Abstract
Intestinal ischemic events, which are followed by reperfusion induce significant tissue damage and frequently result in multiple organ failure with over 70% mortality. Upon reperfusion, excessive inflammation leads to the exacerbated tissue damage. Previous studies indicated that binding of the serum protein, β2-glycoprotein I, to the endothelium initiates a cascade of inflammatory molecules that are required for damage. We hypothesized that peptides derived from the binding domain (Domain V) of β2-glycoprotein I would attenuate ischemia/reperfusion-induced damage and inflammation in a therapeutic manner. Using a mouse model of intestinal ischemia/reperfusion, we administered peptides either prior to ischemia or at clinically relevant time points during reperfusion and evaluated intestinal tissue damage and inflammation after two hours reperfusion. We demonstrate that multiple peptides attenuate injury and inflammation in a dose dependent manner and perhaps more significantly are efficacious when administered up to 30 min after the onset of reperfusion. In addition, an all D-amino acid retro-inverso peptide was also biologically active. Thus, the β2-glycoprotein I-derived peptides attenuate injury and inflammation when administered in a therapeutic manner in intestinal ischemia/reperfusion injury.
Introduction
Although a lack of blood flow (ischemia) and therefore lack of oxygen (hypoxia) result in cellular death and tissue damage, the return of blood flow (reperfusion) to the cell or organ significantly magnifies the tissue damage and may lead to damage in other organs resulting in multiple organ failure. Several clinical conditions result in ischemia/reperfusion (I/R)3-induced injury ranging from myocardial infarction, intestinal I/R, cardiac bypass surgery, stroke, hemorrhage and transplantation. As myocardial infarction and stroke are two of the leading causes of death in the US and approximately 15,000 transplants and 30,000 cases of intestinal I/R (mortality rate of 60–80%) occur each year in the US [1], I/R is a significant clinical condition.
Multiple components of the innate immune response play a role in reperfusion-induced injury, including activation of the complement cascade, cellular infiltrations, and secretion of cytokines and eicosanoids [2–8]. The current literature suggests that during the ischemic period, reactive oxygen species, nitric oxide, Ca2+, lipases and mitochondrial changes expose neoantigens on the cellular surface [9–11]. During reperfusion, naturally occurring antibody(NAb) recognize newly expressed neoantigens including multiple phospholipid binding proteins and the serum protein, β2-glycoprotein I (β2-GPI) [6, 7, 12, 13]. The NAb are critical to initiating an excessive complement response and subsequent inflammation and tissue damage [6, 7]. Additional data indicate that both neutrophil infiltration and complement activation are required for tissue damage as the absence of either attenuates tissue injury (reviewed in [14]).
As a component of the innate immune system, β2-GPI, also known as apolipoprotein H, is a 43 kDa serum protein abundant in the plasma (4–5μM) [15–17]. The protein contains five short consensus repeats with homology to the complement regulatory domains. Each domain consists of ~60 amino acids with Domain V containing additional residues on the C-terminus [18]. Domain V binds to anionic phospholipids, DNA or other negatively charged molecules [18]. Multiple carbohydrate structures stabilize the different conformations of β2-GPI [19]. Each conformation is proposed to have distinct biological activity [19, 20]. The primary conformation circulating in the blood is a closed or circular form. Within the plasma, β2-GPI exists in multiple oxidation states, which appear to regulate platelet adhesion and protect endothelial cells from oxidative stress [21, 22]. Recent studies also identified β2-GPI as an inhibitor of angiogenesis [23, 24]. Activation of β2-GPI induces a “fishhook” conformation when Domain V binds to anionic phospholipids and exposes a neoantigen in Domains I and II which is recognized by NAb [19]. Subsequently, the NAb-β2-GPI complex acts as an opsonin for the clearance of apoptotic cells by phagocytes [18, 25].
The normal physiological function of β2-GPI remains unclear [26]. Anti-β2-GPI Ab are associated with autoimmune diseases and β2-GPI is the major antigenic target for anti-phospholipid Ab found in the serum of anti-phospholipid Ab syndrome and systemic lupus erythematosus patients [27]. Furthermore, increased anti-β2-GPI Ab titer correlated with increased risk of ischemic stroke or heart disease in anti-phospholipid Ab syndrome or systemic lupus erythematosus patients, respectively [28, 29].
Previously, we demonstrated that during reperfusion β2-GPI binds to ischemic cell membranes allowing Ab recognition necessary for subsequent complement activation and inflammation [13]. Importantly, long peptides (25 amino acid) derived from the β2-GPI sequence attenuated β2-GPI binding to hypoxic endothelial cells and attenuated intestinal damage and inflammation when administered prior to I/R [13]. Other β2-GPI-derived peptides also decrease fetal loss in a murine model of anti-phospholipid syndrome [30, 31]. However, the previous studies administered peptide prior to an ischemic event [13] or simultaneously with the Ab which induce fetal loss [30, 31]. These treatment regimens are not feasible therapeutic interventions. Therefore, studies administering peptide at time points after the ischemic event or Ab induction are needed. In addition, reducing the size of the peptide would provide a more cost effective therapeutic, as cost of production is not a linear function relative to length of the peptide. Finally, the peptides provide a useful tool for examining additional mechanisms of β2-GPI-induced damage in response to I/R.
We hypothesized that smaller peptides derived from β2-GPI would therapeutically attenuate the injury and inflammation in a mouse model of intestinal I/R. We demonstrate that the peptides (and smaller derivatives) are biologically active not only when administered prior to ischemia but also when administered during the reperfusion event at 1–10 fold excess of the native protein concentrations. Additionally, biologically active, reversed sequences of D-amino acids (retro inverso) appear to be more effective possibly due to an increased half-life. These data indicate that the β2-GPI-derived peptides are clinically relevant and may provide a therapy for intestinal I/R injury.
Materials and Methods
Mice
Originally obtained from Jackson Laboratory, C57Bl/6 mice were bred and maintained in the Division of Biology at Kansas State University. Housed in a 12-hour light-to-dark, temperature-controlled room, mice were allowed food and water ad libitum. All mice were kept in specific pathogen free conditions (Helicobacter species, mouse hepatitis virus, minute virus of mice, mouse parvovirus, Sendai virus, murine norovirus, Mycoplasma pulmonis, Theiler’s murine encephalomyelitis virus, and endo- and ecto-parasites). All research was approved by the Institutional Animal Care and Use Committee and conducted in compliance with the Animal Welfare Act and other Federal statutes and regulations concerning animals.
Peptides
Previously reported studies employing peptides 296 and 305 attenuated tissue injury [13]. Smaller peptides contained within these regions of the β2-GPI sequence were designed and synthesized (Table I). Peptide p7 contained the consensus sequence for lipid binding found in peptide 296 with a Cys to Ser substitution (SKNKEKK) [32]. Peptides p9 (SSYTVEAHS) and p11 (KKSSYTVEAHS) from peptide 305 also contained the Cys to Ser substitution from original peptide 305. Additional forms of p9 were created using D-amino acids (D-p9) or the retro-inverso of D-p9 (Retro D-p9: SHAEVTYSS). All peptides were generated at the KSU Biochemistry Core Lab by solid-phase synthesis with 9-fluorenylmethoxycarbinyl chemistry in the Biotechnology Core Lab at Kansas State University as described previously [33]. The peptides were purified by reversed phase HPLC and characterized by matrix-assisted laser desorption time of flight mass spectroscopy (MALDI TOF/TOF). All lyophilized peptides were stored at −20°C until time of use. Immediately prior to use, peptides were solubilized in normal saline.
Table I.
