Abstract
It has long been known that subjective tinnitus, a constant or intermittent phantom sound perceived by 10 to 15 % of the adult population, is not a purely auditory phenomenon but is also tied to limbic-related brain regions. Supporting evidence comes from data indicating that stress and emotion can modulate tinnitus, and from brain imaging studies showing functional and anatomical differences in limbic-related brain regions of tinnitus patients and controls. Recent studies from our lab revealed altered blood oxygen level-dependent (BOLD) responses to stimulation at the tinnitus frequency in the ventral striatum (specifically, the nucleus accumbens) and gray-matter reductions (i.e. anatomical changes) in ventromedial prefrontal cortex (vmPFC), of tinnitus patients compared to controls. The present study extended these findings by demonstrating functional differences in vmPFC between 20 tinnitus patients and 20 age-matched controls. Importantly, the observed BOLD response in vmPFC was positively correlated with tinnitus characteristics such as subjective loudness and the percent of time during which the tinnitus was perceived, whereas correlations with Tinnitus Handicap Inventory scores and other variables known to be affected in tinnitus (e.g. depression, anxiety, noise sensitivity, hearing loss) were weaker or absent. This suggests that the observed group differences are indeed related to the tinnitus percept and not to an affective reaction to tinnitus. The results further corroborate vmPFC as a region of high interest for tinnitus research.
Keywords: tinnitus, fMRI, ventromedial prefrontal cortex, auditory gating, limbic, “noise cancellation”
1. Introduction
Subjective tinnitus, an auditory disorder affecting 10 to 15 % of the adult population (Henry et al., 2005), is the constant or intermittent perception of sound (often described as ringing, hissing, whining, pure tone, “cricket sounds”, or noise) in the absence of a corresponding sound source. Despite its relatively simple perceptual manifestation, the neuro-otological mechanisms behind tinnitus are complex and only partly understood, and to date there is no cure or treatment that reliably works for every patient. In most cases tinnitus is associated with damage to the auditory periphery, most commonly loss of hair cells due to loud noise exposure or aging, but also sudden-onset hearing loss, head trauma, ear infections, and certain ototoxic drugs. As a consequence of peripheral auditory damage, less auditory input reaches central auditory neurons within the affected frequency range, which in turn leads to compensatory changes in the central auditory system. As animal models for sensorineural hearing loss and tinnitus have demonstrated, these changes may consist of an upregulation of spontaneous firing rates (Kaltenbach, 2002; Noreña & Eggermont, 2003; Mulders & Robertson, 2009), an invasion of lesion-edge frequency processing into the deafferented regions of central tonotopic maps (Robertson & Irvine, 1989; Rajan & Irvine, 1998; Dietrich et al., 2001), a reduction of lateral inhibition (Rajan, 1998), or increased neural synchrony (Seki & Eggermont, 2003). Although it is not completely clear whether all of these changes contribute to tinnitus, at least one of them seems to be responsible for generating the aberrant central auditory activity that we will refer to as the “tinnitus signal.” It has also been reported that a tinnitus signal can arise in the absence of auditory damage when the auditory system is over-excited via cross-talk from non-auditory structures (somatic tinnitus – Levine, 2003; Shore et al., 2007).
However, tinnitus does not consistently occur under conditions that are assumed to favor the generation of a tinnitus signal. For example, hair cell loss, often associated with measurable hearing loss, occurs in the vast majority of the population either due to loud noise exposure or aging, yet only a subset of people with hearing loss also suffer from tinnitus (Hoffman & Reed, 2004). In addition, the frequent co-occurrence of tinnitus with depression and anxiety (Halford & Anderson, 1991; Andersson et al., 2003), as well as the observation that stressful life events can trigger or exacerbate tinnitus (Schmitt et al., 2000; Alpini & Cesarani, 2006), suggest that tinnitus perception is subject to non-auditory, limbic influences. Support for this idea also comes from neuroimaging studies revealing functional and anatomical differences between tinnitus patients and control participants without tinnitus not only in auditory, but also in limbic-related brain areas (Lockwood et al., 1998; Mirz et al., 2000; Mühlau et al., 2006; Landgrebe et al., 2009; Schlee et al., 2009; Leaver et al., 2011). In addition, a recent study has demonstrated that electrical stimulation of the caudate nucleus in the striatum (area LC) of human tinnitus patients can directly modulate tinnitus perception (Cheung & Larson, 2010).
