Abstract
Recent research suggests an involvement of pro-opiomelanocortin (POMC) gene products in modulating cocaine reward and addiction-like behaviors in rodents. In this study, we investigated whether cocaine-induced conditioned place preference (CPP) alters POMC gene expression in the brain or pituitary of rats. Sprague-Dawley rats were conditioned with 4 injections of 0, 10 or 30 mg/kg cocaine (i.p.) over 8 days and tested 4 days after the last conditioning session. Another group received the same pattern of cocaine injections without conditioning. POMC mRNA levels in the hypothalamus (including arcuate nucleus), amygdala and anterior pituitary, as well as plasma ACTH and corticosterone levels were measured. Cocaine place conditioning at 10 and 30 mg/kg doses increased POMC mRNA levels in a dose-dependent manner in the hypothalamus, with no effect in the amygdala. Cocaine CPP had no effect on POMC mRNA levels in the anterior pituitary or on plasma ACTH or corticosterone levels. In rats that received cocaine at 30 mg/kg without conditioning, there was no such effect on hypothalamic POMC mRNA levels. Alteration of POMC gene expression in the hypothalamus is region-specific after cocaine place conditioning, and dose-dependent. The increased POMC gene expression in the hypothalamus suggests that it is involved in the reward/learning process of cocaine-induced conditioning.
Keywords: POMC, hypothalamus, cocaine, conditioned place preference
Introduction
Pro-opiomelanocortin (POMC) is a large peptide precursor that gives rise to several biologically active neuropeptides, including beta-endorphin, adrenocorticotropic hormone (ACTH), beta-lipotropin and alpha-melanocyte-stimulating hormone. The presence of POMC neurons was originally found to be mainly restricted to the rodent hypothalamus (arcuate nucleus), nucleus of the solitary tract and pituitary [9]. The opioid peptide beta-endorphin is distributed in the hypothalamus, and the dopaminergic mesocorticolimbic regions, probably from hypothalamic POMC neuronal projections. Since activation of the mu opioid receptor by beta-endorphin is rewarding and modulates dopamine release in the nucleus accumbens [16], beta-endorphin may be involved in the reinforcing effects and motivational behaviors of several drugs of abuse [5,14]. For instance, intracerebroventricular beta-endorphin administration has been shown to induce conditioned place preference (CPP) in rats [1].
Opioid receptor antagonists (especially mu-opioid receptor selective antagonists) have been shown to reduce both the rewarding action of cocaine using the CPP model [3,4,12,17], and cocaine reinforcement using the self-administration model [13]. These findings raise the possibility that cocaine may cause the release of endogenous opioid peptides and further suggest that these neuropeptides play a functional role in the actions of cocaine. Of interest, a recent study has demonstrated that cocaine-induced CPP is reduced in beta-endorphin deficient mice, indicating a reduced rewarding effect of cocaine with less endogenous beta-endorphin [10]. Together, these studies demonstrate a modulatory role for beta-endorphin in cocaine reward and reinforcement.
Since the early 1990’s, our laboratory and others have investigated the effect of drugs of abuse on opioid peptides and their receptors. Alterations of preprodynorphin (ppDyn), preproenkephalin, kappa and mu opioid receptor gene expression in mesolimbic brain areas of mice or rats after chronic cocaine exposure or across long-term withdrawal have been broadly studied (see recent reviews [6,7]). However, it is unclear if POMC gene expression in specific brain regions is altered by cocaine, particularly in the setting of cocaine-induced place conditioning [20].
To extend our research to the effects on POMC gene expression, we here report a set of experiments addressing the research question: are POMC mRNA levels in the hypothalamus, amygdala (where POMC expression is relatively abundant [20]), or pituitary (which produces peripheral beta-endorphin) altered after the development of cocaine CPP? The inclusion of the amygdala was based on recent studies showing an involvement of different amygdalar nuclei (basolateral and central nucleus) in cocaine CPP [e.g., 8]. On the basis of evidence implicating POMC-derived beta-endorphin in the rewarding property of cocaine in mice [10], we predicted that POMC gene expression would be elevated in animals tested using cocaine CPP.
Materials and methods
Animals
Male Sprague–Dawley rats (190–220 g, Charles River Labs) were housed under a standard 12-h light/dark cycle (lights on from 7:00 h to 19:00 h) with free access to food and water for 7 days, and habituated to the environment with daily handling for 5 days. The room temperature was maintained at 22 ± 1 °C. The habituation, training, and testing were conducted during the light phase of the cycle. The experimental procedures were approved by the Committee on Animal Care and Use of the Rockefeller University. The Sprague–Dawley rat was chosen based on our previous study, in which the rat showed significant CPP for cocaine at 10 mg/kg [19].