β2-GPI peptide sequences
| Peptide name | Sequence | Residue numbers | MWb (Da) |
|---|---|---|---|
| 296 Cys-Ser | H-IHFYSKNKEKKSSYTVEAHSRDGTI-OHa | 296–320 | 2925 |
| p7 | H-SKNKEKK-OHa | 300–306 | 861 |
| 305 | H-KKCSYTVEAHCRDGTIEIPSCFKEHS-OHa | 305–330 | 2969 |
| 322 | H-IPSCFKEHSSLAFWKTDASELTPC-NH2 | 322–345 | 2698 |
| p9 | H-SSYTVEAHS-OHa | 307–315 | 980 |
| p11 | H-KKSSYTVEAHS-OHa | 305–315 | 1236 |
| D-p9 | H-SSYTVEAHS-OH with D-amino acids | 307–315 | 980 |
| Retro D-p9 | H-SHAEVTYSS-OH with D-amino acids | 315–307 | 980 |
| Scrambled | H-AQCMPDVRIQTA-NH2 | 1331 |
Amino acid sequence is a modification of NCBI sequence, AAB30789, as described in the Materials and Methods.
MW is Molecular weight
Ischemia/Reperfusion Procedure
As described previously, ischemia/reperfusion was performed on ketamine (16 mg/kg) / xylazine (80 mg/kg) anesthetized male mice (8–16 wks old) with buprenorphine administered for pain [34]. Following a midline laparotomy, the body temperature of the mice was equilibrated for 30 min on a 37°C water-circulating heat pad and moistened gauze prevented desiccation. The superior mesenteric artery was identified, isolated, and a small vascular clamp applied for 30 min and removed prior to 2 h of reperfusion. Ischemia was confirmed by observing the intestine changing from a pink to a gray color. Sham treated animals with or without peptide underwent the same procedure as the ischemic mice, without the occlusion of the superior mesenteric artery. Sham treated survival rate was 100% with or without peptide treatment. After two hours of reperfusion, the mice were euthanized and multiple sections of the small intestine, beginning approximately 10 cm distal to the gastroduodenal junction, were collected for histological and other analyses. The sequence of sections used for each analysis was maintained for each animal. All mice received 80–100 μL normal saline or peptide. Mice treated with the various β2-GPI peptides underwent the same procedure with i.v. administration of the peptides. Most studies administered 40 μM (80 nmol/mouse) peptide at 5 min prior to ischemia. Dose response studies administered 1–40 μM peptide at 5 min prior to ischemia. These concentrations are 1–10 fold excess of the native protein concentration [15–17]. Time course studies administered 40 μM peptide at 5 min prior to ischemia, or 15, 30 or 60 min post ischemia.
Histology and Injury Scoring
After euthanasia, a 2 cm mid-jejunum tissue section approximately 10 cm distal to the gastroduodenal junction was immediately fixed in 10% buffered formalin, embedded in paraffin, and 8 μm sections were cut transversely and hematoxylin & eosin stained. Mucosal injury was graded on a six-tiered scale adapted from Chiu et al. [35], as described previously [34]. Briefly, the average damage score of each intestinal section (75–150 villi) was determined after grading each villus from 0–6. Normal villi were assigned a score of zero; villi with tip distortion were assigned a score of 1; a score of 2 was assigned when Guggenheims’ spaces were present; villi with patchy disruption of the epithelial cells were assigned a score of 3; a score of 4 was assigned to villi with exposed but intact lamina propria with epithelial sloughing; a score of 5 was assigned when the lamina propria was exuding; last, villi that displayed hemorrhage or were denuded were assigned a score of 6. Using the same slides, the villus height/crypt depth ratio (V/C) was measured at 200x from 20–25 villi per animal using a Nikon DS camera with a DS-L2 Controller and software (Nikon, Melville, NY). The software was calibrated with a slide micrometer. Cryosections were stained with rat anti-mouse C3, IgM or myeloperoxidase mAb followed by Texas Red conjugated donkey anti-rat Ab and mounted with Prolong Gold (Invitrogen, Grand Island, NY). Photomicrographs were obtained at room temperature using a Plan Fluor 20x/0.5 objective on a Nikon 80i microscope equipped with a Photometrics CoolSnap cf camera using MetaVue software. All microphotographs were analyzed by Image J software (National Institutes of Health, Bethesda, MD) using the fluorescent area fraction after setting threshold for each experiment. The average of the isotype control was subtracted from each photo. The average of 5 photos/tissue from three to four animals per treatment group is reported.
Ex vivo eicosanoid, and cytokine generation
Immediately after collection, a 2 cm intestinal section approximately 14 cm distal to the gastroduodenal junction was minced, washed, resuspended in 37°C oxygenated Tyrode’s buffer (Sigma-Aldrich, St. Louis, MO), incubated at 37°C for 20 min and the supernatants collected. LTB4 and PGE2 concentrations were determined using enzyme immunoassay kits (Cayman Chemical, Ann Arbor, MI). Cytokines present in the supernatants was determined by using a Milliplex MAP immunoassay kit (Millipore, Bedford, MA) and read on a Milliplex Analyzer (Millipore). All LTB4, PGE2 and cytokines concentrations were standardized to the total tissue protein content determined by BCA assay (Pierce, Rockford, IL) adapted to microtiter plates.
Statistical analysis
Data are presented as mean ± SEM and significance (p < 0.05) determined by one-way ANOVA with Newman-Keuls post hoc analysis (GraphPad/Instat Software).
Results
Therapeutically administered peptides 296c-s and 305 protect from I/R-induced damage
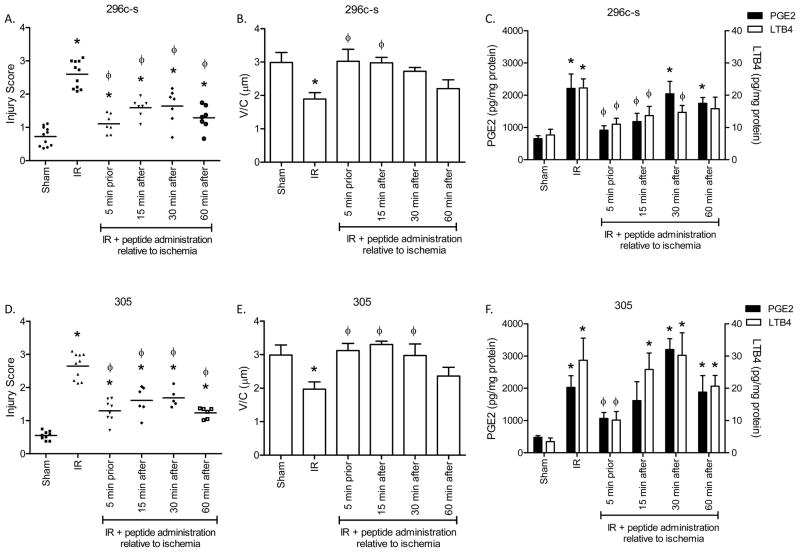
Previous studies indicated that both peptide 296c-s and peptide 305 prevented intestinal I/R-induced tissue injury and PGE2 production [13]. However, the peptides were administered prior to I/R-induced injury, which is not possible in most clinical situations. Therefore, we determined the optimal time of peptide administration. Similar to previous studies, I/R induced significant injury with an average injury score of 2.5 ± 0.12 compared to Sham treatment injury at 0.7 ± 0.06 (Fig. 1A). The V/C decreased with I/R as another measure of intestinal injury (Fig. 1B). As indicated in Figure 1 and Supplementary Figure 1, both peptides appear to be efficacious in preventing intestinal injury when administered at either 5 min prior to reperfusion or 15 min into the reperfusion period in this 2 h evaluation period. In addition, treatment at both 30 min and 60 min post ischemia significantly decreased intestinal injury (Fig. 1A, D). Specifically, peptide 296c-s decreased injury to 1.64 ± 0.19 and 1.40 ± 0.11 when administered at 30 and 60 min, respectively after initiation of reperfusion (Fig. 1A). In addition, the V/C remained high at 30 min post-ischemia indicating tall villi (Fig. 1B). When administered at similar time points, peptide 305 decreased injury to 1.58 ± 0.08 (30 min) and 1.24 ± 0.06 (60 min) (Fig. 1D). The V/C also remained high when peptide 305 was administered at 30 min into the reperfusion period but was shortened by 60 min post-ischemia (Fig. 1E).
Figure 1. Peptides 296c-s and 305 attenuate injury and eicosanoid production when administered after ischemia.
