Abstract
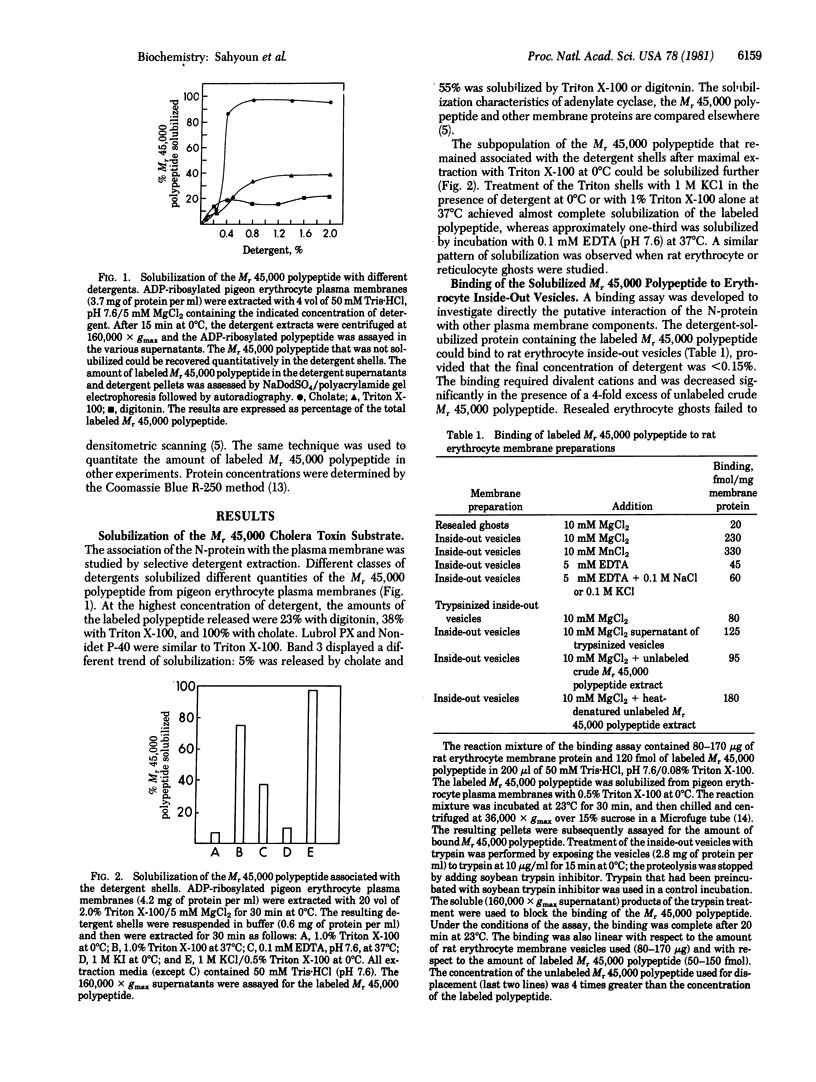
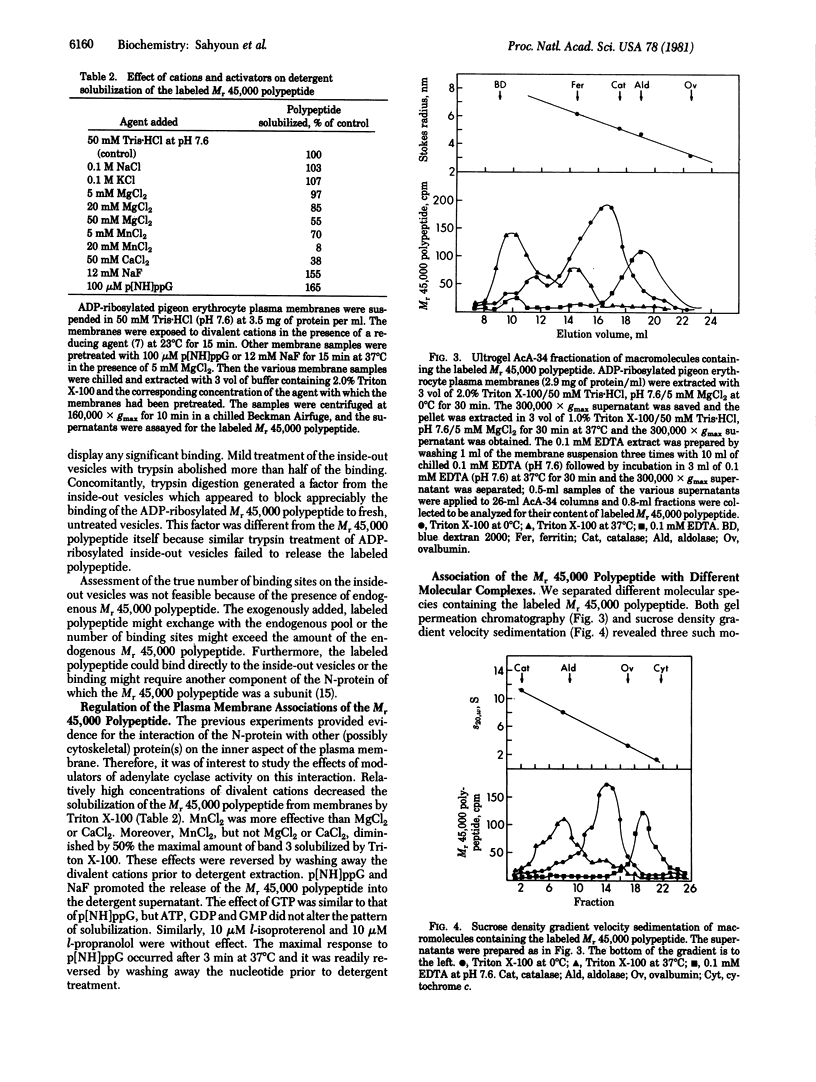
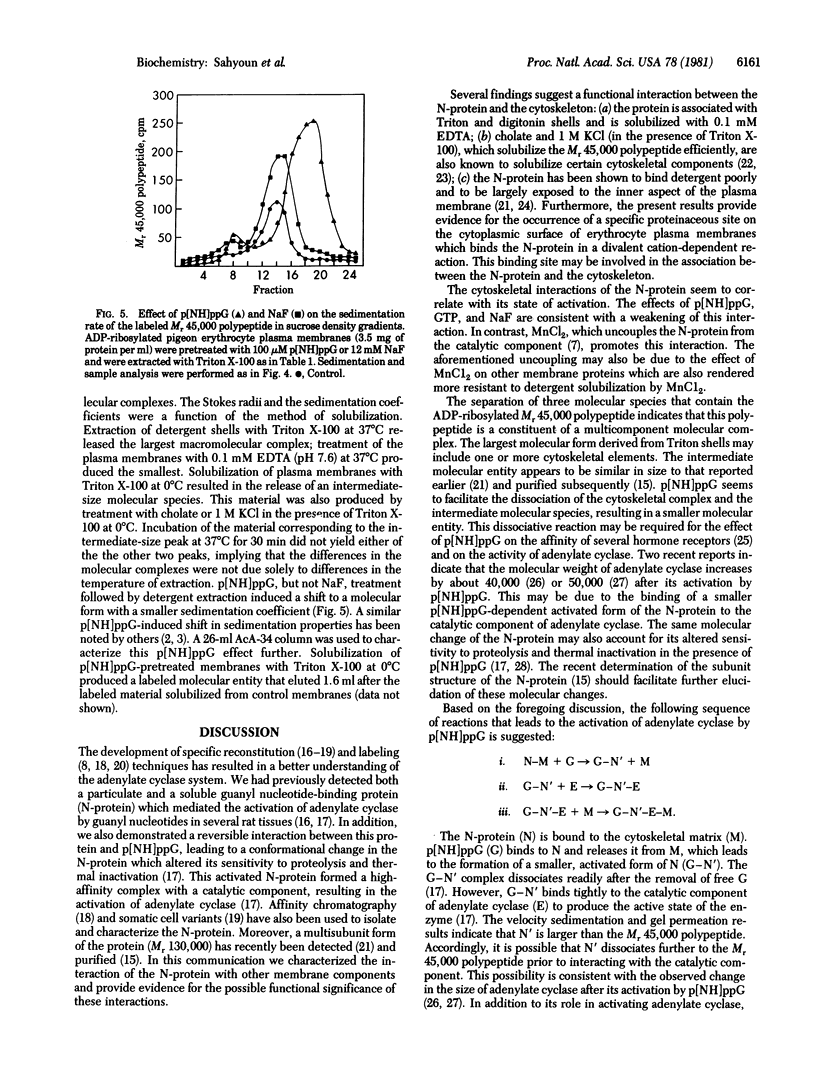
Selective extraction of the adenylate cyclase regulatory protein (N-protein) from pigeon erythrocyte plasma membranes provided evidence for its cytoskeletal association. Cholate, but not Triton X-100 or digitonin, was effective in solubilizing the ADP-ribosylated N-protein. The labeled protein complex or components thereof that were associated with the Triton-insoluble cytoskeleton (shells) could be partly released by 0.1 mM EDTA; 1 M KCl in the presence of Triton X-100 achieved complete solubilization. 5'-Guanylyl imidodiphosphate (p[NH]ppG) and NaF, activators of adenylate cyclase, promoted the release of the regulatory protein from the cytoskeleton but MnCl2, an "uncoupler" of the adenylate cyclase system, had the opposite effect. The solubilized, labeled N-protein was able to bind specifically to rat erythrocyte inside-out vesicles in the presence of divalent cations. A proteolytic product of inside-out vesicles inhibited the binding of the N-protein to fresh vesicles. Three molecular species which contained the Mr 45,000 polypeptide component of the N-protein were identified by gel permeation chromatography and by sucrose density gradient velocity sedimentation. p[NH]ppG appeared to convert the two larger molecular complexes to a smaller molecular entity. Such a molecular dissociation might be relevant to the effects of guanyl nucleotides on the activity of adenylate cyclase and on the affinity of hormone receptors.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett V., Branton D. Selective association of spectrin with the cytoplasmic surface of human erythrocyte plasma membranes. Quantitative determination with purified (32P)spectrin. J Biol Chem. 1977 Apr 25;252(8):2753–2763. [PubMed] [Google Scholar]
- Bennett V., Stenbuck P. J. Identification and partial purification of ankyrin, the high affinity membrane attachment site for human erythrocyte spectrin. J Biol Chem. 1979 Apr 10;254(7):2533–2541. [PubMed] [Google Scholar]