Apparatus
The place conditioning apparatus (model ENV-013MD, Med Associates, VT) was a PVC plastic rectangular chamber that consisted of three compartments. Two conditioning compartments were separated by a smaller middle one. One of the conditioning compartments had white walls and a stainless steel mesh floor; the other had black walls and a ‘grid’ floor, which consisted of stainless-steel rods. The middle compartment had gray walls and a plain gray floor. Animals could access the entire apparatus when the guillotine doors were removed. Through a computer interface, time spent in each compartment was recorded by sets of infrared beams located near the floor of each compartment.
Cocaine CPP procedure
The place conditioning procedure consisted of three phases: pre-conditioning, conditioning and post-conditioning tests, as previously described [19].
Pre-conditioning
Before the onset of the place conditioning, rats were placed in the middle compartment with the guillotine doors removed, allowing free access to two conditioning compartments for a 30 min period daily for 3 consecutive days. The time spent in each conditioning compartment was recorded on the third day. The conditioning compartments occupied for more and less time were designated as the preferred and non-preferred sides, respectively. All animals were included, including the ones that showed differences in preference for each conditioning compartment in the pre-conditioning test. The rats were then assigned to two experimental groups (saline and cocaine), resulting in each group with approximately equal mean times spent for each conditioning compartment in the pre-conditioning test. During drug conditioning sessions, assignment for cocaine conditioning compartment was made in a counterbalanced manner.
Conditioning
The conditioning phase began on the day following the baseline testing. During conditioning, rats were injected intraperitoneally (i.p.) with 10 or 30 mg/kg of cocaine (Sigma Chemical Co, St. Louis, MO, dissolved in 0.9% saline) once, and immediately confined to one conditioning compartment for 30 min. On alternate days, rats received saline injections and were confined to the other compartment for 30 min. The conditioning phase, with cocaine and saline on alternative days, lasted for 8 days. Control animals received saline injections in both conditioning compartments.
Post-conditioning test
Four days after the last conditioning trial, animals were placed in the middle compartment with free access to both conditioning compartments for 30 min. The time spent in each compartment was recorded. Thirty min following the post-conditioning test, tissues were collected for subsequent mRNA or plasma hormone analysis.
Procedure of cocaine administration in CPP regimen without conditioning
The dose of drug exposure (10 or 30 mg/kg of cocaine or saline), and the pattern, route and timing of injections were identical to those of cocaine administration in CPP conditioning groups. The only difference from CPP conditioning groups was that the rats received injections in the home cage, without conditioning procedures. Four days after the last injection, the tissues were collected for subsequent mRNA or plasma hormone analysis.
Experiment I. Rats with 10 mg/kg of cocaine with or without conditioning
One group of rats were trained in this CPP paradigm with cocaine conditioning at 10 mg/kg of cocaine or saline on alternate days (n = 13). The control group received saline in both compartments (n = 8). In parallel, two groups of the SD rats were injected in this CPP pattern regimen with cocaine at 10 mg/kg (n = 7) or with saline (n = 7) without conditioning.
Experiment II. Rats with 30 mg/kg of cocaine with or without conditioning
One group of rats were trained in this CPP paradigm with cocaine conditioning at 30 mg/kg of cocaine or saline on alternate days (n = 10). The control group received saline in both compartments (n = 10). In parallel, two groups of the SD rats were injected in this CPP pattern regimen with cocaine at 30 mg/kg (n = 6) or with saline (n = 6) without conditioning.
Preparation of RNA extracts
In each experiment, 30 min following the post-conditioning test or identical time point after cocaine or saline injections, all rats were immediately sacrificed and each rat brain was prepared for RNA extracts (Supplementary Information [SI] section).
Solution hybridization ribonuclease (RNase) protection-trichloroacetic acid (TCA) precipitation assay
The solution hybridization RNase protection-TCA precipitation protocol has been described in detail in an earlier report [19]. See SI Methods section.
Radioimmunoassays
See SI Methods section for details.