C57Bl/6 mice were subjected to Sham or I/R with or without injection of 40 μM β2-GPI peptides, either prior to ischemia or 5, 15 or 30 min post ischemia. A, B, D, E) Mid-jejunal sections were scored (75–150 villi per animal) (A, D) and villus height/crypt depth ratios measured (B, E) from C57Bl/6 mice with or without injection of 40 μM β2-GPI peptides 296c-s (A, B) or 305 (D, E) at appropriate time points. C, F) PGE2 or LTB4 production was measured in intestinal sections from C57Bl/6 mice with or without injection of β2-GPI peptides 296c-s (C) or 305 (F) prior to Sham or I/R treatment. Values are represented as pg/mg of intestinal protein * = p ≤ 0.05 compared to Sham + peptide, Φ = p ≤ 0.05 compared to I/R treatment animals not receiving peptides. Each symbol in A and D represents an individual animal. B, C, E, F. each bar is representative of 5–10 animals and each treatment was performed on at least 2 separate days.
As eicosanoids, LTB4 and PGE2 play significant roles in I/R-induced intestinal inflammation, we evaluated these molecules in peptide-treated mice. Treatment with peptides 296c-s and 305 attenuated PGE2 production when administered at 15 min post-ischemia (Fig. 1C, F). However, PGE2 increased when peptide was administered at later time points (Fig. 1C, F). I/R-induced LTB4 was significantly inhibited by peptide 296c-s at 15 and 30 min post-ischemia (Fig. 1C). Peptide 305 only attenuated LTB4, a chemotactic factor for inflammatory cells, when administered prior to ischemia (Fig. 1F). These results suggest that both peptides may be appropriate therapeutics for I/R-induced injury.
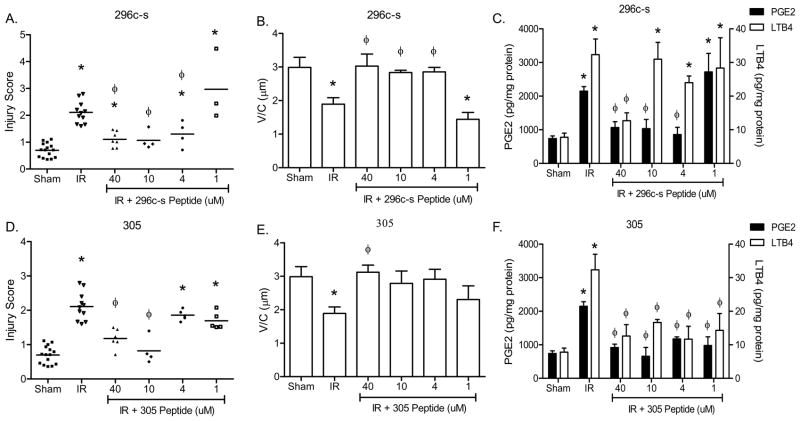
Peptides 296c-s and 305 attenuate I/R-induced injury in a dose dependent manner
To determine the optimal dose of each peptide when administered prior to I/R, we treated at least 3 mice with 1, 4, 10, or 40 μM of peptides 296c-s or 305. As indicated in Figure 2 and Supplementary Figure 2A, 10 μM treatment of either peptide optimally attenuated injury and PGE2 production. Peptide 296c-s also attenuated intestinal damage at 4 μM as determined by low injury scores and high V/C (Fig. 2A, B). In contrast, at 4 μM peptide 305 did not provide protection as determined by injury score despite maintaining high V/C (Fig. 2D, E). In addition, similar concentrations of peptide 296c-s attenuated PGE2 production (Fig. 2C, F). Interestingly, peptide 305 reduced eicosanoid production at all concentrations tested, despite being unable to prevent injury at 1–4μM peptide (Fig. 2D, F). Peptide 296c-s treatment only attenuated LTB4 production at 40 μM peptide while peptide 305 attenuated LTB4 production at all concentrations tested (Fig. 2C, F). These studies indicate that both peptides, 296c-s and 305, are effective when administered at lower concentrations than previously reported and suggest that the peptides differentially alter the inflammatory response.
Figure 2. Peptides 296c-s and305 attenuate I/R-induced injury and inflammation in a dose dependent manner.
C57Bl/6 mice were subjected to Sham or I/R with or without injection of β2-GPI peptides, ranging from 1–40 μM final concentration. A, B, D, E) Mid-jejunal sections were scored (75–150 villi per animal) (A, D) and villus height/crypt depth ratios measured (B, E) from C57Bl/6 mice with or without injection of β2-GPI peptide 296c-s (A, B) or 305 (D, E) prior to Sham or I/R treatment. PGE2 or LTB4 (C, F) production was measured in intestinal sections from C57Bl/6 mice with or without injection of β2-GPI peptide 296c-s (C) or 305 (F) prior to Sham or I/R treatment. Values are represented as pg/mg of intestinal protein * = p ≤ 0.05 compared to Sham + peptide, φ = p ≤ 0.05 compared to I/R treatment animals not receiving peptides. Each symbol in A and D represents an individual animal. Each bar (B, C, E, F) is representative of 3–10 animals and each treatment was performed on at least 2 separate days.
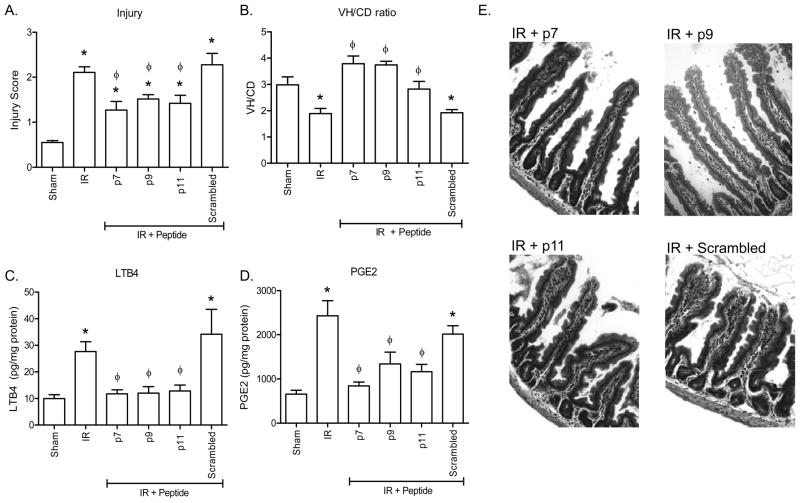
Multiple short peptides attenuate I/R-induced tissue damage and inflammation
As smaller peptides are therapeutically more cost effective, we examined peptides 296c-s and 305, which share 16 of 25 amino acids, for smaller sequences that contain similar inhibitory activity. As such, we tested three peptides, 7–11 amino acids in length, for the ability to provide intestinal protection from I/R-induced injury and inflammation (Fig. 3 and Table I). Multiple short peptides (40 μM; 80 nmol/animal) were administered i.v. to wildtype, C57Bl/6 mice 5 min prior to ischemia. As indicated in Figures 3A, B and E, peptide treatment resulted in significantly decreased intestinal epithelial injury compared to similar mice subjected to I/R in the presence of saline or a scrambled peptide. Importantly, none of the mice treated with short peptides p7, p9, p11 sustained injury scores or V/C ratios significantly different than mice treated with 40 μM peptide 296c-s prior to ischemia (Figs. 1A, B, 3A, E). In addition, the V/C ratios were similar to Sham treated animals, indicating the small peptides attenuated intestinal I/R-induced tissue damage (Fig. 3B).
Figure 3. Shorter peptides attenuate I/R-induced intestinal injury and inflammation.
Prior to Sham or I/R treatment C57Bl/6 mice were treated with or without injection of 40 μM β2-GPI peptides, p7, p9, p11, D-p9 and Retro D-p9. A, B) Mid-jejunal sections from each animal were scored for injury (A) and villus height/crypt depth ratio (V/C) measured using Metavue microscopic software calibrated with a slide micrometer (B). C, D) Ex vivo intestinal LTB4 (C) or PGE2 (D) production was measured from each treated animal. Values are represented as pg/mg of intestinal protein. E) Representative H+E stained sections of each treatment are presented. Magnification for all photomicrographs and measurements were obtained at 200x. * = p ≤ 0.05 compared to Sham + peptide, Φ = p ≤ 0.05 compared to I/R treatment animals not receiving peptides. Each bar is representative of 5–10 animals and each treatment was performed on at least 2 separate days.