Consistent with these findings, our lab has recently proposed that tinnitus perception arises only if two conditions are met: 1) a tinnitus signal is being generated, and 2) this uninformative signal fails to be suppressed by a cortico-striatal limbic network (Rauschecker et al., 2010). At the core of this model is the assumption that in an intact cortico-striatal limbic network, ventromedial prefrontal cortex (vmPFC) can suppress the tinnitus signal (once it has been evaluated and deemed irrelevant) via known excitatory connections to a part of the thalamic reticular nucleus (TRN) that is located near the auditory thalamus (medial geniculate nucleus, MGN; Zikopoulos & Barbas, 2006). The TRN, in turn, can inhibit the MGN, through which the tinnitus signal passes on its way to auditory cortex. If this “noise cancellation system” (Rauschecker et al., 2010) works properly, the tinnitus signal reaches awareness only transiently, before the suppression mechanisms kicks in. However, if the noise cancellation system fails, patients become aware of their tinnitus.
This model can explain various conditions of chronic and intermittent tinnitus and tinnitus with sudden onset: In chronic tinnitus, the noise cancellation failure is permanent and associated with vmPFC gray-matter reductions (Leaver et al., 2011; Leaver et al., 2012). In intermittent tinnitus, the failure occurs transiently whenever the cortico-striatal limbic network is compromised (e.g. as a consequence of stress or sleep deprivation, which could alter the levels of relevant neurotransmitters in limbic regions). When hearing loss is pre-existing, adverse life events can trigger immediate tinnitus onset by affecting the limbic system. Conversely, cases of immediate tinnitus onset after sudden hearing loss can be explained by pre-existing damage to the limbic-related noise cancellation system due to independent causes.
The purpose of the present study was to confirm and extend previous results suggesting involvement of the cortico-striatal limbic network in tinnitus in a new sample of participants, using a modified design derived from our previous study (Leaver et al., 2011). First, the present study focused on whether the previously found anatomical changes in vmPFC might be reflected in corresponding functional changes. Addressing this question using functional magnetic resonance imaging (fMRI) has been complicated by the fact that the vicinity of the sinuses can lead to significant signal dropout in ventral prefrontal areas. To ameliorate this problem, the present data acquisition used a tilted field of view that avoided the sinuses (Deichmann et al., 2003; for illustration, see Figure 1B), which resulted in clearly visible vmPFC signal improvements (for illustration, see Supplementary Figure S1). Second, the present study also assessed several tinnitus-related variables, such as subjective loudness, impact, and the proportion of awake time during which patients were aware of their tinnitus (“tinnitus awareness”), as well as variables known to correlate with tinnitus perception, such as hearing loss, noise sensitivity, depression, and anxiety. Correlations between these variables and the BOLD response can further elucidate the role of a given brain region in tinnitus. Third, the present study used a patient-guided tinnitus matching procedure allowing patients to adjust frequency, loudness, and bandwidth of a test tone. Compared to the previous, experimenter-guided frequency match (for details, see Leaver et al., 2011), this procedure should yield more accurate estimates of the tinnitus frequency. Fourth, patients and controls in the present study were matched for sex and age. This also resulted in an approximate matching of hearing profiles between tinnitus patients and controls, and we took additional precautions to rule out differences in hearing loss, rather than tinnitus, as an alternative explanation for group differences observed in the MRI data. Lastly, the present study employed a modified “sparse sampling” scanning scheme that allowed us to investigate the time-course of the BOLD response while still presenting auditory stimuli in the silent intervals between bursts of scanner noise.
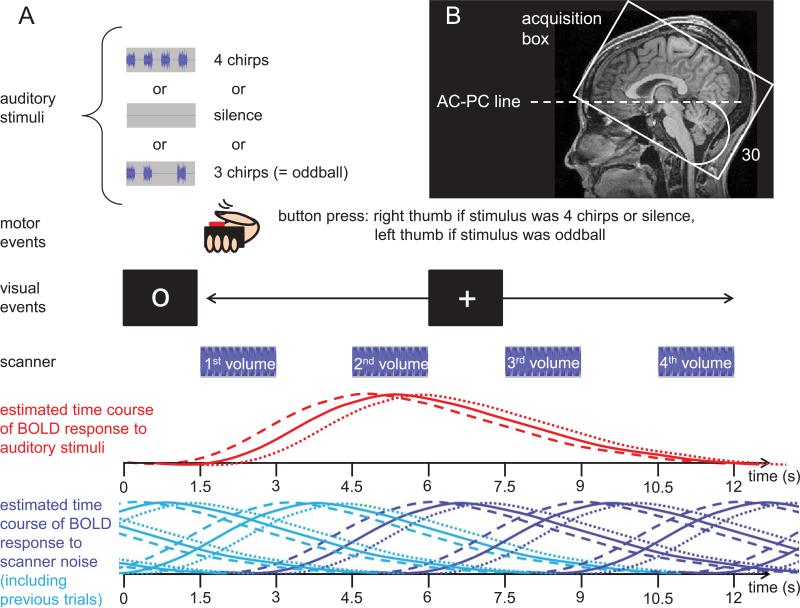
Figure 1.
