Abstract
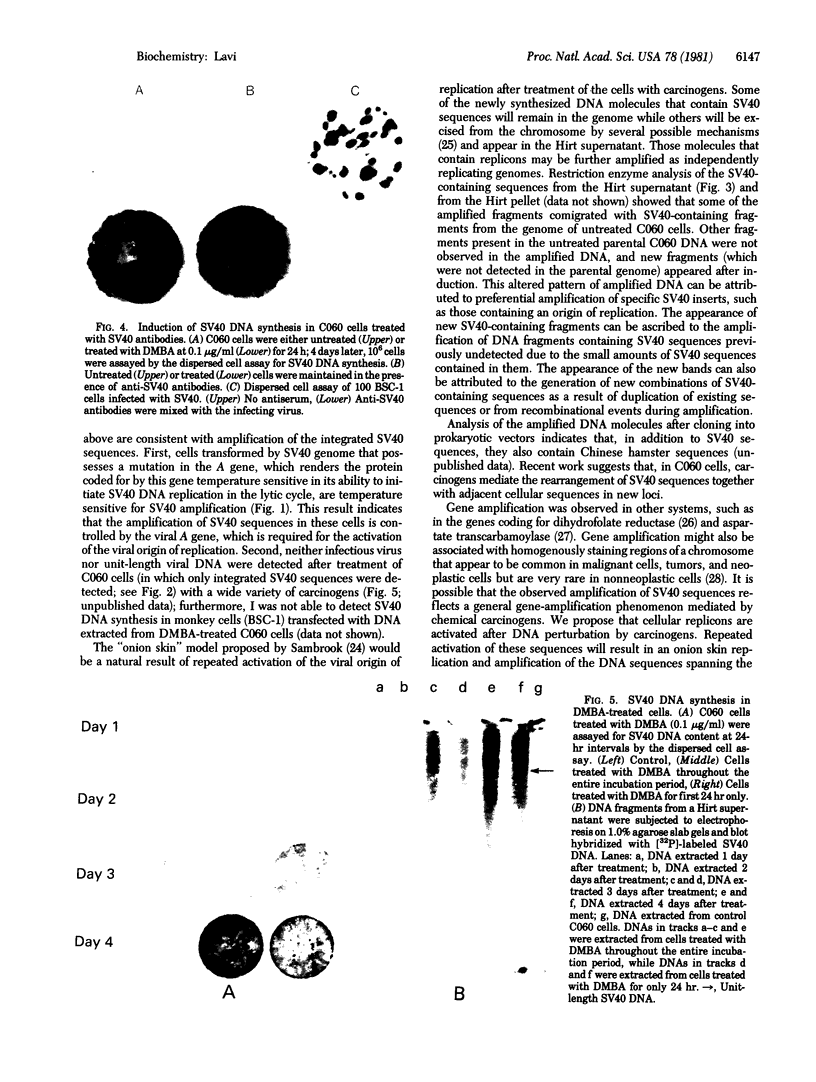
Exposure of simian virus 40 (SV40)-transformed Chinese hamster embryo cells to various chemical and physical carcinogens induced SV40 DNA synthesis. Although the carcinogen-mediated amplification of SV40 DNA is regulated by the viral A gene, the induction of viral DNA synthesis does not result in the rescue of infectious virus or the formation of complete viral DNA molecules. Instead, a heterogeneous collection of DNA molecules containing SV40 sequences was generated by treatment with 7,12-dimethylbenz[a]anthracene. Restriction enzyme analysis of the amplified DNA molecules in the Hirt supernatant showed that not all sequences in the integrated SV40 inserts are present. The possibility that amplification of SV40 sequences is a reflection of a general-gene-amplification phenomenon mediated by carcinogens is discussed.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alt F. W., Kellems R. E., Bertino J. R., Schimke R. T. Selective multiplication of dihydrofolate reductase genes in methotrexate-resistant variants of cultured murine cells. J Biol Chem. 1978 Mar 10;253(5):1357–1370. [PubMed] [Google Scholar]
- Botchan M., Topp W., Sambrook J. Studies on simian virus 40 excision from cellular chromosomes. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 2):709–719. doi: 10.1101/sqb.1979.043.01.079. [DOI] [PubMed] [Google Scholar]
- Cairns J. The origin of human cancers. Nature. 1981 Jan 29;289(5796):353–357. doi: 10.1038/289353a0. [DOI] [PubMed] [Google Scholar]
- Cooper G. M., Okenquist S., Silverman L. Transforming activity of DNA of chemically transformed and normal cells. Nature. 1980 Apr 3;284(5755):418–421. doi: 10.1038/284418a0. [DOI] [PubMed] [Google Scholar]
- Fogel M., Sachs L. Induction of virus synthesis in polyoma transformed cells by ultraviolet light and mitomycin C. Virology. 1970 Jan;40(1):174–177. doi: 10.1016/0042-6822(70)90391-0. [DOI] [PubMed] [Google Scholar]
- Folk W. R. Induction of virus synthesis in polyoma-transformed BHK-21 cells. J Virol. 1973 Mar;11(3):424–431. doi: 10.1128/jvi.11.3.424-431.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Huttner K. M., Ruddle F. H. Study of mitomycin C-induced chromosomal exchange. Chromosoma. 1976 Jun 30;56(1):1–13. doi: 10.1007/BF00293724. [DOI] [PubMed] [Google Scholar]
- Kaplan J. C., Wilbert S. M., Collins J. J., Rakusanova T., Zamansky G. B., Black P. H. Isolation of simian virus 40-transformed inbred hamster cell lines heterogeneous for virus induction by chemicals or radiation. Virology. 1975 Nov;68(1):200–214. doi: 10.1016/0042-6822(75)90161-0. [DOI] [PubMed] [Google Scholar]
- Kinsella A. R., Radman M. Inhibition of carcinogen-induced chromosomal aberrations by an anticarcinogenic protease inhibitor. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3544–3547. doi: 10.1073/pnas.77.6.3544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieg P., Amtmann E., Sauer G., Lavi S., Kleinberger T., Winocour E. The integrated SV40 genome in permissive transformed monkey cells. Virology. 1981 Jan 30;108(2):453–461. doi: 10.1016/0042-6822(81)90452-9. [DOI] [PubMed] [Google Scholar]
- Lavi S., Etkin S. Carcinogen-mediated induction of SV40 DNA synthesis in SV40 transformed Chinese hamster embryo cells. Carcinogenesis. 1981;2(5):417–423. doi: 10.1093/carcin/2.5.417. [DOI] [PubMed] [Google Scholar]