Similar to the large peptides, we evaluated the eicosanoid production in shorter peptide-treated mice. Similar to the intestinal damage, the intestines of p7, p9 or p11 treated mice secreted similar concentrations of LTB4 (Fig. 3C). In addition, the LTB4 secreted differed significantly from I/R treated or scrambled peptide treated mice and was similar to that found in mice subjected to Sham treatment in the presence or absence of peptide treatment (Fig. 3C). Interestingly, all peptides attenuated I/R-induced PGE2 production but only peptide p7 returned PGE2 levels to those found in Sham treated animals (Fig. 3D). These data suggest that the shorter peptides may provide therapeutic efficacy similar to or better than the larger peptides.
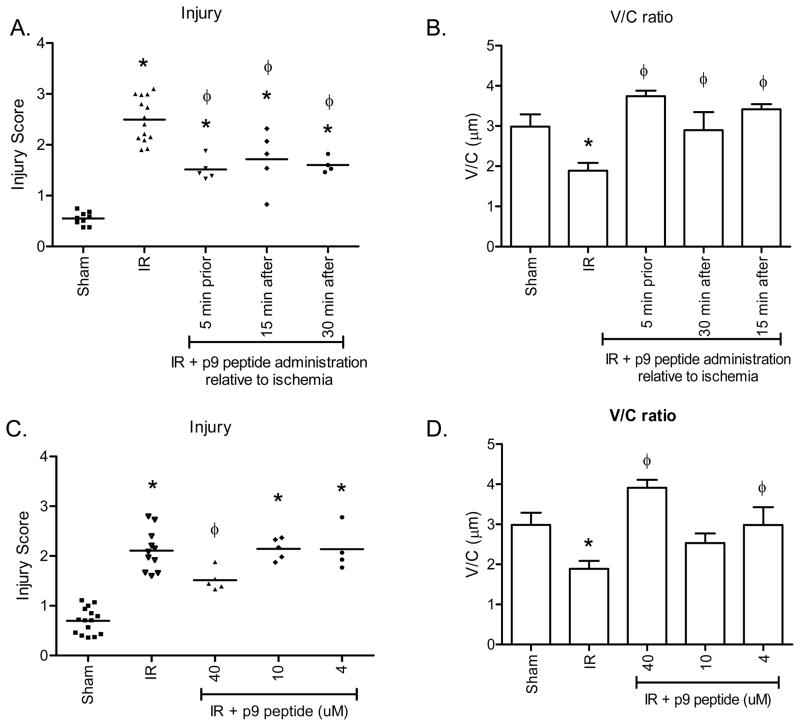
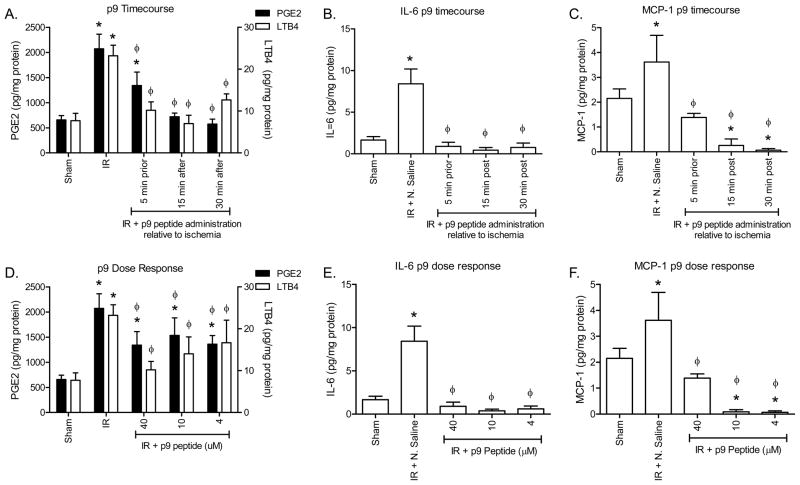
Similar to the larger peptides, time course and concentration studies were performed to determine the clinical relevance of peptide p9 (Fig. 4). P9 appeared efficacious in preventing intestinal injury when administered at either 5 min prior to reperfusion (1.50 ± 0.09) or 15 min post ischemia (1.72 ± 0.25) in this 2 h evaluation period (Fig. 4A, Supplemental Fig. 2B). In addition, treatment at 30 min post ischemia also significantly decreased intestinal injury with injury scores of 1.60 ± 0.08 (Fig. 4A). Finally, the V/C remained elevated even after 30 min post-ischemia (Fig. 4B). When the dose response was evaluated, only 40μM attenuated intestinal damage as determined by decreased injury score and increased V/C (Fig. 4C, D and Supplemental Fig. 2B).
Figure 4. Peptide p9 attenuates I/R-induced injury in a therapeutic and dose dependent manner.
C57Bl/6 mice were subjected to Sham or I/R with or without injection of 40 μM p9 peptides, either prior to ischemia or 5, 15 or 30 min post ischemia (A–B). Additional mice were subjected to Sham or I/R with or without injection of 4–40 μM p9 peptide (C–D). Mid-jejunal sections from appropriately treated C57Bl/6 mice were scored for injury (A, C) and the villus height/crypt depth ratio determined (B, D). * = p ≤ 0.05 compared to Sham + peptide, Φ = p ≤ 0.05 compared to I/R treatment animals not receiving peptides. Each symbol in A and C represents an individual animal. Each bar (B–D) is representative of 4–10 animals and each treatment was performed on at least 2 separate days.
Similar to the larger peptides, peptide p9 attenuated injury and PGE2 production, even when administered at 30 min post ischemia (Fig. 5A). In addition, LTB4 production was significantly decreased after administration of peptide p9 at 15 and 30 min post ischemia (Fig. 5A). Surprisingly, attenuated injury required 80 ng (40 μM) of p9 per mouse, whereas as little as 8 ng (4 μM) reduced eicosanoid production (Fig. 5D). During the inflammatory process, the ischemic tissue also releases cytokines in response to I/R-induced NF-κB mobilization [36]. Analysis of IL-6 and MCP-1 indicated that I/R induced intestinal secretion of both cytokines. Importantly, peptide p9 attenuated cytokine release when administered at any of the 3 time points or at lower concentrations (Fig. 5B, C, E, F). Together, these data indicate that peptide p9 inhibits the I/R-induced inflammatory response.
Figure 5. Peptide p9 attenuates I/R-induced intestinal eicosanoid and cytokine release in a therapeutic and dose dependent manner.
C57Bl/6 mice were subjected to Sham or I/R with or without injection of 40 μM p9 peptides, either 5 min prior to ischemia or 15 or 30 min post-ischemia (A–C). Additional mice were subjected to Sham or I/R with or without injection of 4–40 μM p9 peptide (D–F). PGE2 and LTB4 (A, D) production was measured in intestinal sections from C57Bl/6 mice with or without injection of peptides prior to Sham or I/R treatment. IL-6 (B, E) and MCP-1 (C, F) release was determined by multiplex analysis of intestinal supernatants. Values are represented as pg/mg of intestinal protein in 20 min. * = p ≤ 0.05 compared to Sham + peptide, Φ = p ≤ 0.05 compared to I/R treatment animals not receiving peptides. Each bar is representative of 4–10 animals and each treatment was performed on at least 2 separate days.