Illustration of the scanning paradigm. (A) Time-course of a trial. At the beginning of each trial, an auditory stimulus was presented, accompanied by a fixation circle. After 1.5 seconds, the fixation circle changed to a + sign, signaling participants to indicate via button press whether the stimulus they had just heard was “normal” or an “oddball”. At the same time, the first volume acquisition started. After three additional volume acquisitions (each preceded by 1.5 seconds of silence), the next trial began. Volume acquisitions were timed so as to capture the rise and fall of the expected hemodynamic response to the auditory stimuli. The dashed red line indicates the response locked to chirp onset, the dotted line the response to chirp offset, and the solid line represents the hemodynamic response relative to the middle of the auditory stimulus, which was used for modeling the hemodynamic response function in the GLM. The dark blue lines indicate the BOLD responses to the noise associated with each of the scans shown. The light blue lines indicate the BOLD response to scanner noise in a preceding trial. As becomes evident from this illustration, the frequent and regular occurrence of scanner noise creates a continuously elevated level of activation. Any effects evoked by experimental stimuli and/or task are measured relative to this elevated baseline level. (B) To improve signal in inferior frontal areas, the volume acquisition box was tilted 30 degrees from the line connecting the anterior and posterior commissure (AC-PC line) to avoid intersection of MRI slices with the sinuses.
Twenty tinnitus patients (TPs) and twenty controls (CTs) matched by age and sex underwent fMRI. While in the scanner, they performed a simple button press task in response to three auditory conditions, which were presented repeatedly and in random order. In the “no-stim” condition, no auditory stimulus was presented. In condition “tinn-stim,” each tinnitus patient and his or her stimulus-matched control heard a stimulus at a frequency that corresponded to the patient's tinnitus frequency. Condition “other-stim” comprised trials with stimulation at a single non-tinnitus frequency chosen randomly on each trial from a set of three standard frequencies (375, 1500, and 6000 Hz) of which the one closest to the tinnitus frequency was omitted. Thus, each participant received three different stimuli covering a broad frequency range. For the tinnitus patients, one of these stimuli corresponded to the dominant frequency of their tinnitus, and for each tinnitus patient there was a control participant who received stimuli at the exact same frequencies as the patient. For each participant and frequency, stimulus intensity was adjusted relative to the participant's detection threshold for the same stimuli under scanning conditions. The scanning paradigm is illustrated in Figure 1A.
2. Results
2.1. Behavioral data
The pure-tone frequencies determined as best matches for the tinnitus frequency were generally high (mean = 4563.5 Hz), but covered a wide range (min. = 726 Hz, max. = 10617, standard deviation = 3211.5 Hz). More details on the patients’ tinnitus characteristics can be found in Table 1. As groups, tinnitus patients and controls did not differ significantly regarding age, hearing loss, or depression and anxiety; however, there was a nonsignificant tendency for tinnitus patients to have higher hearing thresholds (see Supplementary Figure S2). Moreover, as can be seen in Table 2, pairwise t-tests showed that tinnitus patients had worse hearing than their stimulus-matched controls for the experimental stimuli in the higher frequency ranges. However, these differences did not impact the fMRI results reported here (see section 4.5.4.). Tinnitus patients also showed a non-significant tendency for scoring higher on depression and anxiety questionnaires. In addition, they scored significantly higher on a combined noise sensitivity measure consisting of subjective noise sensitivity ratings and loudness discomfort levels (LDLs, see section 4.3. for statistical results).
Table 1.
Tinnitus characteristics
| Tinnitus patient | Tinnitus description | Tinnitus location | Frequency match (Hz) | Tinnitus onset (years ago) | THI score |
|---|---|---|---|---|---|
| TP1 | tone | both ears | 8484 | 5 | 8 |
| TP2 | tone | both ears | 1605 | 7 | 12 |
| TP3 | noise, crickets | right ear | 5297 | 1 | 38 |
| TP4 | tone | right ear | 788 | 1 | 16 |
| TP5 | crickets | left ear | 1894 | 1.5 | 32 |
| TP6 | tone, noise | both ears, worse in left | 5000 | 10.5 | 34 |
| TP7 | tone | both ears, worse in left | 5946 | 0.75 | 14 |
| TP8 | crickets | both ears, worse in right | 1388 | 4 | 12 |
| TP9 | noise | both ears | 1214 | 5 | 18 |
| TP10 | tone | both ears | 726 | 0.33 | 2 |
| TP11 | tone | both ears | 10617 | 2 | 14 |
| TP12 | tone | both ears, worse in right | 7681 | 22 | 20 |
| TP13 | other | both ears, worse in right | 3929 | 1.25 | 88 |
| TP14 | noise | left ear | 2630 | 1.16 | 70 |
| TP15 | tone | both ears, worse in right | 1644 | 21.5 | 28 |
| TP16 | tone | both ears, worse in right | 8044 | 7 | 30 |
| TP17 | noise | right ear | 4410 | 4 | 38 |
| TP18 | tone | both ears | 6243 | 10 | 14 |
| TP19 | tone | left ear | 10553 | 1 | 50 |
| TP20 | tone | both ears | 3177 | 3 | 0 |
Table 2.
