Abstract
Ubiquitination of the human κ opioid receptor (hKOR) expressed in CHO cells was observed in the presence of the proteasomal inhibitor MG132 and enhanced by the agonists U50,488H and dynorphin A (Dyn A). The dominant negative (DN) mutants GRK2-K220R and β-arrestin (319–418), but not dynamin I-K44A, reduced Dyn A–stimulated hKOR ubiquitination and a phosphorylation-defective hKOR mutant (hKOR-S358N) did not undergo Dyn A-stimulated ubiquitination, indicating that hKOR ubiquitination is enhanced by receptor phosphorylation, but not by receptor internalization. A hKOR mutant (hKOR-10 KR) in which all ten intracellular Lys residues were changed to Arg showed greatly reduced basal and agonist-promoted receptor ubiquitination and substantially decreased Dyn A–induced receptor down-regulation, without changing ligand binding affinity, receptor-G protein coupling or receptor internalization or desensitization. The ubiquitination sites were further determined to be the three Lys residues in the C-terminal domain. The K63R ubiquitin mutant decreased Dyn A-induced hKOR ubiquitination and down-regulation, but the K48R mutant did not. Expression of HN-CYLD, a DN mutant of the deubiquitinating enzyme CYLD that breaks Lys 63-linked polyubiquitin chain, increased Dyn A-induced hKOR ubiquitination and down-regulation. These results indicate that ubiquitinated hKOR following agonist treatment contains predominantly Lys63-linked polyubiquitin chains and ubiquitination of the hKOR involved in agonist-induced down-regulation.
Effects of opioid drugs and endogenous peptides are mediated by opioid receptors. There are at least three types of opioid receptors - μ, δ and κ The opioid receptors, belonging to the rhodopsin sub-family of the seven transmembrane domain receptor (7TMR) family, are coupled to pertussis toxin-sensitive Gi/Go proteins and activation of their effectors include adenylate cyclase, potassium and calcium channels, and mitogen-activated protein kinase pathways [for a review, see (Law et al., 2000)]. Activation of the κ opioid receptor (KOR) produces many effects including analgesia, water diuresis, dysphoria, and antipruritic effects [see (Liu-Chen, 2004) for references].
After exposure to some agonists, the human KOR expressed in CHO cells or HEK293 cells undergoes internalization [for a review, (Liu-Chen, 2004)] via G protein-coupled receptor kinase (GRK)-, arrestin- and dynamin-dependent pathways that likely involve clathrin-coated vesicles (Li et al., 1999), similar to many other 7TMRs. Following internalization, the receptor is recycled to plasma membranes or trafficked to lysosomes and proteasomes for degradation. We have demonstrated that EBP50/NHERF-1 interacts with the C-terminus of the hKOR, probably in a protein complex, and this association appears to serve as a signal for internalized hKOR to be sorted to the recycling pathway (Li et al., 2002b). In contrast, the signals that direct internalized receptors to degradation pathways have not been fully elucidated. Two biochemical mechanisms are associated with sorting of internalized 7TMRs to lysosomes: proteins associated with receptors and ubiquitination of the receptor. Proteins associated with 7TMRs include sorting nexin 1 for protease-activated receptor 1 (PAR1) (Wang et al., 2002) and GPCR-associated sorting protein (GASP) for the δ opioid receptor (Whistler et al., 2002). Ubiquitination of β2-adrenergic (β2-AR) (Shenoy et al., 2001), chemokine CXCR4 (Marchese and Benovic, 2001; Marchese et al., 2003), V2 vasopressin (Martin et al., 2003) and neurokinin-1 (NK-1) (Cottrell et al., 2006) receptors and protease-activated receptor 2 (PAR2) (Jacob et al., 2005) is important for sorting of the internalized receptors to lysosomes; however, ubiquitination appears not to play a role in the trafficking of the δ opioid receptor (Chaturvedi et al., 2001; Tanowitz and von Zastrow, 2002)
Ubiquitination is an important post-translational modification, by which ubiquitin, a 76-amino acid polypeptide, is conjugated to a substrate protein through the formation of an isopeptide bond between the ε amino group of Lys residues in target proteins and the C-terminal glycine of ubiquitin [for a review, see (Hershko and Ciechanover, 1998)]. Ubiquitination is catalyzed by three enzymes acting in tandem: an E1, a ubiquitin-activating enzyme, an E2, a ubiquitin-carrying enzyme and an E3, a ubiquitin ligase. There are about 400 E3 ubiquitin ligases, which interact directly with the target protein or indirectly through an adapter protein and confer specificity. For mono- and multi-mono-ubiquitination, single ubiquitin molecules are conjugated to one or more Lys residues. Since ubiquitin contains Lys residues, multiple ubiquitins can be linked to one or more Lys residues of the substrate sequentially, termed polyubiquitination. Only a small number of 7TMRs have been examined for ubiquitination and both mono- or poly-ubiquitination have been reported. Ubiquitination of 7TMRs has been shown to be involved in agonist-induced internalization, ER-associated degradation of misfolded receptors and sorting of internalized receptor to lysosomes for degradation [for a review, see (Shenoy, 2007)].
Our previous observations that U50,488H-induced down-regulation of the hKOR expressed in CHO cells was partially reduced by the proteasome inhibitor MG132 or the lysosome inhibitor chloroquine, and almost totally abolished by the combination of both indicate that lysosomes as well as proteasomes are involved in agonist-induced down-regulation of the hKOR (Li et al., 2000).
In this study, we found that the hKOR expressed in CHO cells was ubiquitinated following exposure to an agonist. We examined whether agonist-induced ubiquitination of the hKOR is regulated by receptor phosphorylation and internalization. We then determined the functional significance of ubiquitination of the hKOR by generating a hKOR mutant which was not ubiquitinated, and comparing the mutant with the wildtype in receptor function, trafficking and regulation. We also tested the involvement of Lys63- or Lys48-linked polyubiquitination in the ubiquitination and regulation of the hKOR.
Materials and Methods
Materials
[35S]GTPγS (~1,250 Ci/mmol) and [3H]diprenorphine (~58 Ci/mmol) were purchased from Perkin-Elmer Co. (Boston, MA). Dyn A was purchased from Phoenix Pharmaceuticals (Belmont, CA). U50,488H and naloxone were provided by the National Institute on Drug Abuse (Bethesda, MD). The following reagents were bought from the indicated companies: rabbit polyclonal antibody against the FLAG epitope (F7425), anti-FLAG M2 affinity gel, peroxidase-conjugated goat anti-rabbit IgG, chloroquine and MG132 from Sigma Co. (St. Louis, MO), polyclonal rabbit anti-HA antibody from Zymed Co. (San Francisco, CA), anti-HA monoclonal antibody (HA-11) from Covance (Cumbeland, VA), Alexa Fluor® 488-conjugated goat anti-mouse IgG from Molecular Probes (Eugene, OR), and SuperSignal West Pico chemiluminescent substrate kit from Pierce Chemical (Rockford, IL). The HA-ubiquitin (HA-Ub) expression plasmid was kindly provided by Dr. D. Bohmann of University of Rochester. Dynamin I-K44A, β-arrestin (319–418) and GRK2-K220R were gifts from Dr. Jeffrey Benovic of Thomas Jefferson University; HA-Ub, HA-Ub-K63R, HA-Ub-K48R, CYLD and HN-CYLD from Dr. Marie Wooten of Auburn University.
Construction of FLAG-hKOR-10KR cDNA
cDNA encoding human κ opioid receptor with N-terminal FLAG epitope (FLAG-hKOR) in pcDNA3 was generated previously (Xu et al., 2000). A FLAG-hKOR mutant in which all ten predicted intracellular lysines (at positions 89, 91, 165, 174, 176, 254, 265, 338, 349, and 378) were replaced with arginines was generated using point mutations and the overlap polymerase chain reaction (PCR) method with the FLAG-hKOR in pcDNA3 as the template. Mutated cDNA (named FLAG-hKOR-10KR) was cloned into pcDNA3, and DNA sequence determination was performed to ensure correct generations of all mutations and no unwanted mutations.
Stable expression of FLAG-hKOR-10KR in CHO cells
Clonal CHO cells stably transfected with the FLAG-hKOR-10KR cDNA (CHO-FLAG-hKOR-10KR) were established and a cell line with a similar receptor expression level to CHO cells stably transfected with the FLAG-hKOR cDNA (CHO-FLAG-hKOR) (Li et al., 2002a) was selected for this study. CHO-FLAG-hKOR and CHO-FLAG-hKOR-10KR cells were cultured in Dulbecco's modified Eagle's medium F12 HAM supplemented with 10% fetal calf serum, 0.1 mg/ml geneticin, 100 units/ml penicillin and 100 μg/ml streptomycin in a humidified atmosphere consisting of 5% CO2 and 95% air at 37°C.