Peptide retro-inverso D-p9 attenuates intestinal I/R-induced damage and inflammation
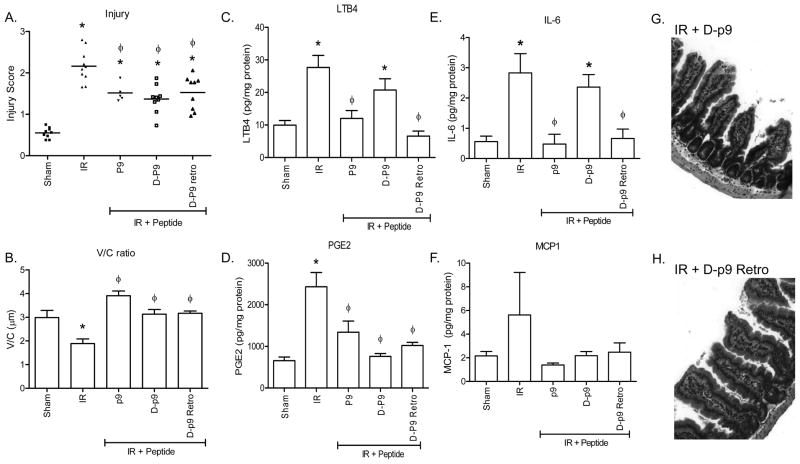
Mammalian proteins consist almost exclusively of L-amino acids. Due to changes in the location of the amino acid side groups, proteins consisting of D-amino acid are generally not biologically active and are not degraded as rapidly by hydrolases. In contrast, reversal of the D-amino acid sequence (retro-inverso) orients the majority of the side groups of a peptide to a conformation that is similar to the L-amino acid sequence. Thus, the retro-inverso sequences frequently have a longer half-life due to the D-amino acids but are functionally similar to the L-amino acid sequence. We hypothesized that D-sequences substituted with all D-amino acids would not protect against I/R-induced damage but that the retro-inverso peptide, may protect from I/R-induced tissue damage similar to p9. Thus, prior to I/R, we treated mice with peptide D-p9 (p9 substituted with D-amino acids) and Retro D-p9 (D-p9 in the reverse sequence; Table I). Surprisingly, treatment with either peptide D-p9 or peptide Retro D-p9 attenuated I/R-induced intestinal injury and maintained V/C similar to peptide p9 treatment (Fig. 6A, B, G, H).
Figure 6. Peptide retro-inverso p9 attenuates I/R-induced injury and inflammation.
C57Bl/6 mice were subjected to Sham or I/R with or without injection of 40 μM peptide p9, D-P9, or RD-p9 prior to ischemia. Mid-jejunal sections from appropriately treated C57Bl/6 mice were scored for injury (A) and the villus height/crypt depth ratio determined (B). LTB4 (C), PGE2 (D), IL-6 (E) and MCP-1 (F) production was measured from intestinal sections from each treatment group. Values are represented as pg/mg of intestinal protein in 20 min. Photomicrographs (G, H) of representative H&E stained intestinal sections of the D-p9 and D-p9 Retro peptide treated animals are provided. Magnification for all photomicrographs and measurements were obtained at 200x. * = p ≤ 0.05 compared to Sham + peptide, Φ = p ≤ 0.05 compared to I/R treatment animals not receiving peptides. Each bar is representative of 4–10 animals and each treatment was performed on at least 2 separate days.
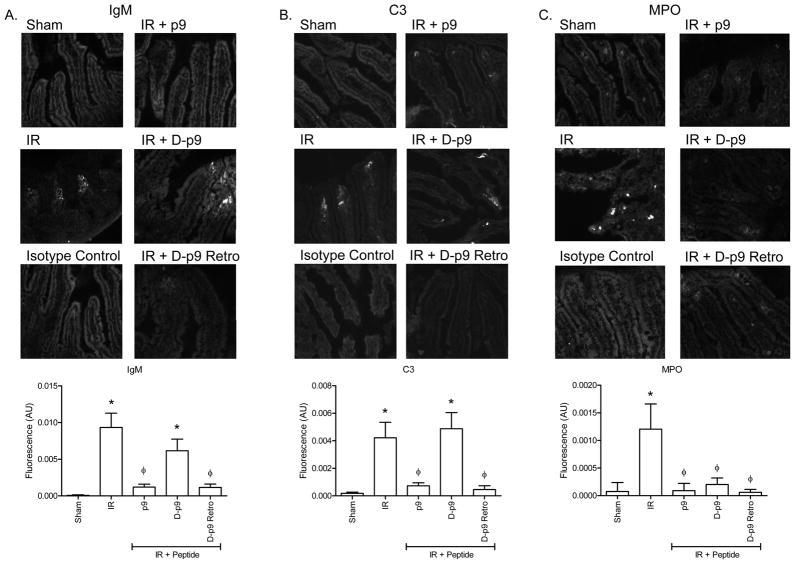
In response to I/R, IgM recognizes and binds multiple neo-antigens prior to complement activation. Complement component C3 is rapidly deposited and required for I/R-induced intestinal damage. Therefore, we examined the ability of the D-stereoisomer of p9 peptides to attenuate IgM binding and C3 deposition after I/R. Intestinal I/R induced both IgM (Fig. 7A) and C3 (Fig. 7B) deposition in untreated mice. Importantly, significantly less IgM and C3 was deposited when mice were treated with p9 and few to no deposits were found in mice treated with Retro D-p9 (Fig. 7A, B). These data suggest that peptide Retro D-p9 has similar function as the L-amino acid peptide, p9.
Figure 7. Peptide Retro D-p9 attenuate IgM and C3 deposition in response to I/R.
Prior to Sham or I/R treatment C57Bl/6 mice were treated with or without injection of 40 μM β2-GPI peptides, p9, D-p9 or Retro D-p9. Representative intestinal sections stained for IgM (A) or C3 (B) deposition. Additional sections were stained for myeloperoxidase (C). Fluorescent intensity was quantitated using Image J with threshold set on isotype control tissues. Each bar is the average ± SEM with 5–8 animals per treatment and 4–6 photographs per animal. Magnification for all photomicrographs and measurements were obtained at 200x. Microphotographs are representative of 3–4 animals stained in at least 3 independent experiments. * = p ≤ 0.05 compared to Sham + peptide, Φ = p ≤ 0.05 compared to I/R treatment animals not receiving peptides.
In contrast to injury, peptide D-p9 did not attenuate IgM deposition (Fig. 7A) or C3deposition (Fig. 7B). To further investigate the mechanism of D-p9 peptide protection from I/R-induced injury, we examined the eicosanoid and cytokine response. As indicated in Figure 6C–F, both p9 and RD-p9 attenuated PGE2 and LTB4 as well as cytokines IL-6 and MCP-1 production compared to untreated I/R or control peptide treated mice. Similar to peptides p9 and RD-p9, D-p9 did not induce PGE2 or MCP-1 production (Fig. 6E, F). However, only L-amino acid containing p9 and Retro D-p9 attenuated LTB4 and IL-6 production in response to I/R (Fig. 6C, D). Peptide D-p9 induced significant LTB4 and IL-6 production (Fig. 6C, D).
As LTB4 is chemotactic for neutrophils and I/R-induced injury requires both complement activation and a neutrophilic inflammatory response, we examined neutrophil infiltration by examining the myeloperoxidase positive cells. As indicated in Figure 7C, I/R induced significant neutrophil infiltration compared to Sham treatment. However, none of the small p9 peptides induced neutrophil infiltration as determined by myeloperoxidase staining. Thus, despite complement and IgM deposition, D-p9 attenuated injury and decreased neutrophil infiltration.
Discussion
Ischemia/reperfusion-induced injury requires activation of a cascade of innate immune components ranging from NAb recognition of a neoantigen, complement activation and inflammatory cell infiltration (reviewed in [14]). Although normally a protective response, during reperfusion, the innate response is excessive and damaging. Thus, inhibition of any of these components (complement activation or neutrophil infiltration) may decrease the immune response to non-damaging levels. We hypothesized that inhibiting an initiator of the cascade would allow each component of the response to remain intact for further insults while preventing the excessive response. The current studies demonstrate that peptide inhibition of β2-GPI attenuates I/R-induced intestinal damage and inflammation when administered up to 30 min post ischemia. To our knowledge this is the first study to therapeutically administer β2-GPI-derived peptides that are small enough to be cost effective for routine use. Finally, the optimized p9 peptide retains activity when synthesized in the retro-inverso form that may extend the therapeutic half-life. Together, these studies extend the possibilities of peptide therapy to intestinal I/R injury and provide evidence that the peptides are clinically relevant.