- Bennett V., Stenbuck P. J. The membrane attachment protein for spectrin is associated with band 3 in human erythrocyte membranes. Nature. 1979 Aug 9;280(5722):468–473. doi: 10.1038/280468a0. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cassel D., Pfeuffer T. Mechanism of cholera toxin action: covalent modification of the guanyl nucleotide-binding protein of the adenylate cyclase system. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2669–2673. doi: 10.1073/pnas.75.6.2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherksey B. D., Zadunaisky J. A., Murphy R. B. Cytoskeletal constraint of the beta-adrenergic receptor in frog erythrocyte membranes. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6401–6405. doi: 10.1073/pnas.77.11.6401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farfel Z., Kaslow H. R., Bourne H. R. A regulatory component of adenylate cyclase is located on the inner surface of human erythrocyte membranes. Biochem Biophys Res Commun. 1979 Oct 29;90(4):1237–1241. doi: 10.1016/0006-291x(79)91169-0. [DOI] [PubMed] [Google Scholar]
- Gill D. M., Meren R. ADP-ribosylation of membrane proteins catalyzed by cholera toxin: basis of the activation of adenylate cyclase. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3050–3054. doi: 10.1073/pnas.75.7.3050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldhammer A., Cook G. H., Wolff J. Preactivation as a determinant for the size of thyroid adenylate cyclase. J Biol Chem. 1980 Jul 25;255(14):6918–6922. [PubMed] [Google Scholar]
- Hebdon G. M., Le Vine H., 3rd, Sahyoun N. E., Schmitges C. J., Cuatrecasas P. Demonstration of choleragen-dependent ADP-ribosylation in whole cells and correlation with the activation of adenylate cyclase. Life Sci. 1980 Apr 28;26(17):1385–1396. doi: 10.1016/0024-3205(80)90041-7. [DOI] [PubMed] [Google Scholar]
- Hebdon M., Le Vine H., 3rd, Sahyoun N., Schmitges C. J., Cuatrecasas P. Properties of the interaction of fluoride- and guanylyl-5'-imidodiphosphate-regulatory proteins with adenylate cyclase. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3693–3697. doi: 10.1073/pnas.75.8.3693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howlett A. C., Gilman A. G. Hydrodynamic properties of the regulatory component of adenylate cyclase. J Biol Chem. 1980 Apr 10;255(7):2861–2866. [PubMed] [Google Scholar]