Participants overview
| HL (averaged across both ears) at | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| pair | sex | TF | int | age | 375 Hz | 1500 Hz | 6000 Hz | TF | |||||
| TP | CT | TP | CT | TP | CT | TP | CT | TP | CT | ||||
| 1*° | F | 8484 | 15 | 57 | 56 | 25.0 | 15.0 | 20.0 | 17.5 | (47.5) | (22.5) | 60.0 | 32.5 |
| 2 | F | 1605 | 30 | 56 | 58 | 7.5 | 12.5 | (15.0) | (12.5) | 12.5 | 52.5 | 15.0 | 15.0 |
| 3 | F | 5297 | 30 | 66 | 66 | 12.5 | 12.5 | 12.5 | 17.5 | (22.5) | (47.5) | 22.5 | 47.5 |
| 4* | M | 788 | 30 | 66 | 64 | 32.5 | 15.0 | (35.0) | (20.0) | 60.0 | 17.5 | 30.0 | 15.0 |
| 5 | F | 1894 | 30 | 28 | 29 | 10.0 | 5.0 | (10.0) | (2.5) | 22.5 | 7.5 | 10.0 | 2.5 |
| 6*° | F | 5000 | 30 | 60 | 61 | 7.5 | 10.0 | 27.5 | 12.5 | (70.0) | (22.5) | 62.5 | 30.0 |
| 7 | M | 5946 | 25 | 65 | 67 | 15.0 | 10.0 | 5.0 | 7.5 | (50.0) | (32.5) | 50.0 | 32.5 |
| 8 | M | 1388 | 30 | 64 | 57 | 22.5 | 15.0 | (12.5) | (7.5) | 20.0 | 17.5 | 22.5 | 10.0 |
| 9 | F | 1214 | 30 | 33 | 41 | 0.0 | 5.0 | (5.0) | (2.5) | 10.0 | 5.0 | 7.5 | 5.0 |
| 10 | M | 726 | 30 | 45 | 50 | (12.5) | (10.0) | 25.0 | 10.0 | 30.0 | 32.5 | 17.5 | 7.5 |
| 11* | F | 10617 | 20 | 48 | 53 | 27.5 | 7.5 | 32.5 | 2.5 | (30.0) | (12.5) | 35.0 | 40.0 |
| 12 | M | 7681 | 30 | 34 | 34 | 10.0 | 5.0 | 10.0 | 17.5 | (10.0) | (17.5) | 7.5 | 22.5 |
| 13*° | F | 3929 | 30 | 38 | 42 | 12.5 | 12.5 | 37.5 | 15.0 | (57.5) | (12.5) | 55.0 | 12.5 |
| 14 | F | 2630 | 30 | 49 | 49 | 7.5 | 5.0 | (12.5) | (7.5) | 20.0 | 20.0 | 22.5 | 40.0 |
| 15° | F | 1644 | 30 | 47 | 53 | 7.5 | 12.5 | (45.0) | (10.0) | 27.5 | 15.0 | 45.0 | 10.0 |
| 16° | M | 8044 | 25 | 43 | 46 | 7.5 | 15.0 | 17.5 | 5.0 | (45.0) | (-2.5) | 50.0 | 15.0 |
| 17 | F | 4410 | 30 | 33 | 30 | 15.0 | 12.5 | 5.0 | 7.5 | (10.0) | (15.0) | 7.5 | 12.5 |
| 18 | M | 6243 | 30 | 42 | 46 | 5.0 | 15.0 | 10.0 | 10.0 | (40.0) | (25.0) | 40.0 | 25.0 |
| 19 | M | 10553 | 30 | 37 | 42 | 5.0 | 15.0 | 7.5 | 10.0 | (20.0) | (15.0) | 17.5 | 10.0 |