Previous studies with β2-GPI-derived peptides 296c-s and 305 demonstrated efficacy when administered prior to ischemic events such as in planned surgical procedures including coronary artery bypass surgery [13]. In a mouse model of anti-phospholipid syndrome, fetal loss was decreased when other β2-GPI-derived peptides were administered either simultaneously with pathogenic Ab or up to 3 h prior to injection of Ab [30, 37]. Finally, in vitro studies also demonstrated an effect when cells were treated with peptide prior to stimulation with anti-β2-GPI Ab [30, 37]. In the current studies peptide treatment was efficacious when administered at 15 and 30 min into the 2 h reperfusion period. Additionally, peptide treatment was efficacious at equal molar concentrations up to a 10-fold excess of the circulating native protein concentrations. Hence, administration of the peptides during the reperfusion period extends the clinical relevance to therapeutics rather than pre-treatments. To our knowledge this is the first demonstration of β2-GPI-derived peptide efficacy in preventing ischemic injury when administered in a clinically relevant mode. Thus, these peptides may be useful in acute scenarios in addition to planned and routine surgical procedures and organ transplants.
Previous studies demonstrated that complement is required for I/R-induced injury [7] , while other studies indicate that neutrophil infiltration is required [3, 4], suggesting that multiple innate components are required for tissue damage and the absence of any one component attenuates injury. The peptides appear to have distinct properties, suggesting that treatment with multiple small peptides may be more effective. For example, peptides 305 and p9 (encompassed within 305) appear to be more critical for inhibiting the inflammatory response (eicosanoids and neutrophil infiltration) and peptides 296c-s and p7 (within 296c-s) attenuate actual epithelial injury more effectively. Hence, a combination of p7 and p9 may be optimal therapeutically.
Previous studies indicated that intestinal PGE2 production is necessary for I/R-induced intestinal injury but that PGE2 alone was not sufficient for I/R-induced intestinal injury [8]. The current data support the correlation at high peptide doses and when administered early in the reperfusion period. In addition, PGE2 production increases prior to intestinal injury in the time course of peptide administration supporting the concept that PGE2 is necessary but not sufficient for I/R-induced injury [8, 38]. Previous studies indicated that 5-lipoxygenase is required for I/R-induced tissue damage [39]. As one of the 5-lipoxygenase products, we examined LTB4 production, a potent chemo-attractant factor and activator of neutrophils [40]. The dose response data suggest that LTB4 is not required for intestinal damage. As LTB4 primarily recruits neutrophils, these data correlate with previous studies indicating the neutrophil infiltration is not sufficient for intestinal damage [5].
Interestingly, the D-amino acid form of p9 (D-p9) attenuated intestinal injury and PGE2 production similar to the L-amino acid form. Although many D-amino acid peptides are not biologically active, other examples of biologically active all D-peptides include the D-PIE12-trimer and an antagonist of p53. As a leading anti-HIV antiviral drug candidate, the D-PIE12-trimer binds with high affinity binding to the region on the HIV virus essential for its successful entry into the human cells [41, 42]. Similarly, an L-enantiomeric peptide antagonist negatively regulates tumor suppressor p53 activity. However, the D-enantiomeric peptide binds the oncoproteins with higher affinity and specificity than the L-enantiomer and the D-peptide activates p53 [43].
β2-GPI and anti-β2-GPI Ab play a role in a wide range of clinical conditions ranging from fetal loss in anti-phospholipid syndrome, lupus, thrombosis, angiogenesis in tumors to Alzheimer’s disease (reviewed in [44, 45]) [46]. It is likely that therapeutic administration of peptides will be efficacious in other models of tissue injury, including autoimmune reactions such as the fetal loss models discussed above and other forms of I/R-induced injury including myocardial infarction, stroke, and transplantation. Inhibitors of another I/R-induced neoantigen, non-muscle myosin, attenuate injury and inflammation (complement deposition and inflammatory cell infiltration) when administered prior to ischemia in muscle, intestinal and heart I/R as well as in a burn model (reviewed in [47]). An additional peptide, N2 with homology to non-muscle myosin heavy chain, attenuated injury in multiple I/R models [48]. Importantly, the peptide was effective at 24 h when administered at 1 h after the onset of reperfusion [49]. The similarities in the intestinal, skeletal muscle and myocardial I/R mechanism of action suggest that the β2-GPI peptides may be effective in these models as well. Due to the breadth of diseases in which β2-GPI and related Ab play a role, the peptides will likely be effective in additional clinical conditions including cancer, thrombosis, lupus and cognitive diseases.
The specific mechanism by which the peptides attenuate injury and inflammation is currently unknown. It appears that the peptides compete with native β2-GPI binding to cell surfaces. The peptides block Ab deposition and complement activation, as the peptides do not contain the Ab binding sites found in Domains I and II. However, the binding of peptide also does not stimulate the proposed β2-GPI receptors, TLR2 [50] or TLR4 [51, 52], as these would likely enhance inflammation. A possible mechanism for attenuation of I/R-induced injury includes peptides preventing β2-GPI binding that may induce externalization of additional neo-antigens (such as annexin IV or non-muscle myosin) on either the endothelium or epithelium. A second possibility is that peptides prevent β2-GPI binding and formation of a complex, which is recognized by the NAb prior to complement activation. Another possibility is that similar to anotherβ2-GPI-derived peptide (EMBI) that overlaps with peptide 296c-s but not with the p9 peptide enantiomers, the peptides may be internalized and regulating protein expression or phosphorylation [30]. In vitro studies indicated that compared to β2-GPI/β2-GPI Ab treatment, EMBI decreased JNK and p38 activation in endothelial cells; however, the activation of JNK and p38 by β2-GPI/β2-GPI Ab treatment on endothelial cells remains unknown [30]. Future explorations are required to define the exact mechanism by which the peptides provide protection.
Supplementary Material
Acknowledgments
We would like to thank Mr. Andrew Fritze for technical assistance with immunohistochemistry and eicosanoid assays.
Footnotes
This work was supported by NIH grant AI061691 and P20 RR017686, and RR016475 from the Institutional Development Award Program of the National Center for Research Resources and the Johnson Center for Basic Cancer Research.
Abbreviations used include: β2-GPI, beta2-glycoprotein I; PGE2, prostaglandin E2; LTB4, leukotriene B4; I/R, ischemia/reperfusion; C3, complement component C3; Ab, antibodies, NAb, naturally occurring antibodies.
