In the axon terminals in rat posterior pituitary, ~60% of KOPR-IR was associated with large secretory vesicles and only ~11% with plasma membrane (Shuster et al., 1999). In our study, male rats were used.
Handling of the rats during experiments may cause acute stress which may trigger the release of dynorphin peptides and affect subcellular distribution of the KOPR. Therefore, all the animals following intrathecal cannulation were acclimated by being handled daily in the same way for at least 5 days as on the experiment day. Furthermore, pretreatment of animals with norBNI did not affect cell surface KOPR-IR, indicating that handling does not affect subcellular distribution of the KOPR.
Differences between spinal cord and transfected cells in % of the KOPR on cell surface
In contrast to the low % of the KOPR on cell membranes in vivo, most of the receptors were found on the cell surface in cell lines transiently or stably transfected with the rat KOPR (Li et al., 1999; Zhang et al., 2002; McLaughlin et al., 2003b). The differences may be due to differences in receptor number and/or levels of the molecules involved in trafficking of the receptor in both the biosynthesis and endocytosis pathways.
The discrepancy in distribution between in vitro and in vivo also exists for DOPR. DOPR is mainly expressed on the cell surface in cell lines (Eisinger and Schulz, 2004). However, 80-90% of DOPR-IR was found intracellularly in dendrites of the rat spinal cord dorsal horn (Cahill et al., 2001a) or striatum (Wang and Pickel, 2001) and even more in somata (Cahill et al., 2001a). In knock-in mice expressing DOPR-EGFP, ~ 60% of DOPR-EGFP was on cell surface (Scherrer et al., 2006).
In contrast, MOPR was predominantly localized on the cell surface both in cultured cells and in vivo, such as the locus coeruleus (Van Bockstaele and Commons, 2001) and striatum (Wang and Pickel, 2001).
Therefore, opioid receptors showed differential subcellular distribution, with the MOPR mostly on cell surface and DOPR and KOPR mainly intracellular, which suggests differential targeting, transport and even mechanisms of action of opioid receptors.
Functional significance of intracellular KOPRs
The large intracellular pool of KOPR in the steady state may be a reserve pool waiting for stimuli to be trafficked to cell surface. The majority of KOPR in vasopressin-containing magnocellular neurosecretory neurons in the rat hypothalamus was associated with large secretory vesicles (Shuster et al., 1999). When salt loading caused release of vasopressin, the KOPR was translocated to cell surface along with the fusion of secretory vesicles with plasma membranes (Shuster et al., 1999). However, the intracellular KOPR in the dorsal horns was mostly dispersed in the cytosol without association with any organelles. Whether the intracellular KOPR is translocated to cell surface under certain physiological or pathophysiological conditions require further investigation.
Intracellular DOPR has been shown to be trafficked to plasma membranes under certain conditions. In rats with chronic inflammation pain induced by subcutaneous injection of complete Freud adjuvant in the hindpaw, the DOPR on the cell surface was increased by one fold in the dendrites of dorsal horn on ipsilateral side (Cahill et al., 2003). A significant subset of DOPR was also recruited to regions close to the plasma membrane (1-199 nm) (Cahill et al., 2003). These findings suggested that the intracellular DOPR can be trafficked to the cell surface upon pain stimuli, leading to enhancement of the analgesic effects of opiates. Chronic morphine treatment has similar effects (Cahill et al., 2001b). It is not known whether KOPR can be regulated in a similar manner. However, the antinociceptive effects of KOPR agonist microinjected into rostral ventromedial medulla were significantly enhanced in rats with chronic inflammation following injection of complete Freud adjuvant in the hindpaw (Schepers et al., 2007). It will be interesting to investigate whether the intracellular KOPR are trafficked to cell surface under such condition.
In summary, we found endogenous KOPR in the rat dorsal horns has a large intracellular pool in the dendrites. KOPR agonists showed differential effects on internalization of endogenous KOPR in vivo.
Acknowledgments
Grant information: This study was supported by the NIH grant DA17302 and Pennsylvania Department of Health (Tobacco Settlement Formula Funds) to Dr. Lee-Yuan Liu-Chen and DA09082 to Dr.Elisabeth J. Van Bockstaele.
