Abstract
Nanoparticles present a new collection of contrast agents for the field of in vivo molecular imaging. This review focuses on promising molecular imaging probes for optical and magnetic resonance imaging based on four representative nanomaterial(s) platforms: quantum dots, upconversion phosphors, superparamagnetic iron oxides, and dendrimer-based agents. Quantum dots are extremely efficient fluorescent nanoparticles with size-tunable emission properties, enabling high sensitivity and greater depth penetration. Their heavy metal composition and long retention in the body, however, pose concerns for clinical translational applications. Upconversion phosphors generate excellent signal-to-background contrast because they emit light with higher energy than the excitation photons and autofluorescence signals. For MRI, iron oxide particles also generate excellent signal and have been used in liver imaging and for cell tracking studies. As they are metabolized through endogenous iron salvage pathways, they have already been introduced as clinical contrast agents. Lastly, dendrimers, a ‘soft’ nanoparticle, can be used as a structural basis for the attachment of small molecule imaging agents and/or targeting groups. This array of nanoparticles should offer insights into the uses and potentials of nanoparticles for the molecular imaging.
Keywords: Molecular imaging, nanomaterial, quantum dot, dendrimer, iron oxide particle
Introduction: General molecular imaging and nanomaterial use, past and current
Molecular imaging is the visualization or measurement of biological processes and targets in living organisms at all physical levels ranging from that of molecules to that of human bodies. Molecular probes for imaging must have both a targeting moiety and an imaging group. An important component is also the carrier molecule to which the targeting moiety and imaging group are attached. The carrier molecule is a major determinant of the biodistribution of a molecular probe. Targeted contrast agents for molecular imaging capitalize on the differential expression of receptors, enzymes, or other molecules in the microenvironment that are unique to a pathologic condition such as cancer. A vast array of targeting ligands, emerging from the science of molecular biology and chemical biology, and a more limited number of known imaging groups emerging from the physical sciences can be employed in molecular imaging probes. Nanoparticles represent an important new innovation in the development of such probes because of their distinct structural and functional properties.
The size of a carrier molecule determines its imaging properties. Nanoparticles fill in the gap in size between low molecular weight small molecules and bulky organic and inorganic particles greater than 100 nm in diameter. The size range of 10–100 nm represents a biologically unique niche for carrier molecules. Despite their small diameters, the surface area of these particles is sufficiently large to enable multifunctional applications. The volume is also small enough to circulate efficiently without rapid sequestration by the reticuloendothelial system by employing appropriate surface modifications. Small adjustments in size and particle coating can lead to changes in routes of excretion and clearance times.
Among the nano-sized materials, hard materials to be discussed include quantum dots (QDs), superparamagnetic iron oxide (SPIO) particles, and upconversion phosphors (UCPs). Alternatively, nanoscale carriers made of soft materials, such as dendrimers, liposomes, and micelles can be used as carrier molecules for various imaging payloads. These nanomaterials can be combined to enable specific targeting, highly functional imaging, and even therapeutic hybrid agents. Physico-chemical characteristics including size, hydrophilicity, and charge of the carrier molecule are primary determinants of bioavailability, but additional selectivity can be achieved by the addition of one or more targeting moieties to the nanoparticle.
Two modalities, magnetic resonance imaging (MRI) and optical imaging, have been typically employed for biomedical imaging with nanoparticles. Compared with conventional small molecular imaging agents, nanomaterials-based contrast agents are generally designed to yield strong signal from a single particle. This strong signal can partly overcome the intrinsic limitations of each imaging modality, e.g., low sensitivity for the MRI or poor depth penetration for the optical imaging. Herein we discuss a selection of these nanoparticle imaging agents, to demonstrate their versatility for molecular imaging.
Classes of nanoparticles
Although many different classes of nanoparticles exist, for purposes of this discussion on molecular imaging we will consider several particles with distinctive characteristics important for molecular imaging.
QDs are nanosized inorganic semiconductors characterized by unique electronic and optical properties. First synthesized and described by Efros and Ekimov in 1982, the synthesis of QDs has greatly improved (Efros 1982, Kim et al. 2003, Jamieson et al. 2007, Hezinger et al. 2008, Delehanty et al. 2009). Though the bandgap energy for electronic excitation of bulk semiconductors depends exclusively on composition, the bandgap energy of QDs is size-dependent (Efros 1982). As a result, particle size can be used to fine-tune the fluorescence emission wavelength. QDs have extremely broad absorption spectra, large absorption cross-sections, narrow and nearly symmetric fluorescence peaks, and are extremely photostable (Smith et al. 2008). The surfaces of QDs can also be modified for improved biocharacteristics, active targeting, or the addition of diagnostic or therapeutic groups (Akerman et al. 2002, Smith et al. 2008, Delehanty et al. 2009).
UCPs are another class of promising optical imaging agents (Zijlmans et al. 1999). These particles are typically composed of phosphor nanocrystals doped with rare earth metals (Yi et al. 2004). Unlike most fluorophores, which absorb excitation light at higher energy and release emission light at lower energy, UCPs are unique because they sequentially absorb low energy photons and emit higher energy photons. For biomedical applications, photons are most practically converted from the near-infrared (NIR) (excitation) to visible to NIR range (emission) which enables deeper tissue excitation penetration (as higher energy photons scatter more), and reduces background signal from the autofluorescence of natural biomaterials which are not excited in the NIR.
Iron oxide particles are another class of contrast agents, investigated since the 1980s, which are generally used in conjunction with MRI (Young et al. 1981). MRI contrast agents are detected by altering the relaxation of surrounding spin-excited protons through magnetic interactions. SPIO particles are composed of an iron oxide core of magnetite or maghemite with a hydrophilic coating for stabilization and dispersion in aqueous media (Thorek et al. 2006). They range in size and classification from ‘ultrasmall’ (USPIO) of 20–40 nm, to ‘small’ (SPIO) 60–150 nm, to larger particles (i.e., micron-sized iron oxide nanoparticles–MPIOs) for cell-labeling and oral-administration. Through their paramagnetic properties iron oxides shorten both the transverse and longitudinal relaxation times, generating image contrast which is dependent on local concentrations of the iron.
Soft materials such as dendrimers represent still another class of nanoparticles. Dendrimers are spherical, branched polymers whose size can be precisely tuned by the selection of cores and interiors, followed by polymerization leading to distinctive ‘generations’ of nanoparticles (Barrett et al. 2009). Two types of dendrimers, the polyamidoamine interior (PAMAM) and the polypropylimine interior (PPI) dendrimers, are highly soluble in aqueous solution and have been commercially available for a decade (Kobayashi and Brechbiel 2004). The large particle surface covered in primary amino or carboxyl groups can be easily conjugated to targeting moieties as well as small molecule imaging probes such as organic fluorophores, radioligands, or gadolinium (Gd) chelates.
Advantages to imaging with nanoparticles
Nanoparticles have a number of advantages for molecular imaging probes. The surface area of nanoparticles enables coordination to other molecules, for targeting and multimodality imaging, while their relatively large size prevents rapid clearance, resulting in adequate time for accumulation at the target. Each class of nanoparticles exhibits qualities which can be useful for different applications. Dendrimer-based agents, for instance, have inherent targeting capabilities with composition- and size-dependent biodistribution, in addition to numerous surface groups to which targeting moieties (such as antibodies) and/or imaging agents can be attached. QDs have size-dependent emission spectra that enable fine tuning for optical imaging and the potential for multi-spectral imaging of several targets simultaneously. UCPs also enable deep-tissue imaging by emitting photons of higher energy than the excitation energy reducing signal contamination due to autofluorescence.