| 20 | M | 3177 | 30 | 23 | 27 | 15.0 | 15.0 | (7.5) | (7.5) | 12.5 | 2.5 | 10.0 | 5.0 |
| mean | 4564 | 28 | 47 | 49 | 12.9 | 11.3 | 17.6 | 10.1 | 30.9 | 19.5 | 29.4 | 19.5 | |
| p (paired t-test) | 0.03 | 0.38 | 0.01 | 0.04 | 0.03 | ||||||||
| p* (paired t-test) | 0.06 | 0.59 | 0.13 | 0.52 | 0.22 | ||||||||
| p° (paired t-test) | 0.13 | 0.27 | 0.12 | 0.51 | 0.62 | ||||||||
Pair – pair of tinnitus patient (TP) and control participant (CT) who received identical stimulation; sex, F – female, M – male; TF – tinnitus frequency, used as one of the stimulus frequencies; int, intensity (in dB SL) at which the stimuli were played; age (in years); HL – hearing loss in dB HL. Hearing loss is shown for the three frequencies used as standard stimuli in the experiment. Numbers in brackets indicate hearing loss for the standard frequency that was replaced by stimulation at the tinnitus frequency. Our efforts to match patients and controls for sex, age, and hearing loss resulted in two groups that did not differ significantly regarding age or mean hearing loss (averaged across all frequencies tested in the extended audiogram). However, at the level of individual TP-CT pairs the matching was suboptimal in some cases, as revealed by paired t-tests. To ensure that the observed fMRI group differences were not due to these cases of suboptimal matching, we repeated the analyses while excluding the five pairs of participants who showed the largest cumulative differences in hearing loss across all stimuli used in the experiment (marked by a *) and while excluding the five pairs of participants who showed the largest differences in hearing loss at the tinnitus frequency (marked by a °).