References
- Arvidsson U, Riedl M, Chakrabarti S, Vulchanova L, Lee JH, Nakano AH, Lin X, Loh HH, Law P-Y, Wessendorf MW, Elde R. The kappa-opioid receptor is primarily postsynaptic: combined immunohistochemical localization of the receptor and endogenous opioids. Proc.Natl.Acad.Sci.U.S.A. 1995;92:5062–5066. doi: 10.1073/pnas.92.11.5062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besse D, Lombard MC, Zajac JM, Roques BP, Besson JM. Pre- and postsynaptic distribution of mu, delta and kappa opioid receptors in the superficial layers of the cervical dorsal horn of the rat spinal cord. Brain Res. 1990;521:15–22. doi: 10.1016/0006-8993(90)91519-m. [DOI] [PubMed] [Google Scholar]
- Cahill CM, McClellan KA, Morinville A, Hoffert C, Hubatsch D, O’Donnell D, Beaudet A. Immunohistochemical distribution of delta opioid receptors in the rat central nervous system: evidence for somatodendritic labeling and antigen-specific cellular compartmentalization. J.Comp Neurol. 2001a;440:65–84. doi: 10.1002/cne.1370. [DOI] [PubMed] [Google Scholar]
- Cahill CM, Morinville A, Hoffert C, O’Donnell D, Beaudet A. Up-regulation and trafficking of delta opioid receptor in a model of chronic inflammation: implications for pain control. Pain. 2003;101:199–208. doi: 10.1016/s0304-3959(02)00333-0. [DOI] [PubMed] [Google Scholar]
- Cahill CM, Morinville A, Lee MC, Vincent JP, Collier B, Beaudet A. Prolonged morphine treatment targets delta opioid receptors to neuronal plasma membranes and enhances delta-mediated antinociception. J.Neurosci. 2001b;21:7598–7607. doi: 10.1523/JNEUROSCI.21-19-07598.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Chen C, Wang Y, Liu-Chen LY. Ligands regulate cell surface level of the human {kappa} opioid receptor by activation-induced down-regulation and pharmacological chaperone-mediated enhancement: Differential effects of nonpeptide and peptide agonists. J.Pharmacol.Exp.Ther. 2006;319:765–775. doi: 10.1124/jpet.106.107987. [DOI] [PubMed] [Google Scholar]
- Dawson-Basoa ME, Gintzler AR. Estrogen and progesterone activate spinal kappa-opiate receptor analgesic mechanisms. Pain. 1996;64:169–177. doi: 10.1016/0304-3959(95)00092-5. [DOI] [PubMed] [Google Scholar]
- Drake CT, Patterson TA, Simmons ML, Chavkin C, Milner TA. Kappa opioid receptor-like immunoreactivity in guinea pig brain: ultrastructural localization in presynaptic terminals in hippocampal formation. J.Comp Neurol. 1996;370:377–395. doi: 10.1002/(SICI)1096-9861(19960701)370:3<377::AID-CNE8>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Eisinger DA, Schulz R. Extracellular signal-regulated kinase/mitogen-activated protein kinases block internalization of delta-opioid receptors. J.Pharmacol.Exp.Ther. 2004;309:776–785. doi: 10.1124/jpet.103.061788. [DOI] [PubMed] [Google Scholar]
- Harris JA, Chang PC, Drake CT. Kappa opioid receptors in rat spinal cord: sex-linked distribution differences. Neuroscience. 2004;124:879–890. doi: 10.1016/j.neuroscience.2003.12.042. [DOI] [PubMed] [Google Scholar]
- Harris JA, Drake CT. Kappa opioid receptor density is consistent along the rostrocaudal axis of the female rat spinal cord. Brain Res. 2001;905:236–239. doi: 10.1016/s0006-8993(01)02515-x. [DOI] [PubMed] [Google Scholar]
- Herman BH, Goldstein A. Antinociception and paralysis induced by intrathecal dynorphin A. J.Pharmacol.Exp.Ther. 1985;232:27–32. [PubMed] [Google Scholar]