Biodistribution of nanoparticles
Each nanoparticle has its own characteristic biodistribution based on size and charge. These are crucial determinants of the type of molecular imaging that is possible with each carrier nanoparticle.
This is best demonstrated by dendrimers that can vary in size from 1–2 nm to 10–15 nm depending on composition and generation. The biodistribution of dendrimer based agents can be significantly altered by changing the core and interior components, increasing the generation number, and adding surface functionalities (Kobayashi and Brechbiel 2003, 2005). These variables directly affect the size, hydrophilicity, net charge, surface charge, and targeting ability (Kobayashi and Brechbiel 2004). Two forms of the highly branched spherical polymers have been commercially available for a decade and have been extensively studied; the PAMAM with an amine or ethylene diamine core, and the PPI with a diaminobutane core, both surrounded by primary amino or carboxyl groups. The conjugation of other groups, such as polyethylene glycol (PEG) chains affects their distribution, likely by altering their effective mass, diameter, hydrophilicity, and/or charge (Kaminskas et al. 2008, Lim et al. 2008). The site of injection can also have strong implications for biodistribution and excretion; intravenous injection often results in accumulation in the liver whereas local or intraperitoneal injections will largely be confined to the injected space (Kobayashi and Brechbiel 2004, Kobayashi et al. 2004b).
Lower generation PAMAM dendrimers (the 2nd and 3rd generation) rapidly leak from the vasculature into the surrounding soft tissue. Medium-sized dendrimers (of the 5th and 6th generation but smaller than 8 nm in diameter) leak more specifically from tumor vasculature while larger dendrimers (6th generation or above) show minimal leakage from the circulation, even within tumors. PAMAM dendrimers larger than 9 nm (6th generation or above) are generally excreted by the liver, though G9 and G10 become trapped in the reticuloendothelial system (Kobayashi and Brechbiel 2003). These higher generation dendrimers are also more difficult to reliably synthesize leading to more polydispersion of size. Dendrimers smaller than 7 nm (5th generation or below) are primarily excreted by the kidneys (Kobayashi and Brechbiel 2004). Linkage to other groups, such as PEG polymers, can significantly alter distribution, pharmacokinetics and excretion patterns (Kojima et al. 2000, Kobayashi et al. 2001a).
The biodistribution of other nanoparticles depends on particle size and on composition, particularly of the outer layer. Biodistribution and circulation times are also affected by nanoparticle interaction with plasma proteins, which increase the effective size of the particles (Aggarwal et al. 2009). Larger nanoparticles, such as QDs, generally circulate for long periods or are taken up by the reticuloendothelial system (Smith et al. 2008). Plasma half-lives can be significantly altered with surface coatings, which affect protein adsorption and particle size, thus escaping from the reticuloendothelial system with a ‘stealth’ feature. In general, hard particles smaller than 7 nm in diameter, including QDs, iron oxide, or silica, can be excreted through the kidney, leading to rapid clearance from blood circulation. Chemical characteristics of the surface coating on these hard particles also can drastically alter their biodistribution and target organs similar to soft nanomaterial(s) including dendrimers (Choi et al. 2010).
Stability of optical agents
The stability of certain nanoparticles enables long-term imaging studies, with particular implications for cell tracking which requires long lived molecular probes. While the base particles are susceptible to degradation, coating layers provide protection and stability. QDs are more biostable than conventional organic fluorophores and can be 100–1000 times more photostable (Smith et al. 2008). As a demonstration, cell-internalized QDs have been imaged weeks after injection. While the fluorescent cores of QDs are relatively unstable when exposed to the environment, shell layers and capping significantly reduce the potential for oxygenation or decomposition (Medintz et al. 2005). Semiconductor shells, which have wider bandgaps than the core material, provide both electrical insulation and protection from oxidation or chemical instability (Smith et al. 2008). The type and thickness of the coating dictates the core protection and long-term stability of the particle. Coating with amphiphilic polymers, for example, not only enables dispersion in aqueous solution but QD stability for long periods. Similarly, with a coating layer, UCPs demonstrate chemical and photostability (Chatteriee et al. 2008a, Jalil and Zhang 2008). Polyethyleneimine (PEI)-coated particles were stable in PBS for weeks after synthesis. They were extremely resistant to photobleaching, with no loss of fluorescence following seven hours of continuous laser excitation (Chatteriee et al. 2008a). From the clinical translation standpoint, however, stability is not always a desirable feature since a clinically viable molecular probe is expected to undergo degradation and elimination in a timely manner.
Optical imaging agents: Upconversion phosphors and quantum dots
The poor penetration of optical light within human tissue poses a significant barrier to the advancement of optical imaging. Light-tissue interactions, particularly light scattering, directly limit the depth of penetration favoring longer wavelength light which can penetrate further into tissue. Additionally, the absorbance of short wavelength ultraviolet (UV) light can cause DNA damage and the absorbance of optical light generates autofluorescence, which can result in a large background signal. A potential solution is the use of longer wavelength NIR excitation of fluorophores, either through two photon fluorescence imaging (TPFI) or by using UCPs. Because some energy is lost in the fluorescence process of typical fluorophores, emitted photons shift towards longer wavelengths. In contrast, UCPs absorb multiple photons, enabling the emission of shorter wavelength, higher energy photons, for example using NIR excitation for visible range emission. TPFI requires the nearly simultaneous absorption of two photons, resulting in a low efficiency. UCPs, however, emit higher energy light following the sequential absorption of multiple photons, enabling a higher efficiency.
UCPs are composed of doped phosphors, among which sodium yttrium fluoride (NaYF4) nanocrystals co-doped with ytterbium (Yb) and erbium (Er) are among the most efficient and widely studied infrared-to-visible nanoparticles (Yi et al. 2004). There is even some potential for tuning the emission wavelength of these crystals by varying the relative concentration of dopants (Wang and Liu 2008). Passive targeting can be used or the surfaces of the particles can be conjugated to targeting groups for active targeting (Zijlmans et al. 1999, Chatteriee et al. 2008b). UCPs further minimize background signal by generating fluorescence that cannot overlap with autofluorescence, as they are at shorter and longer wavelengths, respectively, than the excitation light.
QDs, which emit light in the far-red to NIR range, present another solution for optical imaging. Unlike organic fluorophores, which have broad emission profiles, low photobleaching thresholds and small shifts between absorption and emission, QDs exhibit broad absorption profiles, tunable and narrow emission peaks, and extremely high photostability. They are approximately 2–100 nm in diameter and are typically composed of a CdSe or CdTe core for biological applications (Rzigalinski and Strobl 2009). QDs are 10–20 times brighter than organic fluorophores as a result of comparable quantum efficiencies and significantly larger molar extinction coefficients (10–50 times greater) (Smith et al. 2006). This brightness enables the visualization of low concentration targets that lie deep in tissue.