Strong positive correlations were observed between age and hearing loss, tinnitus handicap (as assessed using the Tinnitus Handicap Inventory; THI, Newman et al., 1996) and “negative mood” (a measure combining depression and anxiety scores from multiple questionnaires, see section 4.3.), general tinnitus loudness ratings (assessing tinnitus loudness as perceived “on an average day”) and post-scan tinnitus loudness ratings, tinnitus awareness ratings and general tinnitus loudness ratings, tinnitus awareness and tinnitus handicap, and general tinnitus loudness ratings and noise sensitivity (see Table S1). Post-scan tinnitus loudness ratings did not differ significantly from general tinnitus loudness ratings (paired t-test, p = 0.50). However, the correlation between the two measures, while large (r = 0.59) was not perfect, indicating that they may measure slightly different things (with the post-scan loudness ratings presumably being a more accurate measure of the tinnitus perceived during the scan).
2.2. Brain areas displaying group differences in response to auditory stimuli
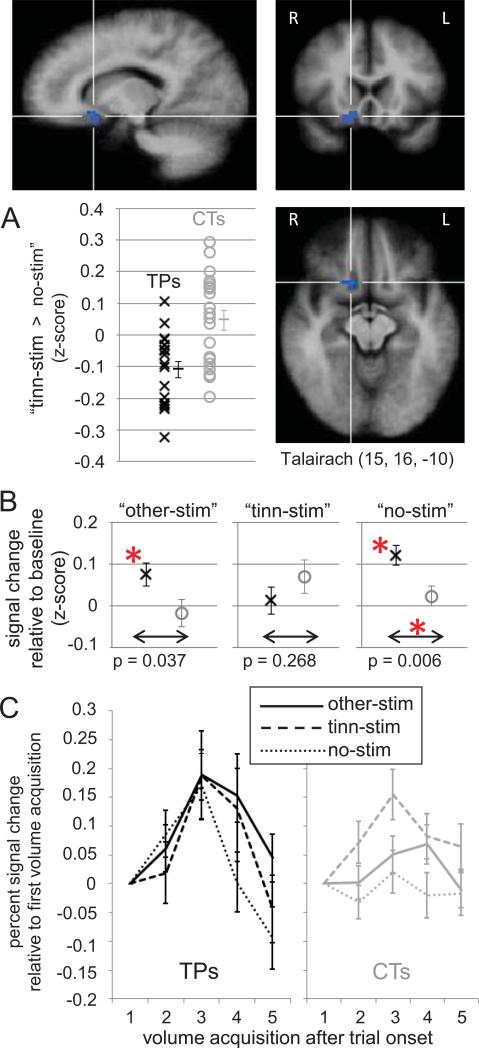
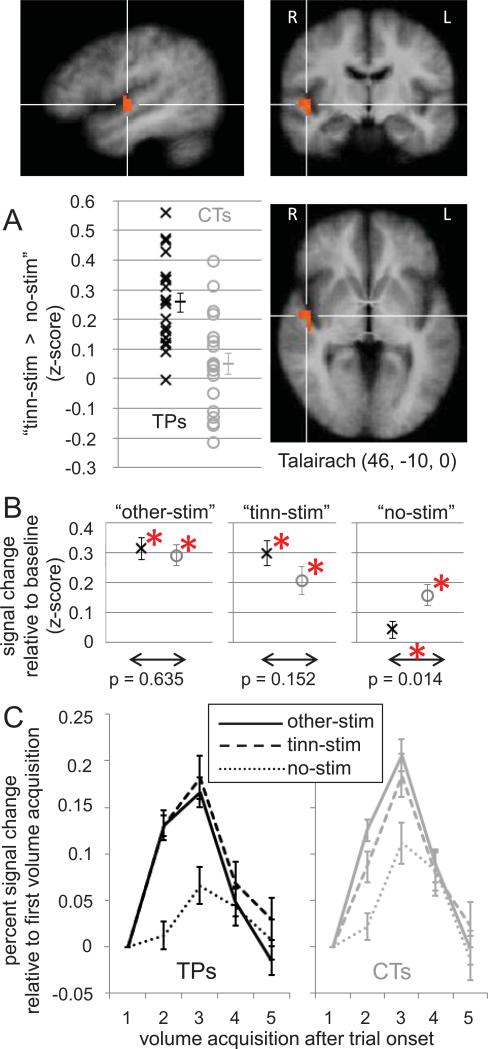
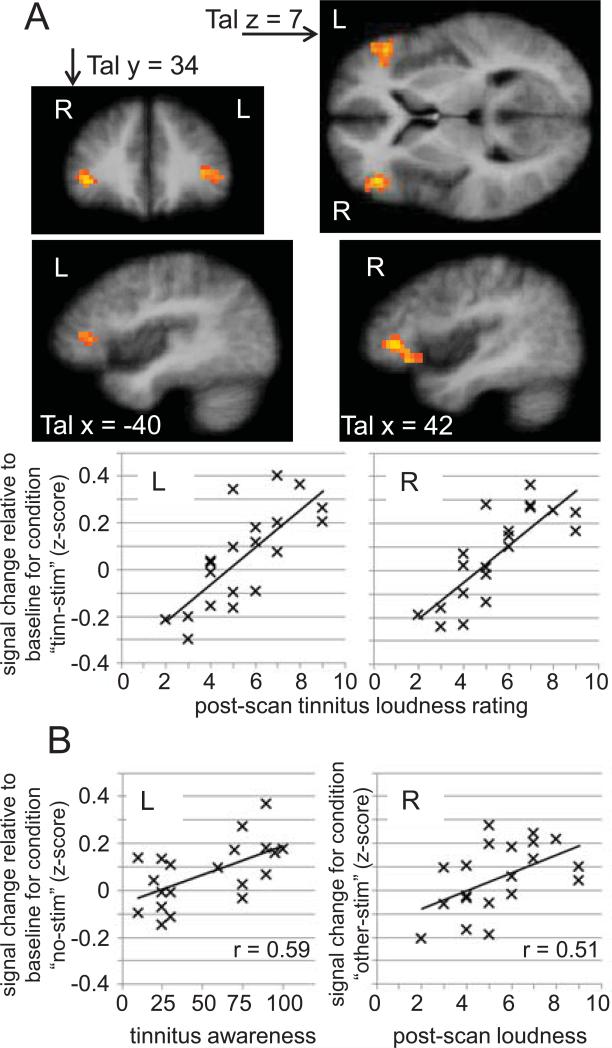
A whole-brain analysis including all functional voxels for which data were available from all participants was performed using a general linear model (GLM) and a random-effects analysis (for details on the GLM and statistical thresholds, see sections 4.5.1. and 4.5.2., respectively). This analysis identified two clusters displaying a significant group difference between tinnitus patients and controls regarding their BOLD response on trials with stimulation at the tinnitus frequency compared to trials without auditory stimulation (contrast “tinn-stim > no-stim”). The first cluster (16 functional voxels, 4323 mm) had its center of gravity (CoG) in right ventro-medial prefrontal cortex (vmPFC, Talairach coordinates 15, 16, - 10, shown in Figure 2A). The second cluster was located in right superior temporal gyrus (STG, CoG Talairach coordinates 46, -10, 0, cluster size 21 voxels, 567 mm3, shown in Figure 5A), including the anterior portion of Heschl's gyrus (Penhune et al. 1996).
Figure 2.