- Hudson T. H., Roeber J. F., Johnson G. L. Conformational changes of adenylate cyclase regulatory proteins mediated by guanine nucleotides. J Biol Chem. 1981 Feb 10;256(3):1459–1465. [PubMed] [Google Scholar]
- Kaslow H. R., Johnson G. L., Brothers V. M., Bourne H. R. A regulatory component of adenylate cyclase from human erythrocyte membranes. J Biol Chem. 1980 Apr 25;255(8):3736–3741. [PubMed] [Google Scholar]
- Lad P. M., Welton A. F., Rodbell M. Evidence for distinct guanine nucleotide sites in the regulation of the glucagon receptor and of adenylate cyclase activity. J Biol Chem. 1977 Sep 10;252(17):5942–5946. [PubMed] [Google Scholar]
- Limbird L. E., Gill D. M., Lefkowitz R. J. Agonist-promoted coupling of the beta-adrenergic receptor with the guanine nucleotide regulatory protein of the adenylate cyclase system. Proc Natl Acad Sci U S A. 1980 Feb;77(2):775–779. doi: 10.1073/pnas.77.2.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limbird L. E., Hickey A. R., Lefkowitz R. J. Unique uncoupling of the frog erythrocyte adenylate cyclase system by manganese. Loss of hormone and guanine nucleotide-sensitive enzyme activities without loss of nucleotide-sensitive, high affinity agonist binding. J Biol Chem. 1979 Apr 25;254(8):2677–2683. [PubMed] [Google Scholar]
- Neer E. J., Echeverria D., Knox S. Increase in the size of soluble brain adenylate cyclase with activation by guanosine 5'-(beta, gamma-imino)triphosphate. J Biol Chem. 1980 Oct 25;255(20):9782–9789. [PubMed] [Google Scholar]
- Northup J. K., Sternweis P. C., Smigel M. D., Schleifer L. S., Ross E. M., Gilman A. G. Purification of the regulatory component of adenylate cyclase. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6516–6520. doi: 10.1073/pnas.77.11.6516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeuffer T. GTP-binding proteins in membranes and the control of adenylate cyclase activity. J Biol Chem. 1977 Oct 25;252(20):7224–7234. [PubMed] [Google Scholar]
- Pfeuffer T. Guanine nucleotide-controlled interactions between components of adenylate cyclase. FEBS Lett. 1979 May 1;101(1):85–89. [PubMed] [Google Scholar]
