Abstract
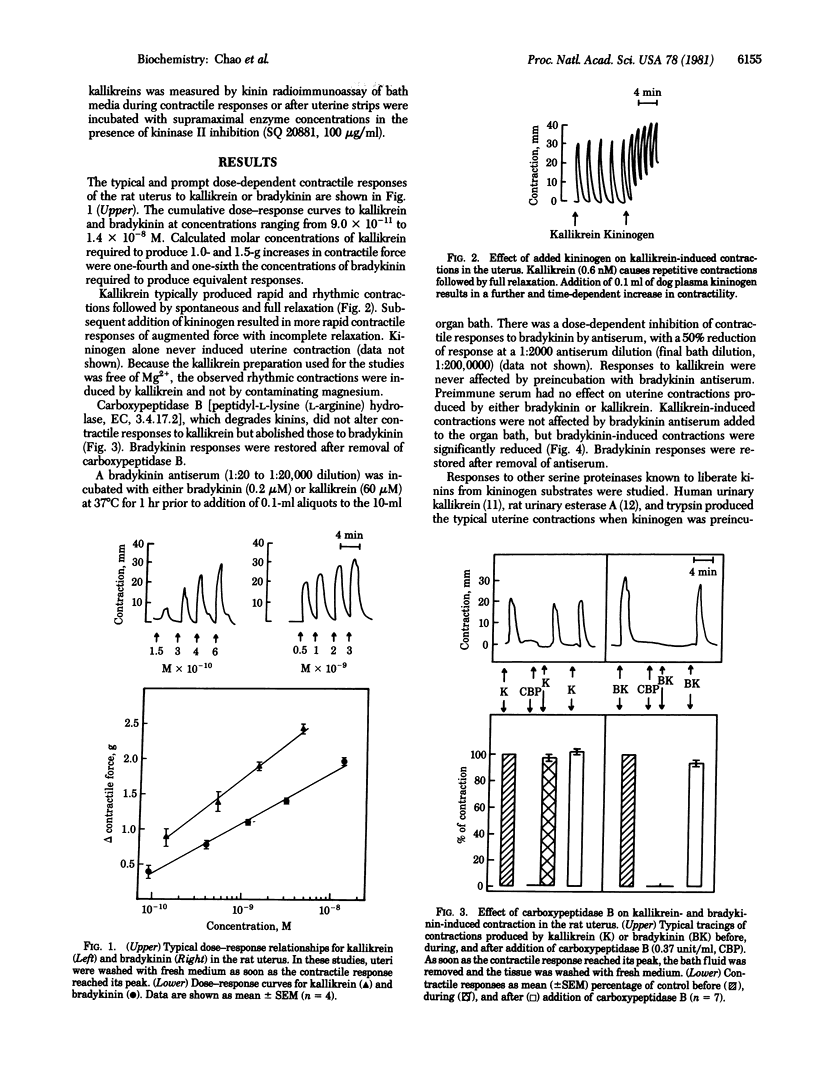
Responses of smooth muscle to kallikreins (EC 3.4.21.8) are generally considered to result from kinin formation. This premise was reexamined with the isolated rat uterus. Rat urinary kallikrein or bradykinin produced dose-dependent contractions of rat uterus but kallikrein was 5-fold more potent than bradykinin. Kallikrein caused an immediate series of rhythmic contractions which could be increased gradually with subsequent addition of kininogen substrate. Kallikrein-induced contractions were unaffected by carboxypeptidase B or a bradykinin antiserum whereas bradykinin-induced contractions were attenuated or abolished. Other serine proteinases, including trypsin, either did not induce contraction in the absence of added kininogen or did so minimally. Although small amounts of kininogen-like substrate were found in uterine tissue, detectable kinin levels (greater than 4 pg) could not be found in bathing media during maximal kallikrein-induced contractions or after uterine tissue was incubated with high concentrations of the enzyme in the presence of SQ 20881, a kininase II inhibitor. The data suggest that uterine contraction produced by a homologous kallikrein does not involve kinin formation but results from an action of this serine proteinase upon other accessible systems coupled to the contractile response.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arakawa K., Maruta H. Ability of kallikrein to generate angiotensin II-like pressor substance and a proposed 'kinin-tensin enzyme system'. Nature. 1980 Dec 25;288(5792):705–706. doi: 10.1038/288705a0. [DOI] [PubMed] [Google Scholar]
- Beaven V. H., Pierce J. V., Pisano J. J. A sensitive isotopic procedure for the assay of esterase activity: measurement of human urinary kallikrein. Clin Chim Acta. 1971 Mar;32(1):67–73. doi: 10.1016/0009-8981(71)90465-7. [DOI] [PubMed] [Google Scholar]
- Beraldo W. T., Araujo R. L., Mares-Guia M. Oxytocic esterase in rat urine. Am J Physiol. 1966 Oct;211(4):975–980. doi: 10.1152/ajplegacy.1966.211.4.975. [DOI] [PubMed] [Google Scholar]
- Chao J., Margolius H. S. Isozymes of rat urinary kallikrein. Biochem Pharmacol. 1979 Jul 1;28(13):2071–2079. doi: 10.1016/0006-2952(79)90226-0. [DOI] [PubMed] [Google Scholar]
- DINIZ C. R., CARVALHO I. F. A micromethod for determination of bradykininogen under several conditions. Ann N Y Acad Sci. 1963 Feb 4;104:77–89. doi: 10.1111/j.1749-6632.1963.tb17654.x. [DOI] [PubMed] [Google Scholar]