Tinnitus-related group differences in ventromedial prefrontal cortex (vmPFC). (A) A cluster of voxels in vmPFC identified in a whole-brain GLM for displaying a significant (p < 0.005, k > 432 mm3) difference between tinnitus patients and controls regarding their BOLD response to contrast “tinn-stim > no-stim”. The Talairach coordinates are provided for the cluster's center of gravity, indicated by the crosshairs. The scatter plot illustrates the signal difference associated with the contrast for all patients (black x'es) and controls (gray o's) averaged across all voxels. Please note that this scatter plot should not be interpreted in terms of the significance of the group difference. Since the voxels whose average is shown here were selected for showing a group difference in the whole-brain analysis, assessing the significance of the illustrated ROI data would constitute a non-independence error (Kriegeskorte et al., 2009). The scatter plot is shown merely to illustrate that the group difference by which the ROI was identified was not driven by a few single outliers. In addition, it is notable that for all except two tinnitus patients, the response to “no-stim” was larger than that to “tinn-stim”, resulting in mostly negative values. (B) Average BOLD signal changes for tinnitus patients and controls in the three different experimental conditions. Error bars indicate standard errors. Differences marked with an asterisk are significant at p < 0.017 (corrected for 3 tests). (C) Illustration of the BOLD time course in the different conditions, shown separately for patients (black) and controls (gray). Signal changes for the time course illustrations were computed relative to the signal measured during the first volume acquisition of each trial (thus, all curves are at zero for that time point).
Figure 5.
Group difference in right STG. (A) A cluster of voxels in STG identified in a whole-brain analysis for displaying a significant (p < 0.005, k > 567 mm3) difference between tinnitus patients and controls regarding their BOLD response to contrast “tinn-stim > no-stim”. The Talairach coordinates are provided for the cluster's center of gravity, indicated by the crosshairs. As in Figure 2, the scatter plot illustrates the signal difference associated with the contrast for all patients (black x'es) and controls (gray o's), averaged across all voxels displaying significance in the whole-brain analysis, and should thus not be interpreted in terms of the significance of the group difference. (B) Average BOLD signal changes for tinnitus patients and controls in the three different experimental conditions. Differences marked with an asterisk are significant at p < 0.0167. (C) Time-course of BOLD signal changes compared to the signal in the first volume acquisition after trial onset. Error bars indicate standard errors.
For contrast “other-stim > no-stim”, group difference maps at a single-voxel threshold of p < 0.005 showed a few noncontiguous voxels along left and right STG in which patients had stronger activation than controls. However, none of these group differences met the cluster size threshold used to correct for multiple comparisons. Thus, these results are not discussed further.
2.3. ROI analyses of the BOLD response in areas displaying group differences
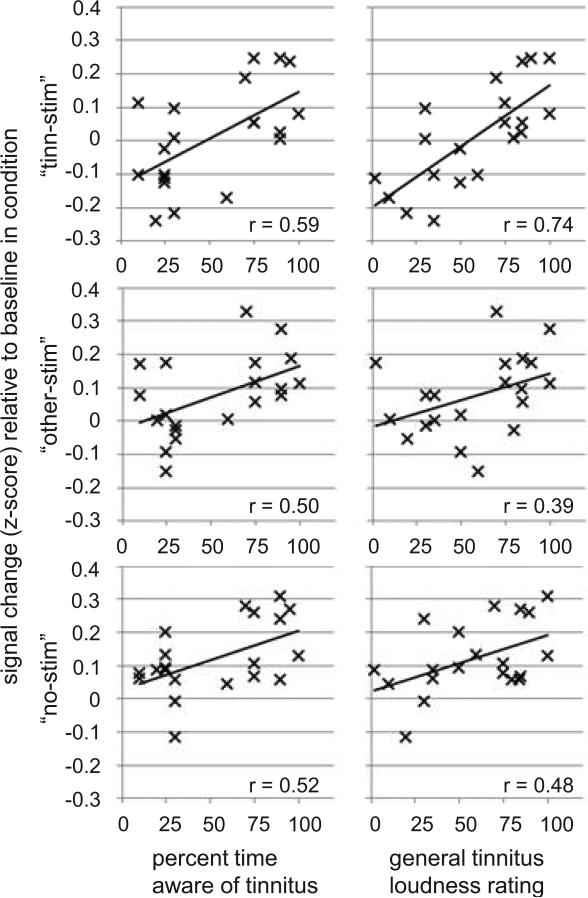
Having identified the above clusters in right vmPFC and right STG as regions of interest (ROIs) in the whole-brain analysis, we performed within-ROI analyses to get a detailed picture on how average BOLD signal changes differed between patients and controls in the three different stimulus conditions (i.e. on trials with stimulation at the tinnitus frequency – “tinn-stim”, on trials with stimulation at non-tinnitus frequencies – “other-stim”, and on trials without auditory stimulation – “no-stim”). The results of this analysis are shown in Figure 2 (B and C) for vmPFC and in Figure 5 (B and C) for STG. In addition, we tested for correlations of the BOLD response in the three different stimulus conditions with tinnitus-related behavioral variables of interest as observed in the group of tinnitus patients (Table 3A, Figure 3).