Semiconductors absorb photons that have energy greater than the bandgap energy. Electronic relaxation is often accompanied by photon emission with energy equal to that of the bandgap (Smith et al. 2006). This bandgap energy becomes size dependent when the charge carriers in the semiconductor are confined to a small enough space, an effect known as quantum confinement. As a result, the emission energy of nanoscale, fluorescent QDs, with the same metallic composition, depend directly on their size, with smaller particles creating more confinement, higher energy bandgaps and thus shorter-wavelength photon emissions. QDs have been synthesized for peak emission from 400–1350 nm (Cai et al. 2007). More recently, composition changes have been used to tune the emission frequency of QDs of the same size, specifically by altering the ratio of employed alloys (Smith et al. 2008). Because of their broad excitation spectra, a single light source can be used to excite QDs with multiple emission spectra (Kobayashi et al. 2007a, Kosaka et al. 2009). Thanks to the tunable narrow emission peaks and broad excitation spectra, which are optimal for spectrally revolved imaging, QDs have the potential to image multiple molecular targets simultaneously (Smith et al. 2006).
Background from the autofluorescence of natural fluorophores can also be eliminated by the combined use of bioluminescent particles to generate ‘self-illuminating’ QDs (So et al. 2006). Such bioluminescence resonance energy transfer (BRET) could be used to activate QDs, thus providing molecular or enzymatic sensitivity (Yao et al. 2007).
In vivo imaging
Nanoparticles enable the development of contrast agents with multiple imaging groups, either of the same type for increased enhancement or of different types for multimodal imaging (Kobayashi and Brechbiel 2004). A variety of applications have emerged for these agents. Because they are inherently macromolecular, molecular probes containing nanoparticles exhibit unique properties that enable them to be useful in particular imaging settings.
Lymphatic imaging
Lymphatic imaging depends on developing imaging probes of a narrow range of sizes that enable rapid uptake of the nanoparticle within the lymphatics. Dendrimer-based contrast agents, for instance, can be designed to passively target the lymphatic system. Using an MRI imaging group (Gd or iron) dendrimers have the potential to not only locate sentinel lymph nodes (SLNs) but provide diagnostic information on lymphatic flow and anatomy. In addition to MRI imaging groups, dendrimers can be conjugated to radionuclides and optical fluorophores. This is particularly significant as each modality offers its own advantages and the addition of multiple agents to a single particle for multimodal probes can combine the advantages of the component particle modalities.
Radionuclide/NIR optical dual modal imaging probes have been synthesized and demonstrated in the lymphatic imaging of mice. Five distinct probes labeled with 111In and one of five NIR optical probes, each emitting at a distinct wavelength, were generated (Kobayashi et al. 2007b). The radionuclide labeling provided good penetration depth and quantitative information while the fluorescent label enabled simultaneous real-time imaging of multiple lymphatic basins (through the spectral resolution of the different fluorophores), and has the potential to be used during surgical procedures. However, it should be noted that from a translational point of view, increasing complexity of nanoparticles makes clinical approval more difficult.
With the proper size, nanoparticles can be passively taken up and retained by the lymphatics. PAMAM dendrimers of the sixth and eighth generations were found to best enhance the lymphatic system, with the former enhancing at a slightly faster pace, especially around the breast tissue (Kobayashi et al. 2006). Dendrimer-based Gd agents were used to visualize the lymphatic drainage from breast tissue following injections around the areola in healthy and cancerbearing mice (Kobayashi et al. 2004b). Dynamic micro-magnetic resonance lymphangiography (MRL) showed enhancement of three major draining lymph vessels and nodes in healthy mice but no enhancement of solid tumor foci within the nodes (Kobayashi et al. 2003b). In addition to their clinical potential, such dendrimer-based agents have been successfully employed for the characterization of animal models of lymphoma (Kobayashi et al. 2005).
Alternatively, USPIO nanoparticles demonstrated negative enhancement of non-tumor-filled lymph nodes 24 h after intravenous injection, as a result of phagocytosis by macrophages within the nodes, that allows us to perform MRL for visualizing normal part of lymph nodes in black. As a result, MRL could potentially be used to locate metastases in the systemic lymph nodes, since the tumor-filled part of the lymph nodes would not change signal at 24 h (Harisinghani et al. 2003).
Optical lymphangiography may provide complimentary information to MRL. Optical agents enable multicolour imaging. The simultaneous imaging of multiple lymphatic basins can be demonstrated with multicolour imaging (Kobayashi et al. 2007b). In this case, each basin is injected with a different optical agent. By performing multispectral imaging, it is possible to discern which agent was injected into which lymphatic basin based on the wavelength of emitted light. This can be accomplished with optical nanoparticles (QD or UCPs) or by labeling dendrimers with small molecule organic fluorophores.
QDs are particularly suited for lymphatic imaging due to their size and visible and NIR QDs can be visualized in real-time with existing and inexpensive technology. The NIR fluorescence also enables deeper lymphatics to be visualized. QDs are significantly brighter and more photostable than organic fluorophores (Kosaka et al. 2009).
A compelling potential application of QDs is for the real-time detection of SLNs during surgery. SLNs mapping followed by histological analysis of the resected node is a common procedure for the detection of cancer metastases especially in breast cancer or melanoma. Currently, SLNs are detected with radiolabelled sulfur colloid which is detected with handheld gamma-ray probes and is known as lymphoscintigraphy. Blue isosulfan dye is also used to confirm the location of the SLN but this requires accurate dissection of tissue down to the node. Lymphoscintigraphy is limited by its poor spatial resolution and blue dye cannot be visualized beyond several millimeters of tissue and additionally poses a cosmetic problem due to skin staining (Jain et al. 2009). QDs, however, could enable the real-time, detection of SLN during surgery, with the possibility of identifying nodes several centimeters deep in the skin.
SLN mapping with QDs was first explored in 2004 in animal models (Kim et al. 2004). Following intradermal injection, QDs migrate rapidly to the lymphatics and colocalize with isosulfan blue in the node. The potential as a surgical aid for SLN mapping was further demonstrated with injections in pig models. The surgeon was able to see lymphatic flow to the SLN and using NIR fluorescence imaging was able to find the SLN even within large lymph node clusters. Further ex vivo analysis of the excised tissue showed the QDs to be exclusively in the SLN, specifically in the outermost rim. A later study demonstrated the migration of QDs to the SLN following injection directly into tumors, further supporting their potential for SLN mapping (Ballou et al. 2007).
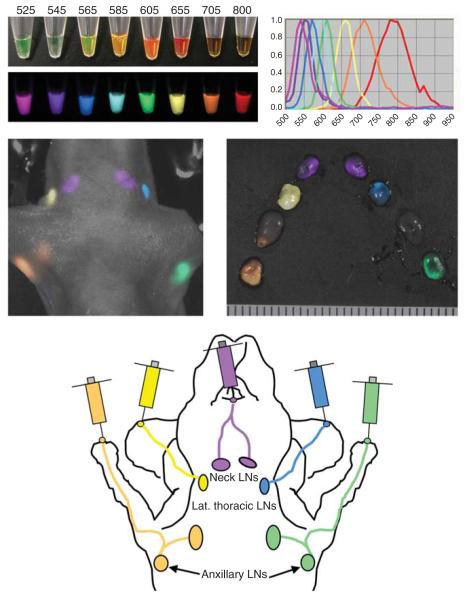
In experimental settings, multicolour imaging has been used to simultaneously image multiple lymphatic basins (Kobayashi et al. 2007a). QDs of five distinct emission wavelengths in the visible and NIR ranges and spectral imaging were used to simultaneously image five different lymphatic basins (Figure 1). Spectral imaging using the Maestro In-Vivo imaging system (CRi, Waltham, MA, USA) enabled the visualization of each QD and thus the lymphatic drainage to the primary lymph nodes of each injection site. By employing visible QDs, the real-time five colour lymphatic imaging achieved with much less expensive installment setting than the spectral imager (Kosaka et al. 2009). Most of the QDs were taken up by macrophages and dendritic cells in the lymphatic sinuses (Kosaka et al. 2009). Imaging was possible at seven days after injection with little signal loss after the first day, a feature unique to QDs.