- Levan A., Levan G., Mitelman F. Chromosomes and cancer. Hereditas. 1977;86(1):15–30. doi: 10.1111/j.1601-5223.1977.tb01208.x. [DOI] [PubMed] [Google Scholar]
- Mark J., Levan G., Mitelman F. Identification by fluorescence of the G chromosome lost in human meningomas. Hereditas. 1972;71(1):163–168. doi: 10.1111/j.1601-5223.1972.tb01017.x. [DOI] [PubMed] [Google Scholar]
- Mark J., Westermark B., Pontén J., Hugosson R. Banding patterns in human glioma cell lines. Hereditas. 1977;87(2):243–260. doi: 10.1111/j.1601-5223.1978.tb01267.x. [DOI] [PubMed] [Google Scholar]
- Max E. E., Seidman J. G., Leder P. Sequences of five potential recombination sites encoded close to an immunoglobulin kappa constant region gene. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3450–3454. doi: 10.1073/pnas.76.7.3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCann J., Choi E., Yamasaki E., Ames B. N. Detection of carcinogens as mutagens in the Salmonella/microsome test: assay of 300 chemicals. Proc Natl Acad Sci U S A. 1975 Dec;72(12):5135–5139. doi: 10.1073/pnas.72.12.5135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perler F., Efstratiadis A., Lomedico P., Gilbert W., Kolodner R., Dodgson J. The evolution of genes: the chicken preproinsulin gene. Cell. 1980 Jun;20(2):555–566. doi: 10.1016/0092-8674(80)90641-8. [DOI] [PubMed] [Google Scholar]
- Peto R., Roe F. J., Lee P. N., Levy L., Clack J. Cancer and ageing in mice and men. Br J Cancer. 1975 Oct;32(4):411–426. doi: 10.1038/bjc.1975.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHAW M. W., COHEN M. M. CHROMOSOME EXCHANGES IN HUMAN LEUKOCYTES INDUCED BY MITOMYCIN C. Genetics. 1965 Feb;51:181–190. doi: 10.1093/genetics/51.2.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakano H., Hüppi K., Heinrich G., Tonegawa S. Sequences at the somatic recombination sites of immunoglobulin light-chain genes. Nature. 1979 Jul 26;280(5720):288–294. doi: 10.1038/280288a0. [DOI] [PubMed] [Google Scholar]
- Sambrook J., Botchan M., Gallimore P., Ozanne B., Pettersson U., Williams J., Sharp P. A. Viral DNA sequences in cells transformed by simian virus 40, adenovirus type 2 and adenovirus type 5. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):615–632. doi: 10.1101/sqb.1974.039.01.075. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Therman E., Kuhn E. M. Cytological demonstration of mitotic crossing-over in man. Cytogenet Cell Genet. 1976;17(5):254–267. doi: 10.1159/000130721. [DOI] [PubMed] [Google Scholar]
- Vogel T., Gluzman Y., Winocour E. Recombination between endogenous and exogenous simian virus 40 genes. II. Biochemical evidence for genetic exchange. J Virol. 1977 Nov;24(2):541–550. doi: 10.1128/jvi.24.2.541-550.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl G. M., Padgett R. A., Stark G. R. Gene amplification causes overproduction of the first three enzymes of UMP synthesis in N-(phosphonacetyl)-L-aspartate-resistant hamster cells. J Biol Chem. 1979 Sep 10;254(17):8679–8689. [PubMed] [Google Scholar]
- Winocour E., Keshet I. Indiscriminate recombination in simian virus 40-infected monkey cells. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4861–4865. doi: 10.1073/pnas.77.8.4861. [DOI] [PMC free article] [PubMed] [Google Scholar]