- Fritz H., Fink E., Truscheit E. Kallikrein inhibitors. Fed Proc. 1979 Dec;38(13):2753–2759. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Marin-Grez M., Carretero O. A. A method for measurement of urinary kallikrein. J Appl Physiol. 1972 Mar;32(3):428–431. doi: 10.1152/jappl.1972.32.3.428. [DOI] [PubMed] [Google Scholar]
- Nustad K., Pierce J. V. Purification of rat urinary kallikreins and their specific antibody. Biochemistry. 1974 May 21;13(11):2312–2319. doi: 10.1021/bi00708a012. [DOI] [PubMed] [Google Scholar]
- Richert N. D., Ryan R. J. Proteolytic enzyme activation of rat ovarian adenylate cyclase. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4857–4861. doi: 10.1073/pnas.74.11.4857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STURMER E., BERDE B. A pharmacological comparison between synthetic bradykinin and synthetic kallidin. J Pharmacol Exp Ther. 1963 Jan;139:38–41. [PubMed] [Google Scholar]
- Sealey J. E., Atlas S. A., Laragh J. H., Oza N. B., Ryan J. W. Human urinary kallikrein converts inactive to active renin and is a possible physiological activator of renin. Nature. 1978 Sep 14;275(5676):144–145. doi: 10.1038/275144a0. [DOI] [PubMed] [Google Scholar]
- Shimamoto K., Ando T., Nakao T., Tanaka S., Sakuma M., Miyahara M. A sensitive radioimmunoassay method for urinary kinins in man. J Lab Clin Med. 1978 May;91(5):721–728. [PubMed] [Google Scholar]
- Shimamoto K., Chao J., Margolius H. S. The radioimmunoassay of human urinary kallikrein and comparisons with kallikrein activity measurements. J Clin Endocrinol Metab. 1980 Oct;51(4):840–848. doi: 10.1210/jcem-51-4-840. [DOI] [PubMed] [Google Scholar]
- Sustarsic D. L., McPartland R. P., Rapp J. P. Total and kallikrein arginine esterase activities in the urine of salt-hypertensive susceptible and resistant rats. Hypertension. 1980 Nov-Dec;2(6):813–820. doi: 10.1161/01.hyp.2.6.813. [DOI] [PubMed] [Google Scholar]
- Suzuki S., Franco-Saenz R., Tan S. Y., Mulrow P. J. Direct action of rat urinary kallikrein on rat kidney to release renin. J Clin Invest. 1980 Oct;66(4):757–762. doi: 10.1172/JCI109913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whalley E. T., Riley A. J. The effect of aprotinin on luteolytic and uterine contractile mechanisms in the pregnant rat at term. J Reprod Fertil. 1979 Mar;55(2):377–384. doi: 10.1530/jrf.0.0550377. [DOI] [PubMed] [Google Scholar]
- ole-MoiYoi O. K., Pinkus G. S., Spragg J., Austen K. F. Identification of human glandular kallikrein in the beta cell of the pancreas. N Engl J Med. 1979 Jun 7;300(23):1289–1294. doi: 10.1056/NEJM197906073002301. [DOI] [PubMed] [Google Scholar]