Figure 1.
Multiple colour lymphatic imaging achieved by the use of multiple different quantum dots. (A) Different quantum dots can be distinguished by theirdistinct colours (emission light wave lengths) even with a single excitation UV light. (B) With simultaneous use of five different quantum dots, both in vivo and ex vivo images depict five different lymphatic basins of distinct colours. (C) Schematic illustration of lymphatic drainages in the head and neck region in a mouse.
UCPs can also be used to optically image the lymphatic system but without background signal from autofluorescence (Kobayashi et al. 2009). Excellent target-to background ratios can be achieved with UCPs. Any autofluorescent signal from biological materials in the body would be shifted to a longer wavelength, while signal from the UCPs would be shifted to a shorter wavelength, making them completely distinct. In addition, theoretically, BRET-QDs, which are of the appropriate size for lymphatic drainage, could also optically image lymph nodes without background signal from autofluorescence, as only the QDs attached to the bioluminescent molecule would be excited.
Cell tracking
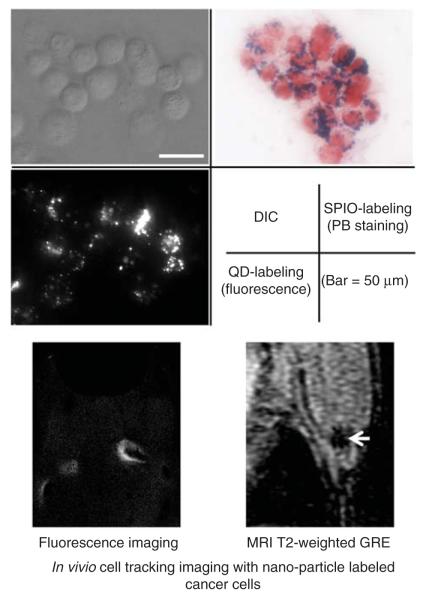
The advent of cell based therapies and the need to understand better the fate of injected cells has stimulated the development of cell tracking techniques (Rogers et al. 2006). Tracking could also offer more important insights into other medical questions, such as how metastatic tumors spread (Voura et al. 2004). Desirable characteristics in a cell tracking imaging agent include that: (1) it is biocompatible, (2) is safe for the injected cells and does not interfere with their function, (3) is highly sensitive (capable of detecting single cells), (4) does not dilute with cell division, (5) does not transfer to other cells, and (6) can be imaged for months to years after initial labeling (Frangioni and Hajjar 2004). For instance, to visualize individual or extremely small populations of cells, the contrast agents must have an ‘effect size’ large enough to be detectable, possible with SPIOs and QDs (Figure 2) (Frangioni and Hajjar 2004). Naturally, no ideal cell tracking agent exists.
Figure 2.
In vivo cell tracking imaging by the use of nanomaterials. (A) Microscopic images of SPIO- or quantum dots-labeled breast cancer cells (MDA-MB468). Both nanoparticles are incorporated into cytoplasm of cells. (B) In vivo cell tracking imaging in the lymphatic system. Labeled cancer cells (B16 melanoma) in lymph nodes can be depicted by both fluorescence and MR imaging.
Iron oxide nanoparticles, however, are particularly well suited for cell tracking with MRI (Rogers et al. 2006). SPIO nanoparticles generate negative contrast by creating a large magnetic field gradient which causes effects far from the individual nanoparticle. Numerous methods have been employed for cellular labeling with these MRI contrast agents, including nonspecific cellular internalization (dependent on the cell type, particle size and particle coating), particle surface modification of nanoparticles including conjugation with the twin arginine translocation (Tat) peptide (Moore et al. 2002), complexes with transfection agents (Arbab et al. 2004), and magnetoelectroporation (Rogers et al. 2006). MRI-based cell tracking with iron oxide particle labeled cells has the potential to detect a single labeled cell in the body under perfect conditions (Shapiro et al. 2004).
QDs are an alternative contrast agent for cell tracking, providing excellent resolution, and the potential for the use of multiple colours to track different cell lines. Though doubts as to the biocompatibility of QDs exist, especially with regard to their retention in the body, many studies have shown them to not interfere with cellular growth, differentiation, or behaviour (Jaiswal et al. 2003, Voura et al. 2004). Although the optical agents can only be imaged through a limited depth of tissue, they could be used for applications of cell tracking on surface tissues through the microscopy of tissue samples and for scientific inquiry in small animal models (Voura et al. 2004, Hayashi et al. 2007). It may also be possible to sample peripheral blood to look for previously tagged cells that are still circulating.
Blood pool imaging
In sufficient doses, larger, hydrophilic dendrimer-based agents can be employed as blood pool contrast agents (Kobayashi and Brechbiel 2004). Mid-size dendrimers are not rapidly excreted nor do they rapidly leak from the vasculature to surrounding tissue and therefore are retained in the vessels for considerable times. Specifically, whereas the smallest G2- and G3-dendrimer agents rapidly leaked to the extravascular tissue, larger G4- and G5-based agents leaked primarily from tumor vessels, while the still larger G6- and G8-based agents were primarily remained in the vessels. Paradoxically even larger G9- and G10-based agents, however, were rapidly cleared from circulation by entrapment in the reticuloendothelial system, prohibiting their use as blood pool contrast agents. Longer plasma half-lives were achieved by the PEGylation of the G4-based agent, which led to decreased liver accumulation and kidney excretion, likely due to the increased hydrophilicity and size of the nanoparticle and the decreased recognition by the immune surveillance system (Kobayashi et al. 2003a).
Organ specific (liver/kidney)
The biodistribution of dendrimers enables their use as organ-specific agents. Corresponding to their route of excretion, low generation dendrimer-based contrast agents (of both the PAMAM and diaminobutane (DAB) composition) are quickly excreted by the kidney (Kobayashi and Brechbiel 2004). Among the dendrimers tested, the G2 DAB dendrimer (DAB-G2) was cleared most rapidly and thus was used in further studies (Kobayashi et al. 2004a).
The utility of these agents was shown in sepsis, ischemia/reperfusion, and cisplatin intoxicated models in mice. Injured kidneys showed delayed excretion with the degree of impairment related to the severity and intra-renal location of the injury. These findings preceded elevation in serum creatinine and histology.
Dendrimer-based contrast agents have also been developed for liver specific imaging (Kobayashi et al. 2001b). As molecules with hydrophobic interior tend to accumulate in the liver, a DAB/PPI dendrimer (with a hydrophobic, pure aliphatic polyamine core) of the fifth generation was evaluated for use as a liver specific agent, as size-dependent biodistribution studies of dendrimers had previously shown that midsize to large dendrimers are excreted by the liver (Kobayashi et al. 2001b). Following administration of the DAB/PPI-G5 agent, the liver parenchyma was highly enhanced for up to 25 min. In tumor-bearing mice, the micrometastases (as small as ~0.3 mm as confirmed by histological analysis) were poorly enhanced, generating large liver:tumor signal differences which were superior to those obtained with conventional intravenous Gd chelates.