- Jordan BA, Cvejic S, Devi LA. Kappa opioid receptor endocytosis by dynorphin peptides. DNA Cell Biol. 2000;19:19–27. doi: 10.1089/104454900314672. [DOI] [PubMed] [Google Scholar]
- Li J, Li J-G, Chen C, Zhang F, Liu-Chen L-Y. Molecular basis of differences in (−)(trans)-3,4-dichloro-N-methyl-N-[2-(1-pyrrolidiny)-cyclohexyl]benzeneacetamide-induced desensitization and phosphorylation between human and rat κ-opioid receptors expressed in Chinese hamster ovary cells. Mol.Pharmacol. 2002;61:73–84. doi: 10.1124/mol.61.1.73. [DOI] [PubMed] [Google Scholar]
- Li J-G, Luo LY, Krupnick JG, Benovic JL, Liu-Chen L-Y. U50,488H-induced internalization of the human kappa opioid receptor involves a beta-arrestin- and dynamin-dependent mechanism. Kappa receptor internalization is not required for mitogen-activated protein kinase activation. J.Biol.Chem. 1999;274:12087–12094. doi: 10.1074/jbc.274.17.12087. [DOI] [PubMed] [Google Scholar]
- Liu-Chen L-Y. Agonist-induced regulation and trafficking of kappa opioid receptors. Life Sci. 2004;75:511–536. doi: 10.1016/j.lfs.2003.10.041. [DOI] [PubMed] [Google Scholar]
- Mansour A, Burke S, Pavlic RJ, Akil H, Watson SJ. Immunohistochemical localization of the cloned kappa 1 receptor in the rat CNS and pituitary. Neuroscience. 1996;71:671–690. doi: 10.1016/0306-4522(95)00464-5. [DOI] [PubMed] [Google Scholar]
- Mansour A, Khachaturian H, Lewis ME, Akil H, Watson SJ. Anatomy of CNS opioid receptors. Trends Neurosci. 1988;11:308–314. doi: 10.1016/0166-2236(88)90093-8. [DOI] [PubMed] [Google Scholar]
- McLaughlin JP, Marton-Popovici M, Chavkin C. Kappa opioid receptor antagonism and prodynorphin gene disruption block stress-induced behavioral responses. J Neurosci. 2003a;23:5674–5683. doi: 10.1523/JNEUROSCI.23-13-05674.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin JP, Xu M, Mackie K, Chavkin C. Phosphorylation of a carboxy-terminal serine within the kappa opioid receptor produces desensitization and internalization. J.Biol.Chem. 2003b;278:34631–34640. doi: 10.1074/jbc.M304022200. [DOI] [PubMed] [Google Scholar]
- Miaskowski C, Taiwo YO, Levine JD. Kappa- and delta-opioid agonists synergize to produce potent analgesia. Brain Res. 1990;509:165–168. doi: 10.1016/0006-8993(90)90327-8. [DOI] [PubMed] [Google Scholar]
- Millan MJ, Czlonkowski A, Lipkowski A, Herz A. Kappa-opioid receptor-mediated antinociception in the rat. II. Supraspinal in addition to spinal sites of action. J.Pharmacol.Exp.Ther. 1989;251:342–350. [PubMed] [Google Scholar]
- Morris BJ, Herz A. Distinct distribution of opioid receptor types in rat lumbar spinal cord. Naunyn Schmiedebergs Arch.Pharmacol. 1987;336:240–243. doi: 10.1007/BF00165811. [DOI] [PubMed] [Google Scholar]
- Pelissier T, Paeile C, Soto-Moyano R, Saavedra H, Hernandez A. Analgesia produced by intrathecal administration of the kappa opioid agonist, U-50,488H, on formalin-evoked cutaneous pain in the rat. Eur.J.Pharmacol. 1990;190:287–293. doi: 10.1016/0014-2999(90)94192-z. [DOI] [PubMed] [Google Scholar]
- Peters A, Palay SL, Webster Hd. The Fine Structure of the Nervous System, neurons and their supporting cells. Oxford University Press; New York: 1991. [Google Scholar]
- Przewlocki R, Shearman GT, Herz A. Mixed opioid/nonopioid effects of dynorphin and dynorphin related peptides after their intrathecal injection in rats. Neuropeptides. 1983a;3:233–240. doi: 10.1016/0143-4179(83)90019-7. [DOI] [PubMed] [Google Scholar]