Ubiquitination of FLAG-hKOR
The experiment was performed according to a modification of our published procedure (Li et al., 2002b). CHO cells in 100-mm dishes were grown to ~90% confluence and transiently transfected with 8 μg HA-Ub and 10 μg FLAG-hKOR, FLAG-hKOR-10KR, FLAG-hKOR-S358N or the vector pcDNA3. CHO-FLAG-hKOR cells were transiently transfected with 8 μg HA-Ub and 10 μg dynamin I-K44A, β-arrestin (319–418) or GRK2-K220R. Approximately two days later, cells were treated with 50 μM MG132 for 3 h and then 1 μM Dyn A was added for another 30 min. Then cells were lysed, solubilized with solubilization buffer (50 mM Tris HCl, 150 mM NaCl, 1 mM EDTA, 2% Triton X-100, 20 μM NEM, pH 7.4) for 1 h at 4°C and centrifuged at 100,000 x g for 1 h. Solubilized materials were incubated for overnight at 4°C with anti-FLAG M2 affinity gel to immunoprecipitate FLAG-hKOR or with HA-11 to immunoprecipitate ubiquitinated hKOR. The immunoprecipitated materials were washed and Laemmli sample buffer was added with 100 mM dithiothreitol (DTT). For removal of proteins immunoprecipitated with FLAG-hKOR, 50 μl 2% SDS sample buffer was added to immunoprecipitated materials. The samples were then incubated with 950 μl solubilization buffer and 50 μl anti-FLAG M2 affinity gel for 1 h at 4°C. Then the immunoprecipitated materials were washed and Laemmli sample buffer was added with 100 mM DTT. Samples were subjected to SDS-PAGE and transferred onto PVDF Immobilon membranes. Membranes were blotted with a rabbit polyclonal antibody against HA or rabbit polyclonal antibody against the FLAG epitope followed by goat anti-rabbit polyclonal IgG conjugated with horseradish peroxidase and reacted with ECL western blotting detection reagents. Images were captured by use of a FujiFilm LAS-1000 plus system.
Quantitation of receptor down-regulation by immunoblotting
Immunoblotting was performed to examine the level of the FLAG-hKOR proteins as described previously (Chen et al., 2006b). Briefly, CHO-FLAG-hKOR and CHO-FLAG-hKOR-10KR cells in 12-well plates were treated with or without 1 μM Dyn A for 4 h at 37°C. Cells were solubilized with 2X Laemmli sample buffer, and then subjected to SDS-PAGE and transferred onto PVDF Immobilon membrane. Immunoblotting was performed with a rabbit polyclonal antibody against FLAG, goat anti-rabbit polyclonal IgG conjugated with horseradish peroxidase and ECL western blotting detection reagents. The results were detected with a Fuji Film LAS-1000 system and quantitated by ImageGauge (version 4.1, Fuji PhotoFilm Co. Ltd.).
Quantitation of cell surface receptors by fluorescence flow cytometry and determination of receptor internalization and down-regulation
were performed according to our published procedure (Li et al., 2003). Briefly, CHO-FLAG-hKOR or CHO-FLAG-hKOR-10KR cells in grown in 12-well plates were treated with or without 10 μM U50,488H for 30 min (for internalization), or 1 μM Dyn A for 4 h (for down-regulation) at 37°C. Cells were washed 3 times with ice-cold PBS buffer (58 mM Na2HPO4, 17 mM NaH2PO4 and 68 mM NaCl), and lifted with PBS buffer containing 0.5 mM EDTA. Cells were incubated with M1 anti-FLAG antibody (1 μg/ml) in 300 μl of Opti-MEMI reduced serum medium containing 1 mM CaCl2 for 45 min at 4°C. After 3 washes with PBS buffer, cells were incubated with Alexa Fluor® 488-conjugated goat anti-mouse IgG (1 μg/ml) in 300 μl of Opti-MEMI reduced serum medium containing 1 mM CaCl2 for 45 min at 4°C. Cells were washed 3 times with ice-cold PBS buffer containing 1 mM CaCl2, and then re-suspended with 300 μl PBS buffer. Immunoreactivity of cell surface receptors was quantitated by fluorescence flow cytometry (FACScan, BD Biosciences, San Jose, CA). Reduction in cell surface immunofluorescence was used to as the measures of receptor internalization and down-regulation.
κ opioid receptor binding
Membrane preparations and receptor binding of CHO-FLAG-hKOR and CHO-FLAG-hKOR-10KR cells was conducted with [3H]diprenorphine in 50 mM Tris-HCl buffer as described previously (Li et al., 1999). Naloxone (10 μM) was used to define nonspecific binding. Saturation experiments were performed with various concentrations of [3H]diprenorphine (ranging from 0.02 nM to 2 nM) to determine total receptor number. Competitive inhibition of [3H]diprenorphine binding was performed with [3H]diprenorphine at a concentration ~0.3 nM and various concentrations of U50,488H or Dyn A to determine the affinity of their binding to receptor. Binding was conducted at 25°C for 60 min in duplicate in a volume of 1 ml with about 15 μg membrane protein. Bound and free [3H]diprenorphine were separated by rapid filtration under reduced pressure over GF/B filters pre-soaked with 0.2% polyethylenimine and 0.1% bovine serum albumin in 50 mM Tris-HCl (pH 7.4) for 1 h. Binding data were analyzed with non-linear regression in Prism 3.0 (GraphPad Software, Inc., San Diego, CA) and the data fitted a one-site binding curve.
[35S]GTPγS Binding and U50,488H-induced desensitization
Membrane preparations and [35S]GTPγS binding were performed according to Li et al. (2002a). Briefly, cells were treated without or with 1 μM U50,488H for 30 min and washed three times with cold PBS buffer. Then, the cells were pelleted and ice-cold lysis buffer (5 mM Tris-HCl, 5 mM EDTA, 5 mM EGTA, 0.1 mM PMSF, 10μM leupeptin, 10 mM sodium fluoride and 10 mM tetrasodium pyrophosphate, pH 7.4) was added. Cell suspension was passed through a 1-ml 29G3/8 syringe needle five times and centrifuged. Pellets were re-suspended in 50 mM Tris-HCl buffer (pH 7.4), passed through the syringe needle and centrifuged and the processes were repeated one more time. Membranes were suspended in 50 mM Tris-HCl buffer (pH 7.4), protein contents were determined by the BCA method, and membranes were frozen at −80°C until use. About 10 μg membrane protein was incubated without or with different concentration of U50,488H, 15 μM GDP and 0.2 nM [35S]GTPγS in reaction buffer (50 mM HEPES, 100 mM NaCl, 5 mM MgCl2, 1 mM EDTA, and 0.1% BSA, pH7.4) in a final volume of 0.5 ml. After 60 min incubation at 30°C, bound and free [35S]GTPγS were separated by filtration with GF/B filters. Radioactivity on filters was determined by liquid scintillation counting.
Statistical analysis
For comparison of multiple groups, data were analyzed with analysis of variance to determine if there were significant differences among groups using Prism 3.0 (GraphPad Software, Inc., San Diego, CA). If so, Dunnett’s post hoc test was performed to determine whether there was significant difference between the control and each treatment group. For comparison of two groups, two-tailed Student's t test was performed. P<0.05 was the level of significance in all statistical analyses.
Results
The hKOR was ubiquitinated and agonists enhanced its ubiquitination
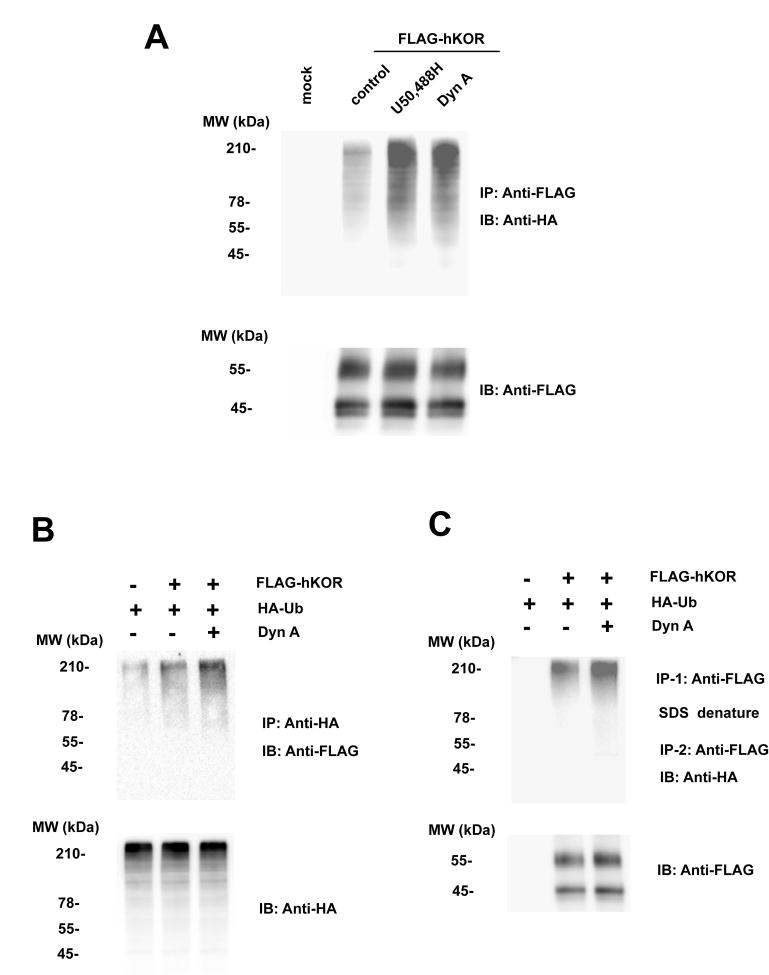
CHO cells were transiently transfected with HA-ubiquitin (HA-Ub) and FLAG-hKOR or the vector pcDNA3 and treated with 50 μM MG132 and then with or without 1 μM U50,488H or 1 μM Dyn A for 30 minutes. Ubiquitination of the receptor was examined by immunoprecipitation of FLAG-hKOR with anti-FLAG antibody and immunoblotting with anti-HA antibody (Fig. 1A). Alternatively, ubiquitinated FLAG-hKOR was detected by immunoprecipitation with anti-HA antibody followed by immunoblotting with anti-FLAG antibody (Fig. 1B). As shown in Fig. 1, compared with pcDNA-transfected control, the hKOR was ubiquitinated in the presence of MG132. There was no detectable ubiquitination of hKOR without MG132 treatment (data not shown). Incubation with U50,488H or Dyn A increased ubiquitination of the receptor (Fig. 1). We also carried out immunoprecipitation under conditions which removed associated proteins. The first immunoprecipitation was performed with anti-FLAG antibody and the immunoprecipitated materials were denatured with 2% SDS to dissociate proteins immunoprecipitated with hKOR. Triton X-100 was added to surround SDS and the second immunoprecipitation was then conducted with anti-FLAG antibody. Immunoblotting was performed with anti-HA antibody. Under these conditions, we also found that Dyn A induced hKOR ubiquitination (Fig. 1C). It appears that ubiquitinated hKOR represents a small % of total receptors.