SPIO particles are taken up by the reticuloendothelial system, particularly Kupffer cells in the liver, and generate negative contrast in liver (Thorek et al. 2006). The signal is less reduced in cirrhotic tissue, presumably due to Kupffer cell dysfunction suggesting a means of functionally evaluating liver tissue (Tanimoto et al. 2002, Margolis et al. 2007).
Like SPIOs, most nano-sized particles over 30 nm in diameter are usually recognized by the reticuloen-dothelial system, particularly the liver and the spleen. As a result, liver-targeted contrast agents are relatively easy to create but agents for other targets must include a ‘stealth feature’ to escape the reticuloendothelial system. In contrast, liver-targeting nano-sized contrast agents are generally excreted into the bile, unlike the large particles engulfed by the macrophages, which are not easily designed. The DAB/PPI-G5 contrast agent therefore remains a promising liver parenchymal contrast agent.
Cancer imaging
The same qualities that make QDs attractive for lymphatic, vascular, and organ targeting potentially make them interesting as cancer imaging agents. The large surface area and multiple surface functionalities enable the conjugation of targeting moieties, in addition to therapeutic agents or other diagnostic agents (Kim et al. 2004). Spectral imaging of multiple QDs also enables the simultaneous imaging of multiple cancer targets.
The targeted imaging of cancer remains a goal for molecular imaging. Progress towards this goal was first made in 2002, when Akerman and co-workers generated QD-peptide conjugates to target specific vascular sites (Akerman et al. 2002). The conjugates specifically detected cellular targets expressing antigens both in vitro and in vivo. While whole organs could not yet bevisualized by QDs in vivo, the ability to target specific antigens with fluorescent QD markers had been demonstrated.
Another step towards molecular imaging of cancer with QDs was achieved soon thereafter, in 2004, with the QD-based imaging of tumor xenografts in live animals (Kim et al. 2004). A marked decrease in fluorescence had been previously observed upon systemic administration of QDs, prohibiting their use as in vivo imaging agents. In this study, QDs were coated with an amphiphilic triblock copolymer to protect and disperse the QDs, PEG molecules for improved biocompatibility and circulation (to make them ‘stealthy’ and avoid recognition by the reticuloendothelial system), and targeting ligands to bind the imaging agents to the cancer. Both passive and active targeting of tumor xenografts expressing the prostate-specific membrane antigen (PSMA) were demonstrated in live animal models. The QDs with only a polymer coating were unable to target the tumor xenografts, while those with surface PEG groups had longer circulation times and slowly accumulated at the tumor sites. This passive accumulation was most likely due to the enhanced permeability and retention (EPR) effect, by which circulating molecules accumulate around tumors due to their leaky neovasculature and poor lymphatic drainage. The QDs conjugated to a anti-PSMA-monoclonal antibody, however, accumulated and were retained by the PSMA-expressing tumors, generating intense signals in whole animal fluorescence imaging. The authors noted that the use of far-red and NIR QDs should permit deeper tissue imaging, due to minimized Rayleigh scattering and natural light absorption.
Toxicity
Generalizations on the toxicity of nanoparticles (NPs) or even classes of NPs are difficult, if not impossible, to make due to the high variability in composition and structure. Many varieties of nanoparticles are potentially toxic or data does not yet exist to prove otherwise. Often, multiple components of the nanoparticles, including the structural base and the imaging moiety, pose individual toxicity risks. In addition, the full nanoparticle might have toxic risks that differ from each component’s individual toxicities. Variables in the toxicity and clearance of NPs include their composition, stability, size, shape, coating, and surface charge. Delayed excretion additionally poses a significant barrier to the approval of nanoparticles for clinical use. Additionally, retention of NPs can interfere with later imaging studies. While a long retention time enables long-term imaging studies, it also poses risks of long-term effects and necessitates long term toxicity studies.
Dendrimers can have toxic effects, depending on the core, surface composition (particularly with the positive charge), interior charge, and generation, all of which also affect the biodistribution (Duncan and Izzo 2005). Concerning the end group charge, cationic dendrimers have been over 10,000-fold more cytotoxic compared with anionic or neutrally charged dendrimers and are therefore avoided for in vivo applications (Duncan and Izzo 2005).
MRI contrast agents also have the potential to cause adverse effects. A well known, adverse effect of Gd based MRI contrast agents is nephrogenic systemic fibrosis (NSF) thought to be related to transmetalation of the chelate which can occur in patients with renal failure (Lin and Brown 2007). The increased retention time of nanoparticles containing Gd poses additional risks of transchelation, making the rapid excretion of such particles particularly important (Duncan and Izzo 2005).
MRI agents based on iron oxide avoid compatibility issues associated with many of the heavy metal-containing nanoparticles. The biodegradable iron can be metabolized through endogenous salvage pathways, though biocompatible surface coatings must also be employed for in vivo use (Bulte and Kraitchman 2004). Iron quantities for diagnostic imaging are significantly less than that stored in the human body (Corot et al. 2006). Several SPIOs have demonstrated good safety profiles following injection in humans. However, there is concern over potential iron overload if the agents are repeatedly administered.
UCPs are extremely new and little data exists on their potential toxicity. Initial biodistribution studies of intravenously injected NaYF4 particles demonstrated rapid accumulation in the lungs but extremely reduced concentrations in all tissues within a day and were undetectable within a week (Chatterjee et al. 2008). Mitochondiral function and membrane leakage of bone marrow stem cells and skeletal myoblast cells were used to assess cell viability as a function of particle concentration, from 1–100 μg/ml (Jalil and Zhang 2008). Though viability decreased for both lines, it remained about 80% for both cell types at the highest tested concentrations.
The issue of biocompatibility of QDs remains unresolved. While numerous studies address the toxicity of QDs in vitro and in vivo, it should be noted that there are large variations in chemical structure among the QD family. The large variety of QDs, particularly in size, core composition, coating, and surface modification, presents a significant hurdle for analyzing their toxicity and biocompatibility as a class of agents. In addition to concerns about the toxicity of individual particles, degradation to the reactive core or to the toxic heavy metals composing the core poses further risks. Dosing parameters and methods of application also vary significantly, making toxicological studies of QDs difficult to compare or combine. The long retention time of QDs also necessitates studies of long-term effects.
QDs are semiconductor nanoparticles composed of composites of atoms from groups II–VI, often CdSe and CdTe for biological applications (Rzigalinski and Strobl 2009). Fluorescent properties are both composition- and size-dependent, factors which also affect the in vivo characteristics of the QD potentially affect their toxic properties. Each of the primary constituent atoms: cadmium, selenium, and tellurium are known to be toxic in humans, so any degradation of the core might have adverse effects (Pelley et al. 2009). Uncapped cores are also highly reactive and subject to photochemical degradation, due to large surface areas often including ‘defect’ sites (Jamieson et al. 2007). Another semiconductor with a wider bandgap (excitation energy), typically ZnS, is often used as ‘shell’ around the core, increasing the chemical stability of the particles and incidentally their fluorescence efficiency (Rzigalinski and Strobl 2009). Additional coating with materials such as mercaptopropionic acid or PEG is used to render the QDs hydrophilic. Decomposition-inducing oxidation can be minimized by the core shell and cap, but not entirely eliminated, particularly with UV exposure (Jamieson et al. 2007). Attached functionalities also have toxic potential, thus a huge variety in the composition of QDs exists, making the study and analysis of QD toxicity a particular challenge.