Data analysis
Differences from pre-conditioning to post-conditioning test (time spent in each compartment) were analyzed using a two-way analysis of variance (ANOVA), Drug side (cocaine, saline) by Phase (pre-conditioning, post-conditioning test) with repeated measures, followed by Newman-Keuls post-hoc tests. Group differences in mRNA levels, plasma ACTH and corticosterone levels were analyzed using a two-way ANOVA, Experiment (Experiment I, Experiment II) by Drug (cocaine, saline), followed by Newman-Keuls post-hoc tests. In other comparisons, differences between two groups were analyzed using a two-tailed Student’s t-test. To determine the correlation between measured variables, linear regression analysis was performed. The accepted level of significance for all tests was p < 0.05. All statistical analyses were performed using Statistica (version 5.5, StatSoft Inc.).
Results
Cocaine CPP expression at 10 or 30 mg/kg
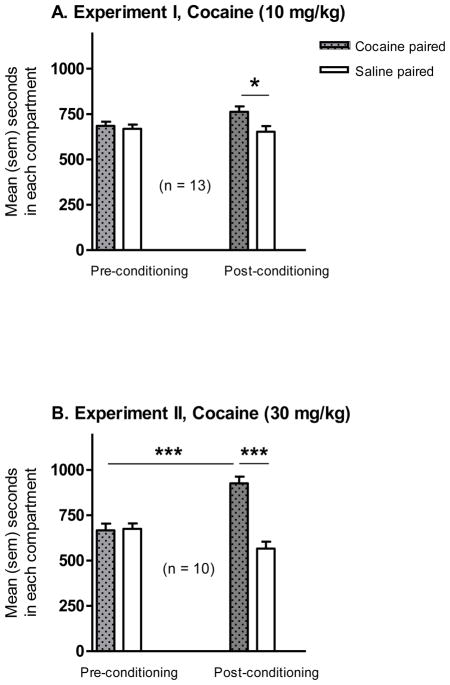
In Experiment 1, one group of rats was trained in a CPP paradigm with cocaine conditioning at 10 mg/kg of cocaine. The mean time spent in the cocaine-paired and saline-paired compartment on the pre-conditioning and post-conditioning test days is shown in Fig. 1A. A two-way ANOVA showed a significant effect of the drug side x phase interaction [F(1,48) = 5.31, p < 0.05]. While rats spent similar amounts of time in each compartment in the pre-conditioning test, rats conditioned with cocaine displayed a preference for the cocaine-paired compartment in the post-conditioning test [Newman-Keuls post-hoc tests: cocaine post-conditioning vs. saline post-conditioning, p < 0.05; cocaine post-conditioning vs. cocaine pre-conditioning, p = 0.05]. As control, another group of rats conditioned with saline showed no preference (data not shown).
Figure 1.
Expression of conditioned place preference (CPP) induced by cocaine in Sprague-Dawley rats. Mean (SEM) seconds spent in the saline- and cocaine-paired compartments were recorded on a 30-min CPP test 4 days after the last cocaine conditioning test and compared to the 30-min pre-conditioning test 1 day before the cocaine conditioning phase. During conditioning, animals received cocaine at 10 mg/kg (A, Experiment I) or 30 mg/kg (B, Experiment II) (The data of control rats conditioned with saline in both compartments were not included). In the post-conditioning test, rats conditioned with cocaine displayed a preference for the cocaine-paired compartment. Data shown in graphs are treatment group mean + SEM. Significant differences are indicated: * p < 0.05 or *** p < 0.001.
In Experiment 2, one group was trained with cocaine conditioning at 30 mg/kg of cocaine. As shown in Fig. 1B, a two-way ANOVA identified a significant main effect of drug side [F(1,36) = 21.5, p < 0.001], and the drug side x phase interaction [F(1,36) = 23.6, p < 0.001]. Rats conditioned with cocaine displayed a significant preference for the cocaine-paired compartment in the post-conditioning test [cocaine post-conditioning vs. cocaine pre-conditioning, p < 0.001; cocaine post-conditioning vs. saline post-conditioning, p < 0.001]. As control, another group conditioned with saline showed no preference (data not shown).