Fig. 1. Ubiquitination of the hKOR was enhanced by U50,488H and Dyn A.
CHO cells were transiently transfected with HA-Ub and FLAG-hKOR or the vector pcDNA3. Approximately two days later, cells were treated with 50 μM MG132 for 3 h and then without or with 10 μM U50,488H or 1 μM Dyn A for another 30 min. (A) Cells were lysed, solubilized and immunoprecipitated with anti-FLAG M2 affinity gel and western blot was performed using rabbit anti-HA antibody (upper) or rabbit anti-FLAG antibody (lower) as described in Materials and Methods. (B) Cells were lysed, solubilized and immunoprecipitated with anti-HA monoclonal antibody and western blot was performed using rabbit anti-FLAG antibody (upper) or rabbit anti-HA antibody (lower) as described in Materials and Methods. (C) Cells were lysed, solubilized and immunoprecipitated with anti-FLAG M2 affinity gel, treated with 2% SDS Laemmli sample buffer followed by addition of excess Triton and then immunoprecipitation with anti-FLAG M2 affinity gel again. Immnoblotting was performed using rabbit anti-HA antibody (upper) or rabbit anti-FLAG antibody (lower) as described in Materials and Methods. Each figure represents one of three independent experiments performed with similar results.
FLAG-hKOR expressed in CHO cells migrated as two bands with Mr’s of 45 kDa and 55 kDa (Fig. 1), similar to our previous findings (Chen et al., 2006a). In addition, the 55-kDa band represents the fully glycosylated FLAG-hKORs (mature form), most of which locates in plasma membranes, whereas the 45-kDa band represents N-linked glycosylated high-mannose intermediates (immature form), which mainly locate in ER and cis-Golgi (Chen et al., 2006a). Treatment with 1 μM U50,488H or 1 μM Dyn A for 30 minutes did not change the level of either protein band (Figs. 1A and 1C).
Ubiquitination of the hKOR was enhanced by receptor phosphorylation, but not by receptor internalization
To investigate whether agonist-induced ubiquitination of the hKOR requires receptor phosphorylation and internalization, we examined effects of dominant negative (DN) mutants of GRK2, β-arrestin and dynamin I. Transient co-transfection of CHO-FLAG-hKOR cells with HA-Ub and GRK2-K220R or β-arrestin (319–418), but not dynamin I-K44A, significantly reduced Dyn A-stimulated hKOR ubiquitination (Fig. 2A and 2B). However, the basal ubiquitination of the hKOR was not affected by expressions of DN mutants of GRK2, β-arrestin and dynamin I (see supplemental data 1). To further examine the role of phosphorylation, we tested whether a phosphorylation-defective hKOR mutant (hKOR-S358N) (Li et al., 2002a) underwent ubiquitination. CHO cells were transiently transfected with HA-Ub and FLAG-hKOR or FLAG-hKOR-S358N to similar receptor expression levels and receptor ubiquitination was detected. The hKOR-S358N mutant showed greatly reduced Dyn A (1 μM)-stimulated ubiquitination, compared with the wildtype (Fig. 2C). These results indicate that agonist-stimulated ubiquitination of the hKOR is enhanced by receptor phosphorylation and association of phosphorylated receptor with clathrin via β-arrestin, but not by receptor internalization. Thus, hKOR ubiquitination occurs to receptors mostly in plasma membranes.
Fig. 2. Ubiquitination of the hKOR was regulated by receptor phosphorylation, but not by receptor internalization.
(A) (B) CHO-FLAG-hKOR cells were transiently transfected with HA-Ub and dynamin I-K44A, β-arrestin (319-418), GRK2-K220R or pcDNA3.
(C) CHO cells were transiently transfected with HA-Ub and FLAG-hKOR or FLAG-hKOR-S358N to similar receptor expression levels. (A) (C) Approximately two days later, cells were treated with 50 μM MG132 for 3 h and then 1 μM Dyn A was added for another 30 min. Cells were lysed, solubilized and immunoprecipitated with anti-FLAG M2 affinity gel and western blot was performed using rabbit anti-HA antibody (upper) or rabbit anti-FLAG antibody (lower) as described in Materials and Methods. Each figure represents one of three independent experiments performed with similar results. (B) Quantitation of results from (A). Densitometric analysis was performed using the OptiQuant software. Each point represents the mean ± s.e.m. of three independent experiments. * P<0.05, compared with the pcDNA3 control group by two-tailed Student’s t test.
Mutations of all ten intracellular Lys residues to Arg (10KR) greatly reduced ubiquitination of the hKOR
To examine the functional importance of hKOR ubiquitination, we generated a hKOR mutant, named hKOR-10KR, in which all ten Lys residues in the intracellular loops and C- terminal domain, the potential ubiquitination sites, were mutated to Arg. Figure 3A shows a schematic representation of the hKOR and the ten intracellular Lys residues denoted by asterisks. CHO cells were transiently transfected with HA-Ub and FLAG-hKOR or FLAG-hKOR-10KR and agonist-stimulated receptor ubiquitination was examined. 10KR mutations greatly reduced basal and Dyn A-stimulated receptor ubiquitination, compared with the wildtype (Fig. 3B). These results indicate that ubiquitination occurs on Lys residues in intracellular domains of the hKOR.
Fig. 3. Mutations of the intracellular Lys residues abolished ubiquitination of the hKOR.
(A) Schematic representation of the hKOR and the intracellular Lys residues mutated. A hKOR mutant (hKOR-10 KR) was generated by changing all ten Lys residues to Arg (at positions 89, 91, 165, 174, 176, 254, 265, 338, 349, and 378) denoted by Asterisks in the intracellular loops and C-terminal domain.
(B) CHO cells were transiently transfected with HA-Ub and FLAG-hKOR or FLAG-hKOR-10KR 3 as indicated. Approximately two days later, cells were treated with 50 μM MG132 for 3 h and then without or with 1 μM U50,488H or 1 μM Dyn A for another 30 min. The cells were lysed, solubilized and immunoprecipitated with anti-FLAG M2 affinity gel and western blot was performed using rabbit anti-HA antibody (upper) or rabbit anti-FLAG antibody (lower) as described in Materials and Methods. Each figure represents one of three independent experiments performed with similar results.
10KR mutations of the hKOR did not change the binding affinity for [3H]diprenorphine, U50,488H and Dyn A, receptor-G protein coupling and agonist-induced desensitization
CHO cells stably expressing FLAG-hKOR-10KR were established and one clonal cell line with similar receptor expression level as CHO-FLAG-hKOR cells was selected for the subsequent experiments. Saturation binding of [3H]diprenorphine binding and competitive inhibition of [3H]diprenorphine binding by U50,488H and Dyn A were performed on membranes prepared from both cell lines (Table 1). The wildtype and the 10KR mutant had similar Kd values for [3H]diprenorphine and comparable Ki values for U50,488H and Dyn A (Table1). These results indicate that mutations of all the intracellular Lys to Arg did not overtly change the receptor binding pocket.
Table 1. Receptor expression levels and ligand binding affinities of the hKOR-WT and hKOR-10KR.