References
- 1.Lock G. Acute intestinal ischaemia. Best Pract Res Clin Gastroenterol. 2001;15:83–98. doi: 10.1053/bega.2000.0157. [DOI] [PubMed] [Google Scholar]
- 2.Crawford MH, Grover FL, Kolb WP, McMahan CA, O’Rourke RA, McManus LM, Pinckard RN. Complement and neutrophil activation in the pathogenesis of ischemic myocardial injury. Circulation. 1988;78:1449–1458. doi: 10.1161/01.cir.78.6.1449. [DOI] [PubMed] [Google Scholar]
- 3.Hernandez LA, Grisham MB, Twohig B, Arfors KE, Harlan JM, Granger DN. Role of neutrophils in ischemia-reperfusion-induced microvascular injury. Am J Physiol. 1987;253:H699–H703. doi: 10.1152/ajpheart.1987.253.3.H699. [DOI] [PubMed] [Google Scholar]
- 4.Simpson R, Alon R, Kobzik L, Valeri CR, Shepro D, Hechtman HB. Neutrophil and nonneutrophil-mediated injury in intestinal ischemia-reperfusion. Ann Surg. 1993;218:444–453. doi: 10.1097/00000658-199310000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rehrig S, Fleming SD, Anderson J, Guthridge JM, Rakstang J, McQueen CE, Holers VM, Tsokos GC, Shea-Donohue T. Complement inhibitor, complement receptor 1-related gene/protein y-Ig attenuates intestinal damage after the onset of mesenteric ischemia/reperfusion injury in mice. J Immunol. 2001;167:5921–5927. doi: 10.4049/jimmunol.167.10.5921. [DOI] [PubMed] [Google Scholar]
- 6.Weiser MR, Williams JP, Moore FD, Kobzik L, Ma M, Hechtman HB, Carroll MC. Reperfusion injury of ischemic skeletal muscle is mediated by natural antibody and complement. J Exp Med. 1996;183:2343–2348. doi: 10.1084/jem.183.5.2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Williams JP, Pechet TTV, Weiser MR, Reid R, Kobzik L, Moore FD, Carroll MC, Hechtman HB. Intestinal reperfusion injury is mediated by IgM and complement. J Appl Physiol. 1999;86:938–942. doi: 10.1152/jappl.1999.86.3.938. [DOI] [PubMed] [Google Scholar]
- 8.Moses T, Wagner L, Fleming SD. TLR4-mediated Cox-2 expression increases intestinal ischemia/reperfusion-induced damage. J Leukoc Biol. 2009;86:971–980. doi: 10.1189/jlb.0708396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Madesh M, Ramachandran A, Pulimood A, Vadranam M, Balasubramanian KA. Attenuation of intestinal ischemia/reperfusion injury with sodium nitroprusside: studies on mitochondrial function and lipid changes. Biochim Biophys Acta. 2000;1500:204–216. doi: 10.1016/s0925-4439(99)00107-6. [DOI] [PubMed] [Google Scholar]
- 10.Ozturk H, Duran H, Uzunlar AK. Mibefradil, a T-type Ca2+ channel blocker, protects against mesenteric ischemia-reperfusion-induced oxidative injury and histologic alterations in intestinal mucosa in rats. Dig Dis Sci. 2006;51:1454–1460. doi: 10.1007/s10620-005-9060-6. [DOI] [PubMed] [Google Scholar]
- 11.Penn AH, Schmid-Schonbein GW. The intestine as source of cytotoxic mediators in shock: free fatty acids and degradation of lipid-binding proteins. Am J Physiol Heart Circ Physiol. 2008;294:H1779–1792. doi: 10.1152/ajpheart.00902.2007. [DOI] [PubMed] [Google Scholar]
- 12.Zhang M, Austen WG, Chiu I, Alicot EM, Humg R, Ma M, Verna N, Xu M, Hechtman HB, Moore FD, Carroll MC. Identification of a specific self-reactive IgM antibody that initiates intestinal ischemia/reperfusion injury. Proc Natl Acad Sci U S A. 2004;101:3886–3891. doi: 10.1073/pnas.0400347101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fleming SD, Pope MR, Hoffman SM, Moses T, Bukovnik U, Tomich JM, Wagner LM, Woods KM. Domain V peptides inhibit beta2-glycoprotein I-mediated mesenteric ischemia/reperfusion-induced tissue damage and inflammation. J Immunol. 2010;185:6168–6178. doi: 10.4049/jimmunol.1002520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fleming SD, Tsokos GC. Complement, natural antibodies, autoantibodies and tissue injury. Autoimmun Rev. 2006;5:89–92. doi: 10.1016/j.autrev.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 15.Lee NS, Brewer HB, Jr, Osborne JC., Jr beta 2-Glycoprotein I. Molecular properties of an unusual apolipoprotein, apolipoprotein H. J Biol Chem. 1983;258:4765–4770. [PubMed] [Google Scholar]
- 16.Polz E, Kostner GM. The binding of beta 2-glycoprotein-I to human serum lipoproteins: distribution among density fractions. FEBS Letters. 1979;102:183–186. doi: 10.1016/0014-5793(79)80955-2. [DOI] [PubMed] [Google Scholar]
- 17.Agar C, de Groot PG, Morgelin M, Monk SD, van Os G, Levels JH, de Laat B, Urbanus RT, Herwald H, van der Poll T, Meijers JC. {beta}2-Glycoprotein I: a novel component of innate immunity. Blood. 2011;117:6939–6947. doi: 10.1182/blood-2010-12-325951. [DOI] [PubMed] [Google Scholar]
- 18.Hagihara Y, Hong DP, Hoshino M, Enjyoji K, Kato H, Goto Y. Aggregation of beta(2)-glycoprotein I induced by sodium lauryl sulfate and lysophospholipids. Biochemistry. 2002;41:1020–1026. doi: 10.1021/bi015693q. [DOI] [PubMed] [Google Scholar]
- 19.Agar C, van Os GM, Morgelin M, Sprenger RR, Marquart JA, Urbanus RT, Derksen RH, Meijers JC, de Groot PG. {beta}2-Glycoprotein I can exist in two conformations: implications for our understanding of the antiphospholipid syndrome. Blood. 2010;116:1336–1343. doi: 10.1182/blood-2009-12-260976. [DOI] [PubMed] [Google Scholar]
- 20.Kondo A, Miyamoto T, Yonekawa O, Giessing AM, Osterlund EC, Jensen ON. Glycopeptide profiling of beta-2-glycoprotein I by mass spectrometry reveals attenuated sialylation in patients with antiphospholipid syndrome. J Proteomics. 2009;73:123–133. doi: 10.1016/j.jprot.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 21.Ioannou Y, Zhang JY, Passam FH, Rahgozar S, Qi JC, Giannakopoulos B, Qi M, Yu P, Yu DM, Hogg PJ, Krilis SA. Naturally occurring free thiols within beta 2-glycoprotein I in vivo: nitrosylation, redox modification by endothelial cells, and regulation of oxidative stress-induced cell injury. Blood. 2010;116:1961–1970. doi: 10.1182/blood-2009-04-215335. [DOI] [PubMed] [Google Scholar]
- 22.Passam FH, Rahgozar S, Qi M, Raftery MJ, Wong JW, Tanaka K, Ioannou Y, Zhang JY, Gemmell R, Qi JC, Giannakopoulos B, Hughes WE, Hogg PJ, Krilis SA. Beta 2 glycoprotein I is a substrate of thiol oxidoreductases. Blood. 2010;116:1995–1997. doi: 10.1182/blood-2010-02-271494. [DOI] [PubMed] [Google Scholar]
- 23.Passam FH, Qi JC, Tanaka K, Matthaei KI, Krilis SA. In vivo modulation of angiogenesis by beta 2 glycoprotein I. J Autoimmun. 2010;35:232–240. doi: 10.1016/j.jaut.2010.06.013. [DOI] [PubMed] [Google Scholar]
- 24.Sakai T, Balasubramanian K, Maiti S, Halder JB, Schroit AJ. Plasmin-cleaved beta-2-glycoprotein 1 is an inhibitor of angiogenesis. Am J Pathol. 2007;171:1659–1669. doi: 10.2353/ajpath.2007.070146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Manfredi AA, Rovere P, Heltai S, Galati G, Nebbia G, Tincani A, Balestrieri G, Sabbadini MG. Apoptotic cell clearance in systemic lupus erythematosus. II. Role of beta2-glycoprotein I. Arthritis Rheum. 1998;41:215–223. doi: 10.1002/1529-0131(199802)41:2<215::AID-ART5>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 26.Valesini G, Shoenfeld Y. A new player in the antiphospholipid syndrome: the beta 2 glycoprotein I cofactor. Autoimmunity. 1992;14:105–110. doi: 10.3109/08916939209083128. [DOI] [PubMed] [Google Scholar]
- 27.Cabiedes J, Cabral AR, Alarcon-Segovia D. Clinical manifestations of the antiphospholipid syndrome in patients with systemic lupus erythematosus associate more strongly with anti-beta 2-glycoprotein-I than with antiphospholipid antibodies. J Rheumatol. 1995;22:1899–1906. [PubMed] [Google Scholar]