POMC mRNA levels in the medial hypothalamus or amygdala
In the medial hypothalamus, a two-way ANOVA showed a significant effect of cocaine conditioning [F(1,35) = 11.0, p < 0.005]. Newman-Keuls post-hoc tests revealed that: (1) in the rats trained with cocaine conditioning at 10 mg/kg, there was no significant increase; and (2) with 30 mg/kg cocaine conditioning, there was a significant increase in POMC mRNA levels when compared with the saline control group (p < 0.005) or the group treated with 10 mg/kg cocaine conditioning (p < 0.01) (Fig. 2A). However, the POMC mRNA levels in the amygdala were unaltered by either dose of cocaine with CPP expression (Table S1). There was no significant effect on POMC mRNA levels after cocaine administration at either 10 or 30 mg/kg dose in a pattern identical to the CPP regimen (Fig. 2B). Furthermore, the POMC mRNA levels in the amygdala were unaltered by either dose of cocaine without CPP conditioning (Table S1).
Figure 2.
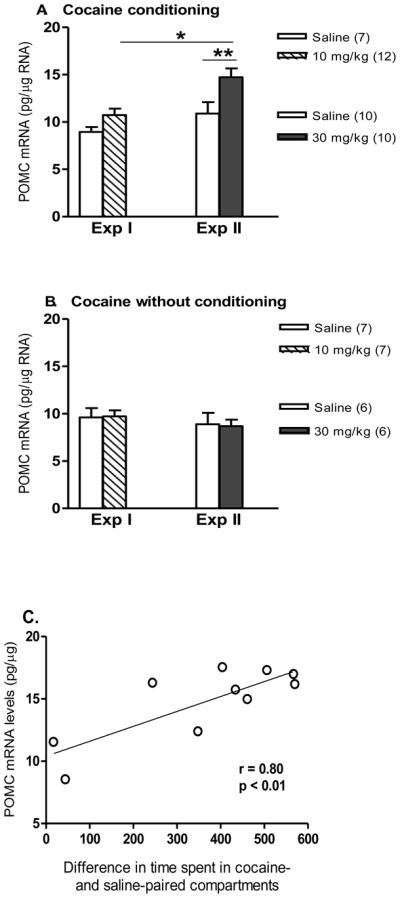
Effects of cocaine on POMC mRNA levels (pg/μg total RNA) in the medial hypothalamus of cocaine (0, 10 or 30 mg/kg) with conditioning (A) or without conditioning (B) in Sprague-Dawley rats in Experiment I and Experiment II. Data shown in graphs are treatment group mean + SEM. Significant differences are indicated: * p < 0.01 or ** p < 0.005. (C) In Experiment 2, regression of POMC mRNA levels in the medial hypothalamus and CPP expression during 30 min post-conditioning test in the rats (n=10) after 30 mg/kg cocaine conditioning. There was a significant positive correlation between the POMC mRNA level and CPP expression (difference in time spent in cocaine-paired and saline-paired compartments).
In each experiment, there was no significant difference in the POMC mRNA levels in the medial hypothalamus between the saline control group with conditioning (Fig. 2A) and without conditioning (Fig. 2B) (Student’s t-test). The relationship between POMC mRNA levels in the medial hypothalamus and CPP expression (difference in time spent in cocaine-paired and saline-paired compartments) was examined, and a significant positive correlation between POMC mRNA levels and CPP expression was found in the animals after cocaine conditioning at 30 mg/kg (r2 = 0.64, n = 13, p < 0.01) (Fig. 2C).
POMC mRNA levels in the anterior pituitary and HPA hormones after cocaine conditioning
As shown in Fig S1A, there was no significant effect on POMC mRNA levels in the anterior pituitary after cocaine conditioning at either 10 or 30 mg/kg dose. Plasma levels of ACTH (Fig S1B) and corticosterone (Fig S1C) showed a similar pattern to that seen in POMC mRNA in the anterior pituitary, especially after cocaine conditioning at 30 mg/kg. However, neither change was significant.
Discussion
The main objective of these experiments was to investigate the effects of cocaine place conditioning on POMC mRNA level in two rat brain regions: hypothalamus and amygdala, as well as in the anterior pituitary. POMC mRNA level modulation by cocaine CPP expression was primarily found in the hypothalamus, whereas that in the amygdala or anterior pituitary was not affected, suggesting that hypothalamic POMC gene expression is altered by cocaine-conditioned behaviors in a region-specific manner. The POMC mRNA increase suggests an enhanced biosynthesis in the POMC neurons in the arcuate nucleus, although it cannot be determined from assays of mRNA levels alone which steps (gene transcription, processing, and/or degradation of mRNA) are affected. In line with our finding of the POMC mRNA increases, it has been demonstrated that either experimenter-delivered or self-administered cocaine stimulates the release of beta-endorphin in the rat arcuate nucleus [14]. Although the stimulatory factors influencing elevation of POMC mRNA level are not fully elucidated in the present study, it is possible that the increased beta-endorphin release by cocaine is responsible for the increase in mRNA to compensate for cocaine-induced peptide depletion. Together, these results suggest that the POMC gene in the neurons of the hypothalamic arcuate nucleus, but not in the amygdala or anterior pituitary, responds to cocaine-induced CPP.