Membranes of CHO cells stably expressing the FLAG-hKOR or FLAG-hKOR-10KR were prepared and Bmax and Kd values of [3H]diprenorphine and Ki values of U50,488H and Dyn A were determined as described in Materials and Methods. Each value represents the mean ± s.e.m. of three independent experiments.
| [3H]diprenorphine | U50,488H | Dyn A | ||
|---|---|---|---|---|
|
|
||||
| Bmax (pmol/mg protein) | Kd (nM) | Ki (nM) | Ki (nM) | |
| hKOR-WT | 1.71 ± 0.17 | 0.12 ± 0.01 | 1.45 ± 0.14 | 0.10 ± 0.01 |
|
| ||||
| HKOR-10KR | 2.20 ± 0.17 | 0.14 ± 0.02 | 1.64 ± 0.26 | 0.11 ± 0.02 |
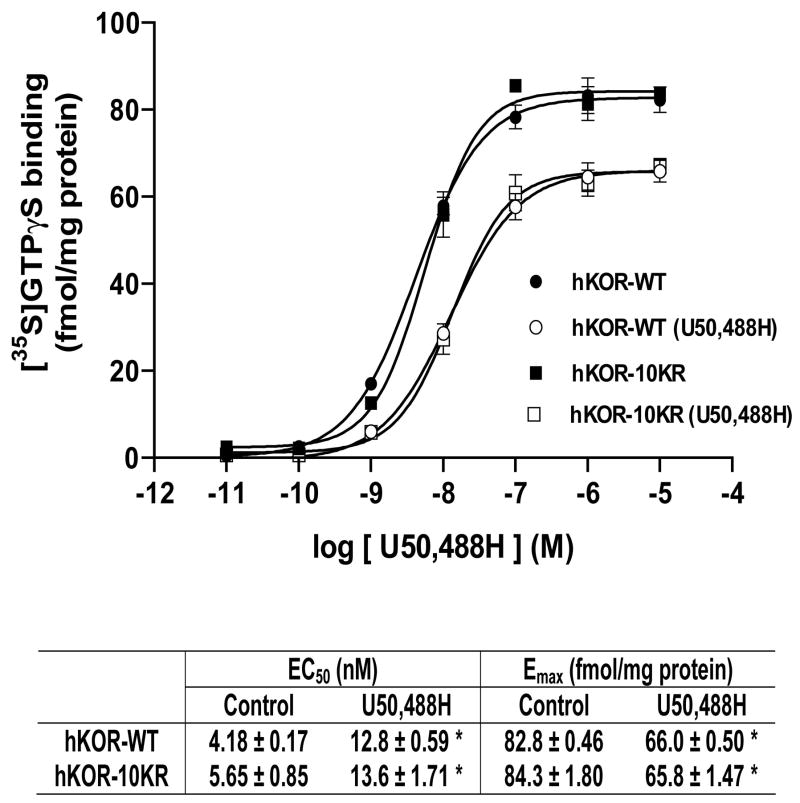
U50,488H-stimulated [35S]GTPγS binding to membranes of both CHO-FLAG-hKOR and CHO-FLAG-hKOR-10KR cells was examined with or without pre-treatment with 1 μM U50,488H (Fig. 4). Without U50,488H pretreatment, the EC50 and Emax values of U50,488H in stimulating [35S]GTPγS binding in both cell lines are similar, indicating that the 10KR mutations do not affect receptor coupling to G protein signaling. Pretreatment with U50,488H reduced the Emax value and increased the EC50 value compared with untreated controls (P<0.05 for both) and hence desensitized both the wildtype and 10KR mutant (Fig. 4). Following pretreatment with U50,488H, EC50 and Emax values are not different between the wildtype and 10KR mutant (P>0.05). The result demonstrate that mutations of the 10 Lys residues to Arg did not affect U50,488H-induced receptor desensitization.
Fig. 4. Mutation of the hKOR ubiquitination sites did not change agonist-induced [35S]GTP γS binding and desensitization.
CHO-FLAG-hKOR or CHO-FLAG-hKOR-10KR cells were incubated without or with 1 μM U50,488H for 30 min and washed. Membranes were prepared and U50,488H-stimulated [35S]GTPγS binding were determined as described in Materials and Methods. The EC50 and Emax values were showed in the table. Each value represents the mean ± s.e.m. of three independent experiments. * P<0.05, compared with the control group by two-tailed Student’s t test.
10KR mutations of the hKOR decreased agonist-induced receptor down-regulation
Both CHO-FLAG-hKOR and CHO-FLAG-hKOR-10KR cells were treated without or with 1 μM Dyn A for 4 h or indicated time periods and receptor down-regulation was determined. We have demonstrated previously that agonist-promoted down-regulation of the hKOR reaches a plateau at 4 h (Zhu et al., 1998). In addition, Dyn A causes a higher degree of hKOR down-regulation than U50,488H since U50,488H and other membrane-permeable ligands act as pharmacological chaperone to enhance hKOR trafficking along the biosynthesis pathway, but Dyn A and other membrane-impermeable ligands do not (Chen et al., 2006b). Therefore, incubation with Dyn A was used in down-regulation studies.
FLAG-hKOR-10 KR expressed in CHO cells migrated as two bands with Mr’s of 45 kDa and 55 kDa (Fig. 5A), similar to FLAG-hKOR (see Fig. 1) (Chen et al., 2006a). Results from immunoblotting followed by quantitation of the 55-kDa form showed that FLAG-hKOR-10KR was down-regulated by Dyn A (4 h) to a much lower extent than FLAG-hKOR (~40% vs. 70%, P<0.01) (Fig. 5A). In contrast, Dyn A treatment did not affect the level of the immature form (45 kDa) of the hKOR and 10 KR mutant (Fig. 5A). We then performed Dyn A-induced down-regulation of the wildtype and 10KR mutant at 2, 3, 4 and 16 hours to determine if the time courses were different. Previously we observed no significant hKOPR down-regulation at 1 h (Zhu et al., 1998); therefore, we started at 2 h. We found that Dyn A-induced down-regulation reached plateau levels at 3 h for both the wildtype and 10KR mutant (Fig 5B), indicating that the time courses of down-regulation are similar. The plateau levels are significantly lower for the 10 KR mutant, compared with the wildtype (Fig 5B). The results indicate that ubiquitination of hKOR is involved in agonist-induced down-regulation. However, since the 10KR mutant still underwent down-regulation, agonist-promoted polyubiquitination is not required for hKOR down-regulation.
Fig. 5. Mutation of the hKOR ubiquitination sites decreased agonist-induced receptor down-regulation, but did not affect agonist-induced internalization.
CHO-FLAG-hKOR or CHO-FLAG-hKOR-10KR cells were treated without or with 1 μM Dyn A for (A) 4 h or (B) indicated time periods for down-regulation or (C) with 10 μM U50,488H for 30 min for internalization. Receptor down-regulation was determined by (A) immunoblotting with anti-FLAG antibody followed by quantitation of the 55-kDa form or (B) fluorescence flow cytometry of cell surface receptors as described in Materials and Methods. (C) For agonist-induced internalization, cell surface receptors were determined using the fluorescence flow cytometry assay. Each value represents the mean ± s.e.m. of three independent experiments. * P<0.05, compared with the control group by two-tailed Student’s t test.
Pretreatment with the lysosomes inhibitor chloroquine (50 μM) for 1 h partially blocked Dyn A-induced 10KR down-regulation (see supplemental data 2). These data indicate that part of the un-ubiquitinated hKOR-10 KR is trafficked to lysosomes for degradation.
10KR mutations of the hKOR did not alter agonist-induced receptor internalization or recycling following agonist removal
Reduced Dyn A-induced down-regulation observed for the 10KR mutant may be attributed to decreased receptor internalization. We thus examined whether 10KR mutations affected internalization. We previously demonstrated that U50,488H and Dyn A promoted similar levels of internalization of the hKOR (Li et al., 2003; Chen et al., 2006b). Both CHO-FLAG-hKOR and CHO-FLAG-hKOR-10KR cells were incubated without or with 10 μM U50,488H for 30 min, and receptor internalization was determined using the fluorescence flow cytometry assay. The extent of receptor internalization induced by U50,488H were similar for the wildtype and hKOR-10KR (Fig. 5C), indicating that receptor ubiquitination is not required for agonist-induced internalization of the hKOR. In addition, the recycling rates of the hKOR and 10KR mutant after removal of U50,488H were not significantly different as determined by the fluorescence flow cytometry assay (see supplemental data 3).
Ubiquitination of the hKOR occurred predominantly at K338, K349 and K378 in the C- terminal domain
To narrow down which intracellular Lys residues of the hKOR are important in receptor ubiquitination, we mutated the three Lys residues (K338, K349 and K378) in the C- terminal domain of the hKOR to Arg to get the hKOR-3KR mutant. The hKOR-3KR mutant, when transfected into CHO cells, showed greatly decreased agonist-induced ubiquitination, compared with the wildtype (Fig. 6). We then generated a CHO cell line stably expressing the FLAG-hKOR-3KR at a similar receptor level as CHO-FLAG-hKOR. The 3KR mutations greatly decreased Dyn A-induced down-regulation of the hKOR (Fig. 6), indicating that ubiquitination occurs predominantly at these three Lys residues, which plays a significant role in agonist-induced down-regulation.