The sizes, coatings and functional groups strongly affect the cellular localization, biodistribution, and retention of nanoparticles, which in turn have implications for short and long-term toxicity (Choi et al. 2007).
Summary
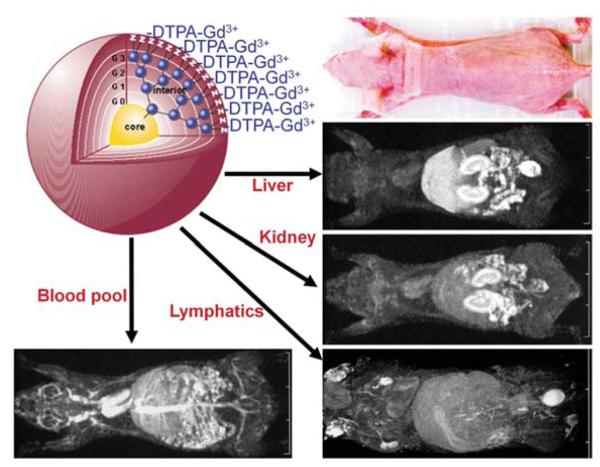
Herein we have focused on four distinct nanoparticles that have been used in imaging probes: QDs, UCPs, iron oxides and dendrimers. Each represents a separate class of nanoparticle. QDs are highly efficient emitters of light and offer the possibility of strong signal and thus improved depth penetration. However, there are concerns about their fate in the body and whether they can be translated into the clinical arena based on the heavy metals that make up their core. Similarly, UCPs offer very high signal with almost no background because they are excited at NIR wavelengths, well above those used to excited endogenous autofluorophores. Thus, signal to back ground ratios are high. Iron oxide molecules have been used in liver imaging and cell tracking and offer very high signal to background ratios. They are metabolized using endogenous iron salvage pathways and have already been introduced in several forms as clinically viable contrast agents. One application in particular is the labeling of normallymph nodes after intravenous injection of ultra-small particles of iron oxide. Finally, dendrimers are representative of the ‘soft’ nanoparticle, a versatile molecule that can be synthesized to various sizes and which contains numerous functional groups for attaching imaging agents or targeting agents (Figure 3). Taken together, these four classes of nanoparticles offer insight into the state of the art of nanoparticle imaging and hopefully serve as a basis for the understanding of other classes of nanoparticles for imaging that will undoubtedly emerge in the near future.
Figure 3.
In vivo MR images of mice using different dendrimer-based contrast agents. By changing size and interior of the dendrimer-based contrast agent, its biodistribution can be drastically altered that enables organ-specific contrast enhancements in the liver, kidneys, lymphatics, or the blood pool.
Acknowledgements
This research was supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research.
Footnotes
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Aggarwal P, Hall JB, McLeland CB, Dobrovolskaia MA, McNeil SE. Nanoparticle interaction with plasma proteins as it relates to particle biodistribution, biocompatibility and therapeutic efficacy. Adv Drug Deliv Rev. 2009;61:428–437. doi: 10.1016/j.addr.2009.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akerman ME, Chan WCW, Laakkonen P, Bhatia SN, Ruoslahti E. Nanocrystal targeting in vivo. Proc Natl Acad Sci USA. 2002;99:12617–12621. doi: 10.1073/pnas.152463399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbab AS, Yocum GT, Kalish H, Jordan EK, Anderson SA, Khakoo AY, Read EJ, Frank JA. Efficient magnetic cell labeling with protamine sulfate complexed to ferumoxides for cellular MRI. Blood. 2004;104:1217–1223. doi: 10.1182/blood-2004-02-0655. [DOI] [PubMed] [Google Scholar]
- Ballou B, Ernst LA, Andreko S, Harper T, Fitzpatrick JAJ, Waggoner AS, Bruchez MP. Sentinel lymph node imaging using quantum dots in mouse tumor models. Bioconj Chem. 2007;18:389–396. doi: 10.1021/bc060261j. [DOI] [PubMed] [Google Scholar]
- Barrett T, Ravizzini G, Choyke PL, Kobayashi H. Dendrimers in medical nanotechnology application of dendrimer molecules in bioimaging and cancer treatment. IEEE Eng Med Biol Mag. 2009;28:12–22. doi: 10.1109/MEMB.2008.931012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulte JWM, Kraitchman DL. Iron oxide MR contrast agents for molecular and cellular imaging. NMR Biomed. 2004;17:484–499. doi: 10.1002/nbm.924. [DOI] [PubMed] [Google Scholar]
- Cai WB, Hsu AR, Li ZB, Chen XY. Are quantum dots ready for in vivo imaging in human subjects? Nanoscale Res Lett. 2007;2:265–281. doi: 10.1007/s11671-007-9061-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatteriee DK, Rufalhah AJ, Zhang Y. Upconversion fluorescence imaging of cells and small animals using lanthanide doped nanocrystals. Biomaterials. 2008a;29:937–943. doi: 10.1016/j.biomaterials.2007.10.051. [DOI] [PubMed] [Google Scholar]
- Chatterjee DK, Fong LS, Zhang Y. Nanoparticles in photodynamic therapy: An emerging paradigm. Adv Drug Deliv Rev. 2008b;60:1627–1637. doi: 10.1016/j.addr.2008.08.003. [DOI] [PubMed] [Google Scholar]
- Choi HS, Liu W, Liu F, Nasr K, Misra P, Bawendi MG, Frangioni JV. Design considerations for tumour-targeted nanoparticles. Nat Nanotechnol. 2010;5:42–47. doi: 10.1038/nnano.2009.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi HS, Liu W, Misra P, Tanaka E, Zimmer JP, Ipe BI, Bawendi MG, Frangioni JV. Renal clearance of quantum dots. Nature Biotechnol. 2007;25:1165–1170. doi: 10.1038/nbt1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corot C, Robert P, Idee JM, Port M. Recent advances in iron oxide nanocrystal technology for medical imaging. Adv Drug Deliv Rev. 2006;58:1471–1504. doi: 10.1016/j.addr.2006.09.013. [DOI] [PubMed] [Google Scholar]
- Delehanty JB, Boeneman K, Bradburne CE, Robertson K, Medintz IL. Quantum dots: A powerful tool for understanding the intricacies of nanoparticle-mediated drug delivery. Expert Opin Drug Deliv. 2009;6:1091–112. doi: 10.1517/17425240903167934. [DOI] [PubMed] [Google Scholar]
- Duncan R, Izzo L. Dendrimer biocompatibility and toxicity. Adv Drug Deliv Rev. 2005;57:2215–2237. doi: 10.1016/j.addr.2005.09.019. [DOI] [PubMed] [Google Scholar]
- Efros AL. Interband absorption of light in a semiconductor sphere. Sov Phys Semiconductors-USSR. 1982;16:772–775. [Google Scholar]