Place preference occurs following administration of beta-endorphin, while CTOP (a selective mu receptor antagonist) induces place aversion [11]. This implies putative endogenous tonic opioid regulation of the reward system, mediated by mu opioid receptors. Of the endogenous opioids, beta-endorphin is the preferred ligand for mu receptors, and thus is a possible candidate for regulating and/or mediating drug-induced reward and reinforcement. In fact, when injected directly into the brain of rats, beta-endorphin has rewarding and reinforcing properties [1,2,18], further suggesting that this opioid peptide could serve as a neuromodulator of the effects of cocaine reward. Furthermore, a recent study demonstrated that cocaine produced attenuated CPP in beta-endorphin knockout mice, which indicates a reduced rewarding effect, providing direct evidence that beta-endorphin has a modulatory role in cocaine reinforcement [10]. Here, using the CPP model, we found an increase in hypothalamic POMC mRNA levels in rats after cocaine CPP expression in a dose-dependent manner. When rats received cocaine administration in a pattern identical to the CPP regimen, but without cocaine conditioning, the POMC mRNA levels were not altered. In Experiment 2, we observed that a large proportion of the rats (8 out of 10) established cocaine CPP expression at 30 mg/kg of cocaine and spent at least 25% more time in the cocaine-paired compartment than the saline-paired one. However, two rats did not reach this criterion [15], and the hypothalamic POMC mRNA levels in these non-responders to cocaine CPP were not different from the saline controls. Therefore, our data suggest that although cocaine conditioning was necessary, the strong expression of conditioned responses to the cocaine-paired environment at 30 mg/kg dose was associated with increased POMC mRNA expression in the hypothalamus. Our results also indicate that increased POMC gene expression (and therefore possibly increased processing and release of POMC peptides, such as beta-endorphin) may contribute to the expression of cocaine CPP.
In summary, the data presented here show that expression of cocaine CPP was associated with a region-specific increase in hypothalamic POMC expression in rats. Because the POMC-derived opioid peptide beta-endorphin is known to positively modulate brain reward functions, our results suggest that the hypothalamic POMC system is a component of the neural circuitry underlying cocaine CPP.
Supplementary Material
Highlights.
Effect of cocaine on POMC gene expression was studied with or without conditioning;
POMC mRNA levels were measured in rat hypothalamus, amygdala and pituitary;
Cocaine CPP but not cocaine alone increased hypothalamic POMC mRNA levels.
Acknowledgments
We thank Dr. K Niikura for his valuable discussions and Susan Russo for editing when the manuscript was being prepared. The work was supported by NIDA Grant DA-P60-05130 (MJK).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Amalric M, Cline EJ, Martinez JL, Jr, Bloom FE, Koob GF. Rewarding properties of beta-endorphin as measured by conditioned place preference. Psychopharmacology. 1987;91:14–19. doi: 10.1007/BF00690919. [DOI] [PubMed] [Google Scholar]
- 2.Bals-Kubik R, Shippenberg TS, Herz A. Involvement of central mu and delta opioid receptors in mediating the reinforcing effects of beta-endorphin in the rat. Eur J Pharmacol. 1990;175:63–69. doi: 10.1016/0014-2999(90)90153-w. [DOI] [PubMed] [Google Scholar]
- 3.Gerrits MA, Patkina N, Zvartau EE, van Ree JM. Opioid blockade attenuates acquisition and expression of cocaine-induced place preference conditioning in rats. Psychopharmacology. 1995;119:92–98. doi: 10.1007/BF02246059. [DOI] [PubMed] [Google Scholar]
- 4.Houdi AA, Bardo MT, Van Loon GR. Opioid mediation of cocaine-induced hyperactivity and reinforcement. Brain Res. 1989;497:195–198. doi: 10.1016/0006-8993(89)90989-x. [DOI] [PubMed] [Google Scholar]