Fig. 6. Mutations of Lys residues in C-tail of hKOR greatly reduced Dyn A-induced receptor ubiquitination and down-regulation.
(A) CHO cells were transiently transfected with HA-Ub and FLAG-hKOR or FLAG-hKOR-3KR in which all three Lys residues in the C-terminal domain (at positions 338, 349, and 378) were changed to Arg residues. Approximately two days later, cells were treated with 50 μM MG132 for 3 h and then cells were treated with 1 μM Dyn A for another 30 min. The cells were lysed, solubilized and immunoprecipitated with anti-FLAG M2 affinity gel and western blot was performed using rabbit anti-HA antibody (upper) or rabbit anti-FLAG antibody (lower) as described in Materials and Methods. Each figure represents one of three independent experiments performed with similar results.
(B) CHO-FLAG-hKOR and CHO cells stably expressing FLAG-hKOR-3KR (CHO-FLAG-hKOR-3KR) were treated without or with 1 μM Dyn A for 4 h and then receptor down-regulation was determined fluorescence flow cytometry of cell surface receptors as described in Materials and Methods. Each value represents the mean ± s.e.m. of three independent experiments. * P<0.05, compared with the control group by two-tailed Student’s t test.
Effects of K63R and K48R mutants of ubiquitin on agonist-induced ubiquitination and down-regulation of the hKOR
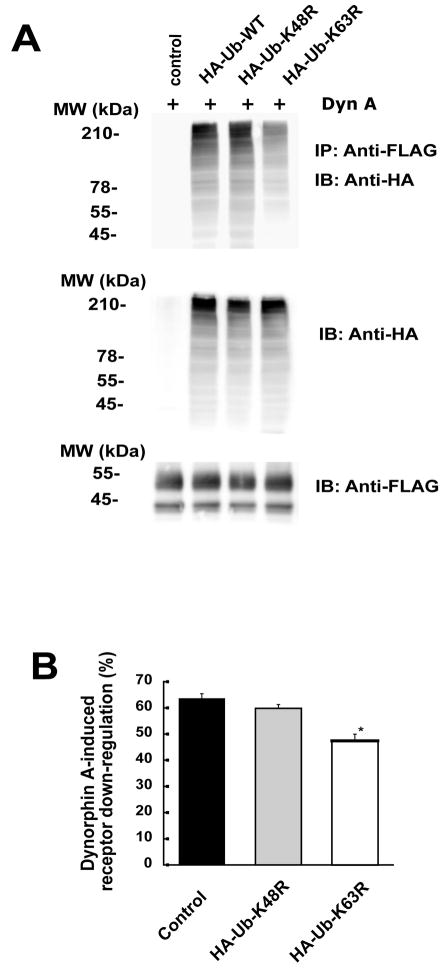
CHO-FLAG-hKOR cells were transiently transfected with HA-Ub wildtype, HA-Ub-K63R or HA-Ub-K48R mutants and Dyn A-stimulated ubiquitination of the hKOR was examined. Compared with HA-Ub, the HA-Ub-K63R mutant decreased Dyn A-promoted hKOR ubiquitination (Fig. 7A), but HA-Ub-K48R mutant did not. HA-Ub wildtype, HA-Ub-K63R and HA-Ub-K48R were expressed at similar levels. These results indicate that K63-linked polyubiquitins is the dominant ones in ubiquitinated hKOR upon agonist stimulation. We then investigated their effects on hKOR down-regulation. Expression of HA-Ub-K63R, but not HA-Ub-K48R, partly reduced Dyn A-induced down-regulation of the hKOR (Fig. 7B).
Fig. 7. Effects of Ub-K63R and Ub-K48R mutants on ubiquitination and down-regulation of the hKOR.
CHO-FLAG-hKOR cells were transiently transfected with HA-Ub wildtype or its K63R (HA-Ub-K63R) or K48R mutant (HA-Ub-K48R). (A) Approximately two days later, cells were treated with 50 μM MG132 for 3 h and then 1 μM Dyn A was added for another 30 min. Cells were lysed, solubilized and immunoprecipitated with anti-FLAG M2 affinity gel and western blot was performed using rabbit anti-HA antibody (upper and middle) or rabbit anti-FLAG antibody (lower) as described in Materials and Methods. Each figure represents one of three independent experiments performed with similar results. (B) About two days after transfection, cells were treated without or with 1 μM Dyn A for 4 h. Receptor down-regulation was determined by fluorescence flow cytometry of cell surface receptors as described in Materials and Methods. Each value represents the mean ± s.e.m. of three independent experiments. * P<0.05, compared with the control group by two-tailed Student’s t test.
Effects of the HN-CYLD mutant on agonist-induced ubiquitination and down-regulation of the hKOR
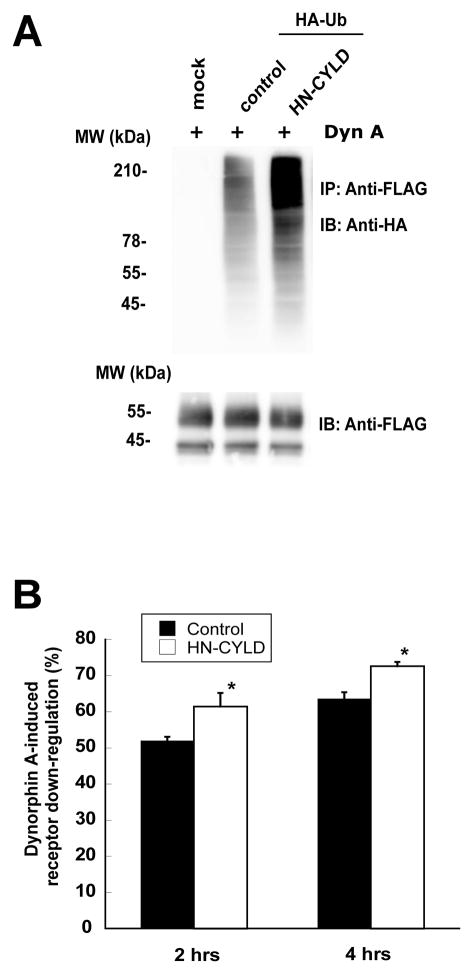
To further examine if the hKOR undergoes K63-linked polyubiquitination, we studied effect of the DN mutant of deubiquitination enzyme cylindromatosis tumor suppressor gene (CYLD), HN-CYLD, on Dyn A-stimulated ubiquitination of the hKOR. CYLD belongs to the family of ubiquitin-specific hydrolases, which are deubiquitinating cysteine proteases (Bignell et al., 2000), and it acts on Lys63-, but not Lys48-, linked polyubiquitin chains (Trompouki et al., 2003; Kovalenko et al., 2003). Transient expression of HN-CYLD greatly increased Dyn A-induced hKOR ubiquitination (Fig. 8A), indicating that the hKOR undergoes K63-linked polyubiquitination. Expression of HN-CYLD in FLAG-hKOR-CHO cells reduced Dyn A-induced down-regulation of the hKOR (Fig. 8B). However, expression of wildtype CYLD did not affect ubiquitination or down-regulation (data not shown). These results indicate that K63-linked polyubiquitination of the hKOR play an important role in agonist-induced receptor down-regulation.
Fig. 8. Effect of HN-CYLD mutant on ubiquitination and down-regulation of the hKOR.
CHO-FLAG-hKOR cells were transiently transfected with HA-Ub and HN-CYLD or pcDNA3. (A) Approximately two days later, cells were treated with 50 μM MG132 for 3 h and then 1 μM Dyn A was added for another 30 min. Cells were lysed, solubilized and immunoprecipitated with anti-FLAG M2 affinity gel and western blot was performed using rabbit anti-HA antibody (upper) or rabbit anti-FLAG antibody (lower) as described in Materials and Methods. Each figure represents one of three independent experiments performed with similar results. (B) About two days after transfection, cells were treated without or with 1 μM Dyn A for 2 or 4 hrs. Receptor down-regulation was determined by fluorescence flow cytometry as described in Materials and Methods. Each value represents the mean ± s.e.m. of three independent experiments. * P<0.05, compared with the control group by two-tailed Student’s t test.
Discussion
The data reported herein provide evidence that hKOR undergoes agonist-dependent ubiquitination and its ubiquitination regulates, but is not required for, sorting of internalized receptor to degradation pathway. Agonist-promoted ubiquitination of the hKOR, which occurs at Lys residues in the C-terminal domain of the hKOR, is enhanced by hKOR phosphorylation, but unchanged by receptor internalization. In addition, agonist-promoted polyubiquitination of the hKOR occurs predominantly as Lys63-linked polyubiquitin chains. To the best of our knowledge, this is the first report that Lys63-linked polyubiquitination of a 7TMR is involved in its down-regulation.