- 28.Ali HY, Abdullah ZA. Anti-beta(2)-glycoprotein I autoantibody expression as a potential biomarker for strokes in patients with anti-phospholipid syndrome. J Immunotoxicol. 2008;5:173–177. doi: 10.1080/15476910802129638. [DOI] [PubMed] [Google Scholar]
- 29.Nojima J, Masuda Y, Iwatani Y, Kuratsune H, Watanabe Y, Suehisa E, Takano T, Hidaka Y, Kanakura Y. Arteriosclerosis obliterans associated with anti-cardiolipin antibody/beta2-glycoprotein I antibodies as a strong risk factor for ischaemic heart disease in patients with systemic lupus erythematosus. Rheumatology (Oxford) 2008;47:684–689. doi: 10.1093/rheumatology/ken124. [DOI] [PubMed] [Google Scholar]
- 30.Blank M, Baraam L, Eisenstein M, Fridkin M, Dardik R, Heldman Y, Katchalski-Katzir E, Shoenfeld Y. beta2-Glycoprotein-I based peptide regulate endothelial-cells tissue-factor expression via negative regulation of pGSK3beta expression and reduces experimental-antiphospholipid-syndrome. J Autoimmun. 2011;37:8–17. doi: 10.1016/j.jaut.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 31.Martinez de la Torre Y, Pregnolato F, D’Amelio F, Grossi C, Disimone N, Pasqualini F, Nebuloni M, Chen P, Pierangeli S, Bassani N, Ambrogi F, Borghi MO, Vecchi A, Locati M, Meroni PL. Anti-phospholipid induced murine fetal loss: Novel protective effect of a peptide targeting the beta2 glycoprotein I phospholipid-binding site. Implications for human fetal loss. J Autoimmun. 2012;38:209–215. doi: 10.1016/j.jaut.2011.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hunt J, Krilis S. The fifth domain of beta 2-glycoprotein I contains a phospholipid binding site (Cys281-Cys288) and a region recognized by anticardiolipin antibodies. J Immunol. 1994;152:653–659. [PubMed] [Google Scholar]
- 33.Tomich JM, Wallace D, Henderson K, Mitchell KE, Radke G, Brandt R, Ambler CA, Scott AJ, Grantham J, Sullivan L, Iwamoto T. Aqueous solubilization of transmembrane peptide sequences with retention of membrane insertion and function. Biophys J. 1998;74:256–267. doi: 10.1016/S0006-3495(98)77784-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fleming SD, Shea-Donohue T, Guthridge JM, Kulik L, Waldschmidt TJ, Gipson MG, Tsokos GC, Holers VM. Mice deficient in complement receptors 1 and 2 lack a tissue injury-inducing subset of the natural antibody repertoire. J Immunol. 2002;169:2126–2133. doi: 10.4049/jimmunol.169.4.2126. [DOI] [PubMed] [Google Scholar]
- 35.Chiu C-J, McArdle AH, Brown R, Scott HJ, Gurd FN. Intestinal mucosal lesion in low-flow states. I. A morphological, hemodynamic, and metabolic reappraisal. Arch Surg. 1970;101:478–483. doi: 10.1001/archsurg.1970.01340280030009. [DOI] [PubMed] [Google Scholar]
- 36.Chen LW, Egan L, Li ZW, Greten FR, Kagnoff MF, Karin M. The two faces of IKK and NF-kappaB inhibition: prevention of systemic inflammation but increased local injury following intestinal ischemia-reperfusion. Nat Med. 2003;9:575–581. doi: 10.1038/nm849. [DOI] [PubMed] [Google Scholar]
- 37.Martinez de la Torre Y, Pregnolato F, D’Amelio F, Grossi C, Disimone N, Pasqualini F, Nebuloni M, Chen P, Pierangeli S, Bassani N, Ambrogi F, Borghi MO, Vecchi A, Locati M, Meroni PL. Anti-phospholipid induced murine fetal loss: Novel protective effect of a peptide targeting the beta2 glycoprotein I phospholipid-binding site. Implications for human fetal loss. J Autoimmun. 2011 doi: 10.1016/j.jaut.2011.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pope MR, Hoffman SM, Tomlinson S, Fleming SD. Complement regulates TLR4-mediated inflammatory responses during intestinal ischemia reperfusion. Mol Immunol. 2010;48:356–364. doi: 10.1016/j.molimm.2010.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mangino MJ, Murphy MK, Anderson CB. Effects of the arachidonate 5-lipoxygenase synthesis inhibitor A-64077 in intestinal ischemia-reperfusion injury. Journal of Pharmacology and Experimental Therapeutics. 1994;269:75–81. [PubMed] [Google Scholar]
- 40.Peters-Golden M, Henderson WR., Jr Leukotrienes. N Engl J Med. 2007;357:1841–1854. doi: 10.1056/NEJMra071371. [DOI] [PubMed] [Google Scholar]
- 41.Welch BD, Francis JN, Redman JS, Paul S, Weinstock MT, Reeves JD, Lie YS, Whitby FG, Eckert DM, Hill CP, Root MJ, Kay MS. Design of a potent D-peptide HIV-1 entry inhibitor with a strong barrier to resistance. J Virol. 2010;84:11235–11244. doi: 10.1128/JVI.01339-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Welch BD, VanDemark AP, Heroux A, Hill CP, Kay MS. Potent D-peptide inhibitors of HIV-1 entry. Proc Natl Acad Sci U S A. 2007;104:16828–16833. doi: 10.1073/pnas.0708109104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu M, Li C, Pazgier M, Mao Y, Lv Y, Gu B, Wei G, Yuan W, Zhan C, Lu WY, Lu W. D-peptide inhibitors of the p53-MDM2 interaction for targeted molecular therapy of malignant neoplasms. Proc Natl Acad Sci U S A. 2010;107:14321–14326. doi: 10.1073/pnas.1008930107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ruiz-Irastorza G, Khamashta MA, Hughes GRV. Hughes syndrome crosses boundaries. Autoimmunity Reviews. 2002:43–48. doi: 10.1016/s1568-9972(01)00005-2. [DOI] [PubMed] [Google Scholar]
- 45.Khamashta MA, Hughes GR. The clinical aspects of the antiphospholipid syndrome. In: Lahita RG, editor. Systemic Lupus Erthematosus. Academic Press; 2004. pp. 1107–1123. [Google Scholar]
- 46.Katzav A, Faust-Socher A, Kvapil F, Michaelson DM, Blank M, Pick CG, Shoenfeld Y, Korczyn AD, Chapman J. Antiphospholipid syndrome induction exacerbates a transgenic Alzheimer disease model on a female background. Neurobiol Aging. 2011;32:272–279. doi: 10.1016/j.neurobiolaging.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 47.Zhang M, Carroll MC. Natural IgM-mediated innate autoimmunity: a new target for early intervention of ischemia-reperfusion injury. Expert Opin Biol Ther. 2007;7:1575–1582. doi: 10.1517/14712598.7.10.1575. [DOI] [PubMed] [Google Scholar]
- 48.Zhang M, Alicot EM, Chiu I, Li J, Verna N, Vorup-Jensen T, Kessler B, Shimaoka M, Chan R, Friend D, Mahmood U, Weissleder R, Moore FD, Carroll MC. Identification of the target self-antigens in reperfusion injury. J Exp Med. 2006;203:141–152. doi: 10.1084/jem.20050390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Haas MS, Alicot EM, Schuerpf F, Chiu I, Li J, Moore FD, Carroll MC. Blockade of self-reactive IgM significantly reduces injury in a murine model of acute myocardial infarction. Cardiovasc Res. 2010;87:618–627. doi: 10.1093/cvr/cvq141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Alard JE, Gaillard F, Daridon C, Shoenfeld Y, Jamin C, Youinou P. TLR2 is one of the endothelial receptors for {beta}2-glycoprotein I. J Immunol. 2010;185:1550–1557. doi: 10.4049/jimmunol.1000526. [DOI] [PubMed] [Google Scholar]
- 51.Meroni PL, Raschi E, Testoni C, Parisio A, Borghi MO. Innate immunity in the antiphospholipid syndrome: role of toll-like receptors in endothelial cell activation by antiphospholipid antibodies. Autoimmun Rev. 2004;3:510–515. doi: 10.1016/j.autrev.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 52.Allen KL, Fonseca FV, Betapudi V, Willard B, Zhang J, McCrae KR. A novel pathway for human endothelial cell activation by antiphospholipid/anti-beta2 glycoprotein I antibodies. Blood. 2011;119:884–893. doi: 10.1182/blood-2011-03-344671. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.