- Frangioni JV, Hajjar RJ. In vivo tracking of stem cells for clinical trials in cardiovascular disease. Circulation. 2004;110:3378–3383. doi: 10.1161/01.CIR.0000149840.46523.FC. [DOI] [PubMed] [Google Scholar]
- Harisinghani MG, Barentsz J, Hahn PF, Deserno WM, Tabatabaei S, van de Kaa CH, de la Rosette J, Weissleder R. Noninvasive detection of clinically occult lymph-node metastases in prostate cancer. N Engl J Med. 2003;348:2491–2499. doi: 10.1056/NEJMoa022749. [DOI] [PubMed] [Google Scholar]
- Hayashi K, Jiang P, Yamauchi K, Yamamoto N, Tsuchiya H, Tomita K, Moossa AR, Bouvet M, Hoffman RM. Real-time imaging of tumor-cell shedding and trafficking in lymphatic channels. Cancer Res. 2007;67:8223–8228. doi: 10.1158/0008-5472.CAN-07-1237. [DOI] [PubMed] [Google Scholar]
- Hezinger AFE, Tessmar J, Gopferich A. Polymer coating of quantum dots – a powerful tool toward diagnostics and sensorics. Eur J Pharmaceut Biopharmaceut. 2008;68:138–152. doi: 10.1016/j.ejpb.2007.05.013. [DOI] [PubMed] [Google Scholar]
- Jain R, Dandekar P, Patravale V. Diagnostic nanocarriers for sentinel lymph node imaging. J Controlled Release. 2009;138:90–102. doi: 10.1016/j.jconrel.2009.05.010. [DOI] [PubMed] [Google Scholar]
- Jaiswal JK, Mattoussi H, Mauro JM, Simon SM. Long-term multiple color imaging of live cells using quantum dot bioconjugates. Nature Biotechnol. 2003;21:47–51. doi: 10.1038/nbt767. [DOI] [PubMed] [Google Scholar]
- Jalil RA, Zhang Y. Biocompatibility of silica coated NaYF4 upconversion fluorescent nanocrystals. Biomaterials. 2008;29:4122–4128. doi: 10.1016/j.biomaterials.2008.07.012. [DOI] [PubMed] [Google Scholar]
- Jamieson T, Bakhshi R, Petrova D, Pocock R, Imani M, Seifalian AM. Biological applications of quantum dots. Biomaterials. 2007;28:4717–4732. doi: 10.1016/j.biomaterials.2007.07.014. [DOI] [PubMed] [Google Scholar]
- Kaminskas LM, Boyd BJ, Karellas P, Krippner GY, Lessene R, Kelly B, Porter CJH. The impact of molecular weight and PEG chain length on the systemic pharmacokinetics of PEGylated poly L-lysine dendrimers. Molec Pharmaceut. 2008;5:449–463. doi: 10.1021/mp7001208. [DOI] [PubMed] [Google Scholar]
- Kim S, Fisher B, Eisler HJ, Bawendi M. Type-II quantum dots: CdTe/CdSe(core/shell) and CdSe/ZinTe(core/shell) heterostructures. J Am Chem Soc. 2003;125:11466–11467. doi: 10.1021/ja0361749. [DOI] [PubMed] [Google Scholar]
- Kim S, Lim YT, Soltesz EG, De Grand AM, Lee J, Nakayama A, Parker JA, Mihaljevic T, Laurence RG, Dor DM, Cohn LH, Bawendi MG, Frangioni JV. Near-infrared fluorescent type II quantum dots for sentinel lymph node mapping. Nature Biotechnol. 2004;22:93–97. doi: 10.1038/nbt920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi H, Brechbiel MW. Dendrimer-based macromolecular MRI contrast agents: Characteristics and application. Molec Imag. 2003;2:1–10. doi: 10.1162/15353500200303100. [DOI] [PubMed] [Google Scholar]
- Kobayashi H, Brechbiel MW. Dendrimer-based nanosized MRI contrast agents. Curr Pharmaceut Biotechnol. 2004;5:539–549. doi: 10.2174/1389201043376571. [DOI] [PubMed] [Google Scholar]
- Kobayashi H, Brechbiel MW. Nano-sized MRI contrast agents with dendrimer cores. Adv Drug Deliv Rev. 2005;57:2271–2286. doi: 10.1016/j.addr.2005.09.016. [DOI] [PubMed] [Google Scholar]
- Kobayashi H, Hama Y, Koyama Y, Barrett T, Regino CAS, Urano Y, Choyke PL. Simultaneous multicolor imaging of five different lymphatic basins using quantum dots. Nano Lett. 2007a;7:1711–1716. doi: 10.1021/nl0707003. [DOI] [PubMed] [Google Scholar]
- Kobayashi H, Jo SK, Kawamoto S, Yasuda H, Hu XZ, Knopp MV, Brechbiel MW, Choyke PL, Star RA. Polyamine dendrimer-based MRI contrast agents for functional kidney imaging to diagnose acute renal failure. J Magnet Res Imag. 2004a;20:512–518. doi: 10.1002/jmri.20147. [DOI] [PubMed] [Google Scholar]
- Kobayashi H, Kawamoto S, Bernardo M, Brechbiel MW, Knopp MV, Choyke PL. Delivery of gadolinium-labeled nanoparticles to the sentinel lymph node: Comparison of the sentinel node visualization and estimations of intra-nodal gadolinium concentration by the magnetic resonance imaging. J Controlled Release. 2006;111:343–351. doi: 10.1016/j.jconrel.2005.12.019. [DOI] [PubMed] [Google Scholar]
- Kobayashi H, Kawamoto S, Brechbiel MW, Bernardo M, Sato N, Waldmann TA, Tagaya Y, Choyke PL. Detection of lymph node involvement in hematologic malignancies using micromagnetic resonance lymphangiography with a gadolinumlabeled dendrimer nanoparticle. Neoplasia. 2005;7:984–991. doi: 10.1593/neo.05454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi H, Kawamoto S, Jo SK, Bryant HL, Brechbiel MW, Star RA. Macromolecular MRI contrast agents with small dendrimers: Pharmacokinetic differences between sizes and cores. Bioconj Chem. 2003a;14:388–394. doi: 10.1021/bc025633c. [DOI] [PubMed] [Google Scholar]
- Kobayashi H, Kawamoto S, Saga T, Sato N, Hiraga A, Ishimori T, Konishi J, Togashi K, Brechbiel MW. Positive effects of polyethylene glycol conjugation to generation-4 polyamidoamine dendrimers as macromolecular MR contrast agents. Magnet Reson Med. 2001a;46:781–788. doi: 10.1002/mrm.1257. [DOI] [PubMed] [Google Scholar]
- Kobayashi H, Kawamoto S, Sakai Y, Choyke PL, Star RA, Brechbiel MW, Sato N, Tagaya Y, Morris JC, Waldmann TA. Lymphatic drainage imaging of breast cancer in mice by micro-magnetic resonance lymphangiography using a nano-size paramagnetic contrast agent. J Nat Cancer Inst. 2004b;96:703–708. doi: 10.1093/jnci/djh124. [DOI] [PubMed] [Google Scholar]
- Kobayashi H, Kawamoto S, Star RA, Waldmann TA, Tagaya Y, Brechbiel MW. Micro-magnetic resonance lymphangiography in mice using a novel dendrimer-based magnetic resonance imaging contrast agent. Cancer Res. 2003b;63:271–276. [PubMed] [Google Scholar]
- Kobayashi H, Kosaka N, Ogawa M, Morgan NY, Smith PD, Murray CB, Ye XC, Collins J, Kumar GA, Bell H, Choyke PL. In vivo multiple color lymphatic imaging using upconverting nanocrystals. J Materials Chem. 2009;19:6481–6484. [Google Scholar]