Ubiquitination of the hKOR regulates, but is not required for, agonist-induced hKOR down-regulation
Ubiquitination of the hKOR was increased substantially following agonist stimulation. The rapid occurrence of ubiquitination of the hKOR following agonist incubation (30 min) is similar to that reported for several 7TMRs, including the β2-AR (within 15 min) (Shenoy et al., 2001). As shown in several figures, it is most likely that the hKOR is polyubiquitinated since ubiquitinated hKOR is of high molecular weights.
Mutations of the all intracellular Lys residues to Arg greatly reduced Dyn A-induced receptor ubiquitination and down-regulation (Fig. 5A, 5B), but did not affect receptor internalization, indicating that intracellular Lys residues and/or ubiquitination of hKOR plays an important role in sorting of internalized hKOR for degradation. We showed that agonist-promoted down-regulation of the hKOR involved trafficking of the receptor from early endosomes to late endosomes to lysosomes (Li et al., 2000). The results indicate that ubiquitination of the hKOR is involved in targeting the receptor from endosomes to lysosomes. These results are consistent with those on several 7TMRs, including β2-AR (Shenoy et al., 2001), chemokine CXCR4 (Marchese and Benovic, 2001; Marchese et al., 2003), V2 vasopressin (Martin et al., 2003), NK-1 (Cottrell et al., 2006) and PAR2 (Jacob et al., 2005), but not those on the δ opioid receptor (Tanowitz and von Zastrow, 2002) and the complex of calcitonin receptor-like receptor and receptor activity-modifying protein 1 (Cottrell et al., 2007). Sorting of CXCR4 from endosomes to lysosomes requires Hrs and Vps4, an AAA ATPase; the latter is involved in regulating the ubiquitination status of both CXCR4 and Hrs (Marchese et al., 2003).
The 10KR mutations of FLAG-hKOR eliminated agonist-promoted ubiquitination and reduced, but did not abolish, Dyn A-induced down-regulation. Similar results were reported for the K-to-R mutants of the V2 vasopressin (Martin et al., 2003) and NK-1 (Cottrell et al., 2006) receptors. These results suggest that ubiquitination of these receptors plays a regulatory, but not a mandatory, role for trafficking of the receptors in multivesicular bodies en route to lysosomes for degradation. For some 7TMRs, ubiquitination is essential. Un-ubiquitinated mutants of β2-AR, PAR2 and CXCR4 were not down-regulated following agonist treatment (Marchese and Benovic, 2001; Shenoy et al., 2001; Jacob et al., 2005). In addition, wildtype β1-AR was reported not to undergo agonist-induced ubiquitination or down-regulation (Liang and Fishman, 2004). In contrast, Tanowitz et al. (2002) reported that mutation of all intracellular Lys residues to Arg in the δ opioid receptor did not affect agonist-induced endocytosis and proteolytic degradation of the receptor and its co-localization with lysosomes, indicating ubiquitination-independent down-regulation. However, Vps4/SKD-1 and Hrs, are still required for sorting of the mutant δ opioid receptor from endosomes to multivesicular bodies to lysosomes (Hislop et al., 2004). Thus, requirement of ubiquitination for down-regulation appears to be receptor-dependent. There are two possibilities for the sorting of un-ubiquitinated hKOR for degradation. The result that pretreatment with the lysosomes inhibitor chloroquine partially blocked Dyn A-induced hKOR-10KR down-regulation (supplemental data 2) indicates that some of the hKOR-10 KR is trafficked to lysosomes for degradation. In addition, there may be other factors involved in the trafficking of the hKOR. GASP has been shown to interact with the C-terminal domains of the δ and κ opioid receptors (Whistler et al., 2002; Simonin et al., 2004) and participate in sorting the δ opioid receptor to lysosomes for degradation (Whistler et al., 2002). These results further support the notion that ubiquitination of hKOR regulates, but is not required for, its down-regulation.
Ubiquitinated hKOR contains predominantly Lys 63-linked polyubiquitin chains
There are seven Lys residues in ubiquitin: K6, K11, K27, K29, K33, K48 and K63. Polyubiquitin chains can be linked by different Lys residues in ubiquitin; however, K48- and K63-linked polyubiquitin chains are the predominant ones. It has been recognized that Lys48-linked polyubiquitination targets proteins to proteasomes for degradation, whereas Lys63-linked polyubiquitination plays important roles in interactions with protein machineries involved in endocytic trafficking, inflammatory response, protein translation, and DNA repair [for a review, see (Pickart and Fushman, 2004)].
We found that the K63R ubiquitin mutant greatly decreased Dyn A-promoted hKOR ubiquitination and down-regulation, but the K48R mutant did not (Fig. 7). These results indicate that agonist-promoted polyubiquitination of the hKOR occurs predominantly as Lys63-linked polyubiquitin chains, which is involved in its agonist-induced down-regulation. This is the first report that Lys63-linked polyubiquitination of a 7TMR is involved in its agonist-promoted down-regulation. Lys63-linked polyubiquitination has been shown to be involved in endocytic trafficking and degradation of epidermal growth factor and Trk A receptors (Geetha et al., 2005; Huang et al., 2006a) and dopamine transporter (Miranda et al., 2005).
Ubiquitination is a reversible process. Deubiquitinating enzymes remove ubiquitins from ubiquitinated proteins, but do not degrade ubiquitins, and, thus, allow ubiquitin to be re-used. Expression of HN-CYLD, a DN mutant of the deubiquitination enzyme CYLD that breaks Lys63-, but not Lys48-, linked polyubiquitin chains, increased Dyn A-induced hKOR ubiquitination and down-regulation (Fig. 8). These results further support the role of Lys63-linked polyubiquitination of hKOR in its down-regulation. To the best of our knowledge, this is the first report showing that deubiquitinating enzymes play a role in 7TMR down-regulation. The role of de-ubiquitinating enzymes in EGFR trafficking has been studied, yielding inconsistent results. Mizuno et al. (2005) reported that UBPY deubiquitinated the EGFR in endosomes and reduced EGFR degradation; however, Row et al. (2006) and Alwan et al (2007) showed that deubiquitination of EGFR by UBPY is required for EGFR degradation.
K63R ubiquitin greatly reduced Dyn A-promoted hKOR ubiquitination, but modestly attenuated hKOR down-regulation. These observation is in accord with the notion that ubiquitination regulates, but is not required for, hKOR down-regulation. In addition, HN-CYLD dramatically enhanced Dyn A-promoted hKOR ubiquitination, but increased hKOR down-regulation to a much lower extent. These results suggest that the level of ubiquitination without HN-CYLD expression may be sufficient to drive down-regulation and higher ubiquitination levels do not increase down-regulation proportionally to the increase in ubiquitination. In addition, the immunoprecipitation procedure we used did not remove proteins associated with the receptor. Therefore, the possibility that HN-CYLD expression enhanced ubiquitination of associated proteins can not be excluded.
Agonist-promoted ubiquitination of the hKOR is regulated by receptor phosphorylation, but not by receptor internalization
We showed previously that GRK2-K220R and β-arrestin (319–418) reduced agonist-induced internalization of the hKOR (Li et al., 1999). In addition, GRK2-K220R attenuated, while S358N mutation in the hKOR eliminated, agonist-induced phosphorylation of hKOR (Li et al., 2002a). β-arrestin (319–418) binds well to clathrin, but it does not bind to receptor (Krupnick et al., 1997). In this study, we found that Dyn A did not stimulate ubiquitination of the hKOR-S358N mutant and the dominant negative mutants GRK2-K220R and β-arrestin (319–418) reduced Dyn A-stimulated hKOR ubiquitination. These results indicate that ubiquitination of the hKOR is enhanced by receptor phosphorylation and association of phosphorylated receptor with clathrin via β-arrestin. Similar observations were reported for the β2-AR (Shenoy et al., 2001) and the yeast α-factor receptor Ste2 (Hicke et al., 1998). Since dynamin I-K44A, an effective inhibitor of hKOR internalization (Li et al., 1999), did not affect its ubiquitination, agonist-stimulated ubiquitination of the hKOR does not require receptor internalization and hence occurs to the hKOR in the plasma membrane. β-arrestin was found to be required for ubiquitination of V2 vasopressin receptor (Martin et al., 2003). How β-arrestin play a role in the ubiquitination of hKOR needs further investigation. It is reasonable to speculate that agonist-promoted hKOR phosphorylation at S358 triggers events ultimately leading to ubiquitination at one or more of Lys residues at 338, 349, and 378 in the hKOR C-terminal domain.
Agonist-dependent and -independent 7TMR ubiquitination
There are two pools of 7TMRs which are ubiquitinated: misfolded newly synthesized receptors and receptors on plasma membranes. The former is agonist-independent and the latter is agonist-dependent.
Since Dyn A is a hydrophilic ligand which activates only cell surface receptors, agonist-induced hKOR ubiquitination occurs to receptors on plasma membranes. Agonist treatment has been found to enhance ubiquitination of several other 7TMRs, including the yeast α-factor (Hicke and Riezman, 1996; Roth and Davis, 1996), β2-AR (Shenoy et al., 2001), and V2 vasopressin (Martin et al., 2003), PAR2 (Jacob et al., 2005), CXCR4 (Marchese and Benovic, 2001) and NK-1 (Cottrell et al., 2006) receptors, which is involved in endocytosis, trafficking of the receptors from endosomes to lysosomes.