- Rodbell M. The role of hormone receptors and GTP-regulatory proteins in membrane transduction. Nature. 1980 Mar 6;284(5751):17–22. doi: 10.1038/284017a0. [DOI] [PubMed] [Google Scholar]
- Ross E. M., Howlett A. C., Ferguson K. M., Gilman A. G. Reconstitution of hormone-sensitive adenylate cyclase activity with resolved components of the enzyme. J Biol Chem. 1978 Sep 25;253(18):6401–6412. [PubMed] [Google Scholar]
- Sahyoun N. E., Le Vine H., 3rd, Hebdon G. M., Hemadah R., Cuatrecasas P. Specific binding of solubilized adenylate cyclase to the erythrocyte cytoskeleton. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2359–2362. doi: 10.1073/pnas.78.4.2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahyoun N. E., LeVine H., 3rd, Hebdon G. M., Khouri R. K., Cuatrecasas P. Evidence for cytoskeletal associations of the adenylate cyclase system obtained by differential extraction of rat erythrocyte ghosts. Biochem Biophys Res Commun. 1981 Aug 14;101(3):1003–1010. doi: 10.1016/0006-291x(81)91848-9. [DOI] [PubMed] [Google Scholar]
- Sahyoun N., Hollenberg M. D., Bennett V., Cuatrecasas P. Topographic separation of adenylate cyclase and hormone receptors in the plasma membrane of toad erythrocyte ghosts. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2860–2864. doi: 10.1073/pnas.74.7.2860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahyoun N., Schmitges C. J., Le Vine H., 3rd, Cuatrecasas P. Molecular resolution and reconstitution of the GPP (NH) P and NAF sensitive adenylate cyclase system. Life Sci. 1977 Dec 15;21(12):1857–1863. doi: 10.1016/0024-3205(77)90169-2. [DOI] [PubMed] [Google Scholar]
- Steck T. L., Kant J. A. Preparation of impermeable ghosts and inside-out vesicles from human erythrocyte membranes. Methods Enzymol. 1974;31:172–180. doi: 10.1016/0076-6879(74)31019-1. [DOI] [PubMed] [Google Scholar]
- Steck T. L., Weinstein R. S., Straus J. H., Wallach D. F. Inside-out red cell membrane vesicles: preparation and purification. Science. 1970 Apr 10;168(3928):255–257. doi: 10.1126/science.168.3928.255. [DOI] [PubMed] [Google Scholar]