- Kobayashi H, Koyama Y, Barrett T, Hama Y, Regino CAS, Shin IS, Jang BS, Le N, Paik CH, Choyke PL, Urano Y. Multimodal nanoprobes for radionuclide and five-color near-infrared optical lymphatic imaging. Acs Nano. 2007b;1:258–264. doi: 10.1021/nn700062z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi H, Saga T, Kawamoto S, Sato N, Hiraga A, Ishimori T, Konishi J, Togashi K, Brechbiel MW. Dynamic micromagnetic resonance imaging of liver micrometastasis in mice with a novel liver macromolecular magnetic resonance contrast agent DAB-Am64-(1B4M-Gd)(64) Cancer Res. 2001b;61:4966–4970. [PubMed] [Google Scholar]
- Kojima C, Kono K, Maruyama K, Takagishi T. Synthesis of polyamidoamine dendrimers having poly(ethylene glycol) grafts and their ability to encapsulate anticancer drugs. Bioconj Chem. 2000;11:910–917. doi: 10.1021/bc0000583. [DOI] [PubMed] [Google Scholar]
- Kosaka N, Ogawa M, Sato N, Choyke PL, Kobayashi H. In vivo real-time, multicolor, quantum dot lymphatic imaging. J Invest Dermatol. 2009;129:2818–2822. doi: 10.1038/jid.2009.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim J, Guo Y, Rostollan CL, Stanfield J, Hsieh JT, Sun XK, Simanek EE. The role of the size and number of polyethylene glycol chains in the biodistribution and tumor localization of triazine dendrimers. Molec Pharmaceut. 2008;5:540–547. doi: 10.1021/mp8000292. [DOI] [PubMed] [Google Scholar]
- Lin SP, Brown JJ. MR contrast agents: Physical and pharmacologic basics. J Magnet Reson Imag. 2007;25:884–899. doi: 10.1002/jmri.20955. [DOI] [PubMed] [Google Scholar]
- Margolis DJA, Hoffman JM, Herfkens RJ, Jeffrey RB, Quon A, Gambhir SS. Molecular imaging techniques in body imaging. Radiology. 2007;245:333–356. doi: 10.1148/radiol.2452061117. [DOI] [PubMed] [Google Scholar]
- Medintz IL, Uyeda HT, Goldman ER, Mattoussi H. Quantum dot bioconjugates for imaging, labelling and sensing. Nature Materials. 2005;4:435–446. doi: 10.1038/nmat1390. [DOI] [PubMed] [Google Scholar]
- Moore A, Sun PZ, Cory D, Hogemann D, Weissleder R, Lipes MA. MRI of insulitis in autoimmune diabetes. Magn Reson Med. 2002;47:751–758. doi: 10.1002/mrm.10110. [DOI] [PubMed] [Google Scholar]
- Pelley JL, Daar AS, Saner MA. State of academic knowledge on toxicity and biological fate of quantum dots. Toxicolog Sci. 2009;112:276–296. doi: 10.1093/toxsci/kfp188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers WJ, Meyer CH, Kramer CM. Technology insight: In vivo cell tracking by use of MRI. Nature Clin Pract Cardiovasc Med. 2006;3:554–562. doi: 10.1038/ncpcardio0659. [DOI] [PubMed] [Google Scholar]
- Rzigalinski BA, Strobl JS. Cadmium-containing nanoparticles: Perspectives on pharmacology and toxicology of quantum dots. Toxicol Appl Pharmacol. 2009;238:280–288. doi: 10.1016/j.taap.2009.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro EM, Skrtic S, Sharer K, Hill JM, Dunbar CE, Koretsky AP. MRI detection of single particles for cellular imaging. Proc Natl Acad Sci USA. 2004;101:10901–10906. doi: 10.1073/pnas.0403918101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AM, Dave S, Nie SM, True L, Gao XH. Multicolor quantum dots for molecular diagnostics of cancer. Exp Rev Molec Diagnost. 2006;6:231–244. doi: 10.1586/14737159.6.2.231. [DOI] [PubMed] [Google Scholar]
- Smith AM, Duan HW, Mohs AM, Nie SM. Bioconjugated quantum dots for in vivo molecular and cellular imaging. Adv Drug Deliv Rev. 2008;60:1226–1240. doi: 10.1016/j.addr.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- So MK, Xu CJ, Loening AM, Gambhir SS, Rao JH. Selfilluminating quantum dot conjugates for in vivo imaging. Nature Biotechnol. 2006;24:339–343. doi: 10.1038/nbt1188. [DOI] [PubMed] [Google Scholar]
- Tanimoto A, Yuasa Y, Shinmoto H, Jinzaki M, Imai Y, Okuda S, Kuribayashi S. Superparamagnetic iron oxide-mediated hepatic signal intensity change in patients with and without cirrhosis: Pulse sequence effects and Kupffer cell function. Radiology. 2002;222:661–666. doi: 10.1148/radiol.2223010690. [DOI] [PubMed] [Google Scholar]
- Thorek DLJ, Chen A, Czupryna J, Tsourkas A. Superparamagnetic iron oxide nanoparticle probes for molecular imaging. Ann Biomed Engine. 2006;34:23–38. doi: 10.1007/s10439-005-9002-7. [DOI] [PubMed] [Google Scholar]
- Voura EB, Jaiswal JK, Mattoussi H, Simon SM. Tracking metastatic tumor cell extravasation with quantum dot nanocrystals and fluorescence emission-scanning microscopy. Nature Med. 2004;10:993–998. doi: 10.1038/nm1096. [DOI] [PubMed] [Google Scholar]
- Wang F, Liu XG. Upconversion multicolor fine-tuning: Visible to near-infrared emission from lanthanide-doped NaYF4 nanoparticles. J Am Chem Soc. 2008;130:5642–5643. doi: 10.1021/ja800868a. [DOI] [PubMed] [Google Scholar]
- Yao HQ, Zhang Y, Xiao F, Xia ZY, Rao JH. Quantum dot/bioluminescence resonance energy transfer based highly sensitive detection of proteases. Angewandte Chem-Int Ed. 2007;46:4346–4349. doi: 10.1002/anie.200700280. [DOI] [PubMed] [Google Scholar]
- Yi GS, Lu HC, Zhao SY, Yue G, Yang WJ, Chen DP, Guo LH. Synthesis, characterization, and biological application of size-controlled nanocrystalline NaYF4: Yb,Er infrared-to-visible up-conversion phosphors. Nano Lett. 2004;4:2191–2196. [Google Scholar]
- Young IR, Clarke GJ, Bailes DR, Pennock JM, Doyle FH, Bydder GM. Enhancement of relaxation rate with paramagnetic contrast agents in NMR imaging. J Comput Tomogr. 1981;5:543–547. doi: 10.1016/0149-936x(81)90089-8. [DOI] [PubMed] [Google Scholar]
- Zijlmans H, Bonnet J, Burton J, Kardos K, Vail T, Niedbala RS, Tanke HJ. Detection of cell and tissue surface antigens using up-converting phosphors: A new reporter technology. Analyt Biochem. 1999;267:30–36. doi: 10.1006/abio.1998.2965. [DOI] [PubMed] [Google Scholar]