There was a low level of basal ubiquitination of the hKOR, which was most likely due to ubiquitination of newly synthesized misfolded receptors for endoplasmic reticulum-related degradation. Agonist-independent ubiquitination has been demonstrated for the δ opioid (Petaja-Repo et al., 2001), TRH (Cook et al., 2003) and calcium-sensing (Huang et al., 2006b) and platelet-activating factor (Dupre et al., 2003) receptors.
The observation that 10KR mutation did not totally eliminate ubiquitination may be due to the possibility that there may be ubiquitinated proteins immunoprecipitated with the hKOR. For example, Shenoy et al. (2001) demonstrated that following activation of the β2-adrenergic receptor, β-arrestin was ubiquitinated which was important for internalization of the receptor.
Methods used for determination of hKOR down-regulation
We used both flow cytometry and immunoblotting to determine receptor down-regulation. Flow cytometry was used to quantify loss of cell surface receptor, whereas immunoblotting followed by quantitation detected reduction in the mature (55-kDa) form of the hKOR. In our previous study (Chen et al., 2006b) and the current study, we found that two methods yielded similar levels of agonist-induced hKOR down-regulation. Radioligand binding was not used to determine down-regulation since it was difficult to remove Dyn A and residual Dyn A interfered with binding.
Conclusion
Agonists promoted ubiquitination of the hKOR, which occurs after receptor phosphorylation, but before internalization. The hKOR was ubiquitinated at the three Lys residues in the C-terminal domain predominantly by Lys63-linked polyubiquitin chains. Agonist-promoted ubiquitination plays a regulatory, but not obligatory, role for agonist-induced down-regulation.
Supplementary Material
Acknowledgments
We thank Dr. D. Bohmann of University of Rochester for HA-Ub expression plasmid; Dr. Jeffrey Benovic of Thomas Jefferson University for the expression constructs of Dynamin I-K44A, β-arrestin (319-418) and GRK2-K220R and Dr. Marie Wooten of Auburn University for HA-Ub, HA-Ub-K63R, HA-Ub-K48R, CYLD and HN-CYLD.
This work was supported by National Institutes of Health Grant DA17302.
Abbreviations
- 7TMR
seven transmembrane domain receptor
- β2-AR
β2-adrenergic receptor
- CHO cells
Chinese hamster ovary cells
- CHO-FLAG-hKOR
CHO cell lines stably expressing FLAG-hKOR
- CHO-FLAG-hKOR-10KR
CHO cell lines stably expressing FLAG-hKOR-10KR
- CYLD
cylindromatosis tumor suppressor gene
- DN
dominant negative mutant
- Dyn A
dynorphin A (1–17)
- ER
Endoplasmic reticulum
- FLAG-hKOR
Flag-tagged human κ opioid receptor
- hKOR
human κ opioid receptor
- hKOR-10KR
a FLAG-hKOR mutant in which all ten intracellular lysines (at positions 89, 91, 165, 174, 176, 254, 265, 338, 349, and 378) were replaced with arginines
- NK-1
neurokinin-1 receptor
- PAF
platelet-activating factor
- PAR1 and PAR2
protease-activated receptor 1 and 2, respectively
- U50
488H, (-)(trans)-3,4-dichloro-N-methyl-N-[2-(1-pyrrolidiny) cyclohexyl] benzeneacetamide
References
- Alwan HA, van Leeuwen JE. UBPY-mediated epidermal growth factor receptor (EGFR) de-ubiquitination promotes EGFR degradation. J Biol Chem. 2007;282:1658–1669. doi: 10.1074/jbc.M604711200. [DOI] [PubMed] [Google Scholar]
- Bignell GR, Warren W, Seal S, Takahashi M, Rapley E, Barfoot R, Green H, Brown C, Biggs PJ, Lakhani SR, Jones C, Hansen J, Blair E, Hofmann B, Siebert R, Turner G, Evans DG, Schrander-Stumpel C, Beemer FA, van Den OA, Halley D, Delpech B, Cleveland MG, Leigh I, Leisti J, Rasmussen S. Identification of the familial cylindromatosis tumour-suppressor gene. Nat Genet. 2000;25:160–165. doi: 10.1038/76006. [DOI] [PubMed] [Google Scholar]
- Chaturvedi K, Bandari P, Chinen N, Howells RD. Proteasome involvement in agonist-induced down-regulation of mu and delta opioid receptors. J Biol Chem. 2001;276:12345–12355. doi: 10.1074/jbc.M008054200. [DOI] [PubMed] [Google Scholar]
- Chen C, Li JG, Chen Y, Huang P, Wang Y, Liu-Chen LY. GEC1 interacts with the kappa opioid receptor and enhances expression of the receptor. J Biol Chem. 2006a;281:7983–7993. doi: 10.1074/jbc.M509805200. [DOI] [PubMed] [Google Scholar]
- Chen Y, Chen C, Wang Y, Liu-Chen LY. Ligands regulate cell surface level of the human {kappa} opioid receptor by activation-induced down-regulation and pharmacological chaperone-mediated enhancement: Differential effects of nonpeptide and peptide agonists. J Pharmacol Exp Ther. 2006b;319:765–775. doi: 10.1124/jpet.106.107987. [DOI] [PubMed] [Google Scholar]
- Cook LB, Zhu CC, Hinkle PM. Thyrotropin-releasing hormone receptor processing: role of ubiquitination and proteasomal degradation. Mol Endocrinol. 2003;17:1777–1791. doi: 10.1210/me.2003-0073. [DOI] [PubMed] [Google Scholar]
- Cottrell GS, Padilla B, Pikios S, Roosterman D, Steinhoff M, Gehringer D, Grady EF, Bunnett NW. Ubiquitin-dependent down-regulation of the neurokinin-1 receptor. J Biol Chem. 2006;281:27773–27783. doi: 10.1074/jbc.M603369200. [DOI] [PubMed] [Google Scholar]
- Cottrell GS, Padilla B, Pikios S, Roosterman D, Steinhoff M, Grady EF, Bunnett NW. Post-endocytic sorting of calcitonin receptor-like receptor and receptor activity-modifying protein 1. J Biol Chem. 2007;282:12260–12271. doi: 10.1074/jbc.M606338200. [DOI] [PubMed] [Google Scholar]
- Dupre DJ, Chen Z, Le Gouill C, Theriault C, Parent JL, Rola-Pleszczynski M, Stankova J. Trafficking, ubiquitination, and down-regulation of the human platelet-activating factor receptor. J Biol Chem. 2003;278:48228–48235. doi: 10.1074/jbc.M304082200. [DOI] [PubMed] [Google Scholar]
- Geetha T, Jiang J, Wooten MW. Lysine 63 polyubiquitination of the nerve growth factor receptor TrkA directs internalization and signaling. Mol Cell. 2005;20:301–312. doi: 10.1016/j.molcel.2005.09.014. [DOI] [PubMed] [Google Scholar]
- Hershko A, Ciechanover A. The ubiquitin system. Annu Rev Biochem. 1998;67:425–479. doi: 10.1146/annurev.biochem.67.1.425. [DOI] [PubMed] [Google Scholar]
- Hicke L, Riezman H. Ubiquitination of a yeast plasma membrane receptor signals its ligand-stimulated endocytosis. Cell. 1996;84:277–287. doi: 10.1016/s0092-8674(00)80982-4. [DOI] [PubMed] [Google Scholar]
- Hicke L, Zanolari B, Riezman H. Cytoplasmic tail phosphorylation of the alpha-factor receptor is required for its ubiquitination and internalization. J Cell Biol. 1998;141:349–358. doi: 10.1083/jcb.141.2.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hislop JN, Marley A, von Zastrow M. Role of mammalian vacuolar protein-sorting proteins in endocytic trafficking of a non-ubiquitinated G protein-coupled receptor to lysosomes. J Biol Chem. 2004;279:22522–22531. doi: 10.1074/jbc.M311062200. [DOI] [PubMed] [Google Scholar]
- Huang F, Kirkpatrick D, Jiang X, Gygi S, Sorkin A. Differential regulation of EGF receptor internalization and degradation by multiubiquitination within the kinase domain. Mol Cell. 2006a;21:737–748. doi: 10.1016/j.molcel.2006.02.018. [DOI] [PubMed] [Google Scholar]
- Huang Y, Niwa J, Sobue G, Breitwieser GE. Calcium-sensing receptor ubiquitination and degradation mediated by the E3 ubiquitin ligase dorfin. J Biol Chem. 2006b;281:11610–11617. doi: 10.1074/jbc.M513552200. [DOI] [PubMed] [Google Scholar]