- Przewlocki R, Stala L, Greczek M, Shearman GT, Przewlocka B, Herz A. Analgesic effects of mu-, delta- and kappa-opiate agonists and, in particular, dynorphin at the spinal level. Life Sci. 1983b;33(Suppl 1):649–652. doi: 10.1016/0024-3205(83)90586-6. [DOI] [PubMed] [Google Scholar]
- Schepers RJ, Mahoney JL, Shippenberg TS. Inflammation-induced changes in rostral ventromedial medulla mu and kappa opioid receptor mediated antinociception. Pain. 2007 doi: 10.1016/j.pain.2007.07.010. [DOI] [PubMed] [Google Scholar]
- Scherrer G, Tryoen-Toth P, Filliol D, Matifas A, Laustriat D, Cao YQ, Basbaum AI, Dierich A, Vonesh JL, Gaveriaux-Ruff C, Kieffer BL. Knockin mice expressing fluorescent delta-opioid receptors uncover G protein-coupled receptor dynamics in vivo. Proc.Natl.Acad.Sci.U.S.A. 2006;103:9691–9696. doi: 10.1073/pnas.0603359103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz R, Wehmeyer A, Schulz K. Visualizing preference of G protein-coupled receptor kinase 3 for the process of kappa-opioid receptor sequestration. Mol.Pharmacol. 2002;61:1444–1452. doi: 10.1124/mol.61.6.1444. [DOI] [PubMed] [Google Scholar]
- Shuster SJ, Riedl M, Li X, Vulchanova L, Elde R. Stimulus-dependent translocation of kappa opioid receptors to the plasma membrane. J.Neurosci. 1999;19:2658–2664. doi: 10.1523/JNEUROSCI.19-07-02658.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spampinato S, Candeletti S. Characterization of dynorphin A-induced antinociception at spinal level. Eur.J.Pharmacol. 1985;110:21–30. doi: 10.1016/0014-2999(85)90024-x. [DOI] [PubMed] [Google Scholar]
- Stroh T, Jackson AC, Sarret P, Dal Farra C, Vincent JP, Kreienkamp HJ, Mazella J, Beaudet A. Intracellular dynamics of sst5 receptors in transfected COS-7 cells: maintenance of cell surface receptors during ligand-induced endocytosis. Endocrinol. 2000;141:354–365. doi: 10.1210/endo.141.1.7259. [DOI] [PubMed] [Google Scholar]
- Vanderah TW, Laughlin T, Lashbrook JM, Nichols ML, Wilcox GL, Ossipov MH, Malan TP, Jr, Porreca F. Single intrathecal injections of dynorphin A or des-Tyr-dynorphins produce long-lasting allodynia in rats: blockade by MK-801 but not naloxone. Pain. 1996;68(2-3):275–81. doi: 10.1016/s0304-3959(96)03225-3. [DOI] [PubMed] [Google Scholar]
- Van Bockstaele EJ, Commons KG. Internalization of mu-opioid receptors produced by etorphine in the rat locus coeruleus. Neuroscience. 2001;108:467–477. doi: 10.1016/s0306-4522(01)00426-2. [DOI] [PubMed] [Google Scholar]
- Wang H, Pickel VM. Preferential cytoplasmic localization of delta-opioid receptors in rat striatal patches: comparison with plasmalemmal mu-opioid receptors. J.Neurosci. 2001;21:3242–3250. doi: 10.1523/JNEUROSCI.21-09-03242.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood PL, Rackham A, Richard J. Spinal analgesia: comparison of the mu agonist morphine and the kappa agonist ethylketazocine. Life Sci. 1981;28:2119–2125. doi: 10.1016/0024-3205(81)90618-4. [DOI] [PubMed] [Google Scholar]
- Zhang F, Li J, Li J-G, Liu-Chen L-Y. (−)U50,488H [(trans)-3,4-dichloro-N-methyl-N-[2-(1-pyrrolidinyl)-cyclohexyl]benzeneace tamide] induces internalization and down-regulation of the human, but not the rat, kappa-opioid receptor: Structural basis for the differential regulation. J.Pharmacol.Exp.Ther. 2002;302:1184–1192. doi: 10.1124/jpet.302.3.1184. [DOI] [PubMed] [Google Scholar]