- 5.Koob GF, Kreek MJ. Stress, dysregulation of drug reward pathways, and the transition to drug dependence. Am J Psychiatry. 2007;164:1149–1159. doi: 10.1176/appi.ajp.2007.05030503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kreek MJ, Zhou Y, Butelman ER, Levran O. Opiate and cocaine addiction: from bench to clinic and back to the bench. Cur Opin Pharmacol. 2009;9:74–80. doi: 10.1016/j.coph.2008.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Le Merrer L, Becker JAJ, Befort K, Kieffer BL. Reward processing by the opioid system in the brain. Physiol Rev. 2009;89:1379–1412. doi: 10.1152/physrev.00005.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li FQ, Xue YX, Wang JS, Fang Q, Li YQ, Zhu WL, He YY, Liu JF, Xue LF, Shaham Y, Lu L. Basolateral amygdala cdk5 activity mediates consolidation and reconsolidation of memories for cocaine cues. J Neurosci. 2010;30:10351–10359. doi: 10.1523/JNEUROSCI.2112-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mansour A, Khachaturian H, Lewis ME, Akil H, Watson SJ. Anatomy of CNS opioid receptors. Trends Neurosci. 1988;11:308–314. doi: 10.1016/0166-2236(88)90093-8. [DOI] [PubMed] [Google Scholar]
- 10.Marquez P, Baliram R, Dabaja I, Gajawada N, Lutfy K. The role of beta-endorphin in acute motor stimulatory and rewarding actions of cocaine in mice. Psychopharmacology. 2008;197:443–448. doi: 10.1007/s00213-007-1053-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mucha RF, Iversen SD. Reinforcing properties of morphine and naloxone revealed by conditioned preferences: a procedural examination. Psychopharmacology. 1984;82:241–247. doi: 10.1007/BF00427782. [DOI] [PubMed] [Google Scholar]
- 12.Rademacher DJ, Steinpreis RE. Effects of the selective mu(1)-opioid receptor antagonist, naloxonazine, on cocaine-induced conditioned place preference and locomotor behavior in rats. Neurosci Lett. 2002;332:159–162. doi: 10.1016/s0304-3940(02)00950-3. [DOI] [PubMed] [Google Scholar]
- 13.Ramsey NF, van Ree JM. Intracerebroventricular naltrexone treatment attenuates acquisition of intravenous cocaine self-administration in rats. Pharmacol Biochem Behav. 1991;40:807–810. doi: 10.1016/0091-3057(91)90090-o. [DOI] [PubMed] [Google Scholar]
- 14.Roth-Deri I, Green-Sadan T, Yadid G. Beta-endorphin and drug-induced reward and reinforcement. Prog Neurobiol. 2008;86:1–21. doi: 10.1016/j.pneurobio.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 15.Seip K, Pereira M, Wansaw M, Reiss J, Dziopa E, Morrell J. Incentive salience of cocaine across postpartum period of the female rat. Psychopharmacology. 2008;199:119–130. doi: 10.1007/s00213-008-1140-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spanagel R, Herz A, Bals-Kubik R, Shippenberg TS. Beta-endorphin-induced locomotor stimulation and reinforcement are associated with an increase in dopamine release in the nucleus accumbens. Psychopharmacology. 1991;104:51–56. doi: 10.1007/BF02244553. [DOI] [PubMed] [Google Scholar]
- 17.Suzuki T, Shiozaki Y, Masukawa Y, Nagase H. Role of mu and kappa -opioid receptors in cocaine-induced conditioned place preference. Jpn J Pharmacol. 1992;58:435–442. doi: 10.1254/jjp.58.435. [DOI] [PubMed] [Google Scholar]
- 18.van Ree JM, Smyth D, Colpaert F. Dependence creating properties of lipotropin C-fragment (beta-endorphin): evidence for its internal control of behavior. Life Sci. 1979;24:495–502. doi: 10.1016/0024-3205(79)90170-x. [DOI] [PubMed] [Google Scholar]
- 19.Zhou Y, Cui CL, Schlussman SD, Choi J, Ho A, Han JS, Kreek MJ. Effect of cocaine place conditioning, chronic escalating-dose binge pattern cocaine and its acute withdrawal on orexin/hypocretin and preprodynorphin gene expressions in the lateral hypothalamus. Neuroscience. 2008;153:1225–1234. doi: 10.1016/j.neuroscience.2008.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou Y, Proudnikov D, Yuferov V, Kreek MJ. Drug-induced and genetic alterations in stress-responsive systems: Implications for specific addictive diseases. Brain Res. 2010;1314:235–252. doi: 10.1016/j.brainres.2009.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.