- Jacob C, Cottrell GS, Gehringer D, Schmidlin F, Grady EF, Bunnett NW. c-Cbl mediates ubiquitination, degradation, and down-regulation of human protease-activated receptor 2. J Biol Chem. 2005;280:16076–16087. doi: 10.1074/jbc.M500109200. [DOI] [PubMed] [Google Scholar]
- Kovalenko A, Chable-Bessia C, Cantarella G, Israel A, Wallach D, Courtois G. The tumour suppressor CYLD negatively regulates NF-kappaB signalling by deubiquitination. Nature. 2003;424:801–805. doi: 10.1038/nature01802. [DOI] [PubMed] [Google Scholar]
- Krupnick JG, Santini F, Gagnon AW, Keen JH, Benovic JL. Modulation of the arrestin-clathrin interaction in cells. Characterization of beta-arrestin dominant-negative mutants. J Biol Chem. 1997;272:32507–32512. doi: 10.1074/jbc.272.51.32507. [DOI] [PubMed] [Google Scholar]
- Law P-Y, Wong YH, Loh HH. Molecular mechanisms and regulation of opioid receptor signaling. Ann Rev Pharmacol Toxicol. 2000;40:389–430. doi: 10.1146/annurev.pharmtox.40.1.389. [DOI] [PubMed] [Google Scholar]
- Li J, Li J-G, Chen C, Zhang F, Liu-Chen L-Y. Molecular basis of differences in (-)(trans)-3,4-dichloro-N-methyl-N-[2-(1-pyrrolidiny)-cyclohexyl]benzeneacetamide-induced desensitization and phosphorylation between human and rat κ-opioid receptors expressed in Chinese hamster ovary cells. Mol Pharmacol. 2002a;61:73–84. doi: 10.1124/mol.61.1.73. [DOI] [PubMed] [Google Scholar]
- Li J-G, Zhang F, Jin XL, Liu-Chen L-Y. Differential regulation of the human kappa opioid receptor by agonists: etorphine and levorphanol reduced dynorphin A- and U50,488H-induced internalization and phosphorylation. J Pharmacol Exp Ther. 2003;305:531–540. doi: 10.1124/jpet.102.045559. [DOI] [PubMed] [Google Scholar]
- Li J-G, Benovic JL, Liu-Chen L-Y. Mechanisms of agonist-induced down-regulation of the human kappa-opioid receptor: internalization is required for down-regulation. Mol Pharmacol. 2000;58:795–801. [PubMed] [Google Scholar]
- Li J-G, Chen C, Liu-Chen L-Y. Ezrin-radixin-moesin-binding phosphoprotein-50/Na+/H+ exchanger regulatory factor (EBP50/NHERF) blocks U50,488H-induced down-regulation of the human kappa opioid receptor by enhancing its recycling rate. J Biol Chem. 2002b;277:27545–27552. doi: 10.1074/jbc.M200058200. [DOI] [PubMed] [Google Scholar]
- Li J-G, Luo LY, Krupnick JG, Benovic JL, Liu-Chen L-Y. U50,488H-induced internalization of the human kappa opioid receptor involves a beta-arrestin- and dynamin-dependent mechanism. Kappa receptor internalization is not required for mitogen-activated protein kinase activation. J Biol Chem. 1999;274:12087–12094. doi: 10.1074/jbc.274.17.12087. [DOI] [PubMed] [Google Scholar]
- Liang W, Fishman PH. Resistance of the human beta1-adrenergic receptor to agonist-induced ubiquitination: a mechanism for impaired receptor degradation. J Biol Chem. 2004;279:46882–46889. doi: 10.1074/jbc.M406501200. [DOI] [PubMed] [Google Scholar]
- Liu-Chen L-Y. Agonist-induced regulation and trafficking of kappa opioid receptors. Life Sci. 2004;75:511–536. doi: 10.1016/j.lfs.2003.10.041. [DOI] [PubMed] [Google Scholar]
- Marchese A, Benovic JL. Agonist-promoted Ubiquitination of the G Protein-coupled Receptor CXCR4 Mediates Lysosomal Sorting. J Biol Chem. 2001;276:45509–45512. doi: 10.1074/jbc.C100527200. [DOI] [PubMed] [Google Scholar]
- Marchese A, Raiborg C, Santini F, Keen JH, Stenmark H, Benovic JL. The E3 Ubiquitin Ligase AIP4 Mediates Ubiquitination and Sorting of the G Protein-Coupled Receptor CXCR4. Dev Cell. 2003;5:709–722. doi: 10.1016/s1534-5807(03)00321-6. [DOI] [PubMed] [Google Scholar]
- Martin NP, Lefkowitz RJ, Shenoy SK. Regulation of V2 vasopressin receptor degradation by agonist-promoted ubiquitination. J Biol Chem. 2003;278:45954–45959. doi: 10.1074/jbc.M308285200. [DOI] [PubMed] [Google Scholar]
- Miranda M, Wu CC, Sorkina T, Korstjens DR, Sorkin A. Enhanced ubiquitylation and accelerated degradation of the dopamine transporter mediated by protein kinase C. J Biol Chem. 2005;280:35617–35624. doi: 10.1074/jbc.M506618200. [DOI] [PubMed] [Google Scholar]
- Mizuno E, Iura T, Mukai A, Yoshimori T, Kitamura N, Komada M. Regulation of epidermal growth factor receptor down-regulation by UBPY-mediated deubiquitination at endosomes. Mol Biol Cell. 2005;16:5163–5174. doi: 10.1091/mbc.E05-06-0560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petaja-Repo UE, Hogue M, Laperriere A, Bhalla S, Walker P, Bouvier M. Newly synthesized human delta opioid receptors retained in the endoplasmic reticulum are retrotranslocated to the cytosol, deglycosylated, ubiquitinated, and degraded by the proteasome. J Biol Chem. 2001;276:4416–4423. doi: 10.1074/jbc.M007151200. [DOI] [PubMed] [Google Scholar]
- Pickart CM, Fushman D. Polyubiquitin chains: polymeric protein signals. Curr Opin Chem Biol. 2004;8:610–616. doi: 10.1016/j.cbpa.2004.09.009. [DOI] [PubMed] [Google Scholar]
- Roth AF, Davis NG. Ubiquitination of the yeast a-factor receptor. J Cell Biol. 1996;134:661–674. doi: 10.1083/jcb.134.3.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Row PE, Prior IA, McCullough J, Clague MJ, Urbe S. The ubiquitin isopeptidase UBPY regulates endosomal ubiquitin dynamics and is essential for receptor down-regulation. J Biol Chem. 2006;281:12618–12624. doi: 10.1074/jbc.M512615200. [DOI] [PubMed] [Google Scholar]
- Shenoy SK. Seven-transmembrane receptors and ubiquitination. Circ Res. 2007;100:1142–1154. doi: 10.1161/01.RES.0000261939.88744.5a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenoy SK, McDonald PH, Kohout TA, Lefkowitz RJ. Regulation of receptor fate by ubiquitination of activated beta 2-adrenergic receptor and beta-arrestin. Science. 2001;294:1307–1313. doi: 10.1126/science.1063866. [DOI] [PubMed] [Google Scholar]
- Simonin F, Karcher P, Boeuf JJ, Matifas A, Kieffer BL. Identification of a novel family of G protein-coupled receptor associated sorting proteins. J Neurochem. 2004;89:766–775. doi: 10.1111/j.1471-4159.2004.02411.x. [DOI] [PubMed] [Google Scholar]
- Tanowitz M, von Zastrow M. Ubiquitination-independent Trafficking of G protein-coupled receptors to lysosomes. J Biol Chem. 2002;277:50219–50222. doi: 10.1074/jbc.C200536200. [DOI] [PubMed] [Google Scholar]
- Trompouki E, Hatzivassiliou E, Tsichritzis T, Farmer H, Ashworth A, Mosialos G. CYLD is a deubiquitinating enzyme that negatively regulates NF-kappaB activation by TNFR family members. Nature. 2003;424:793–796. doi: 10.1038/nature01803. [DOI] [PubMed] [Google Scholar]
- Wang Y, Zhou Y, Szabo K, Haft CR, Trejo J. Down-regulation of protease-activated receptor-1 is regulated by sorting nexin 1. Mol Biol Cell. 2002;13:1965–1976. doi: 10.1091/mbc.E01-11-0131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whistler JL, Enquist J, Marley A, Fong J, Gladher F, Tsuruda P, Murray SR, von Zastrow M. Modulation of postendocytic sorting of G protein-coupled receptors. [see comments.] Science. 2002;297:615–620. doi: 10.1126/science.1073308. [DOI] [PubMed] [Google Scholar]
- Xu W, Chen C, Huang P, Li J, de Riel JK, Javitch JA, Liu-Chen L-Y. The conserved cysteine 7.38 residue is differentially accessible in the binding-site crevices of the mu, delta, and kappa opioid receptors. Biochem. 2000;39:13904–13915. doi: 10.1021/bi001099p. [DOI] [PubMed] [Google Scholar]
- Zhu J, Luo LY, Mao GF, Ashby B, Liu-Chen LY. Agonist-induced desensitization and down-regulation of the human kappa opioid receptor expressed in Chinese hamster ovary cells. J Pharmacol Exp Ther. 1998;285:28–36. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.