Table 3.
Correlations between BOLD response and behavioral variables in the group oftinnitus patients.
| A) Correlations in ROIs displaying group differences between TPs and CTs | ||||||
|---|---|---|---|---|---|---|
| right vmPFC | right STG | |||||
| other-stim | tinn-stim | no-stim | other-stim | tinn-stim | no-stim | |
| age | -0.32 | -0.46 | -0.19 | -0.28 | -0.03 | -0.15 |
| av. hearing loss | 0.01 | -0.02 | 0.27 | -0.36 | 0.06 | -0.32 |
| negative mood | -0.00 | 0.11 | 0.05 | -0.24 | -0.08 | -0.35 |
| noise sensitivity | 0.32 | 0.35 | 0.13 | 0.08 | 0.13 | -0.10 |
| general loudness | 0.39 | 0.74 | 0.48 | -0.09 | -0.08 | -0.14 |
| postscan loudn. | 0.29 | 0.28 | 0.30 | 0.01 | -0.04 | 0.07 |
| tinn. awareness | 0.50 | 0.59 | 0.52 | 0.00 | 0.08 | 0.09 |
| THI | 0.39 | 0.41 | 0.24 | -0.45 | -0.22 | -0.26 |
| B) Correlations in ROIs displaying strong correlations with tinnitus variables | ||||||
|---|---|---|---|---|---|---|
| left IFG | right IFG | |||||
| other-stim | tinn-stim | no-stim | other-stim | tinn-stim | no-stim | |
| age | 0.18 | -0.04 | -0.22 | 0.17 | 0.19 | -0.03 |
| av. hearing loss | 0.17 | 0.13 | -0.13 | 0.21 | 0.33 | 0.13 |
| negative mood | -0.16 | 0.29 | 0.13 | 0.10 | 0.33 | 0.17 |
| noise sensitivity | -0.02 | 0.13 | -0.05 | 0.30 | 0.26 | 0.11 |
| general loudness | 0.14 | 0.39 | 0.25 | 0.27 | 0.46 | 0.24 |
| postscan loudn. | 0.46 | *** | 0.29 | 0.51 | *** | 0.32 |
| tinn. awareness | 0.09 | 0.42 | 0.59 | 0.16 | 0.34 | 0.37 |
| THI | -0.30 | 0.16 | 0.16 | -0.02 | 0.28 | 0.27 |
Bold print highlights correlations exceeding 0.50. Stars mark correlations used to define the associated ROI. “other-stim” – trials with stimuli at a non-tinnitus frequency, “tinn-stim” – trials with stimulation at the tinnitus frequency, “no-stim” – trials without auditory stimulation.
Figure 3.
Correlations between BOLD signal changes and tinnitus characteristics in the right vmPFC ROI identified for displaying group differences regarding contrast “tinn-stim > no-stim”. Correlations with additional variables are listed in Table 3.
2.3.1. Ventromedial prefrontal cortex (vmPFC)
As illustrated in Figure 2, controls did not show significant BOLD responses in vmPFC during any stimulus condition, neither compared to an implicit (“between-trials”) baseline corresponding to the intercept of the GLM (Figure 2B) nor compared to the signal level measured during the first volume acquisition of each trial (Figure 2C). In contrast, tinnitus patients displayed significant BOLD increases on trials with stimulation at non-tinnitus frequencies (“other-stim”) as well as on trials without auditory stimulation (“no-stim”), resulting in significant between-group differences for the latter condition. BOLD increases on trials with stimulation at the patients’ tinnitus frequency (“tinn-stim”) compared to the implicit “between-trials” baseline were not significant for either patients or controls (Figure 2B). Compared to the signal level measured during the first volume acquisition, the BOLD response on “tinn-stim” trials showed a characteristic rise and fall for both groups (particularly in patients; Figure 2C). The apparent discrepancy between the BOLD response for “tinn-stim” in Figure 2C and the “absence” of a BOLD response for “tinn-stim” in Figure 2B arises because the data in Figure 2B reflect how well the observed BOLD time course fits the predicted, canonical BOLD response. The canonical BOLD time course (as implemented in BrainVoyager QX, version 2.3.1) is already near its peak during the second volume acquisition, when the observed BOLD response in vmPFC is just starting to rise (Figure 2C). Thus, the fit between these two was not significant (Figure 2B), despite the presence of a significant BOLD response in this region (Figure 2C). This lag in the observed vmPFC time course could suggest that activity reaches vmPFC only after passing several intermediate processing stations. In this context, it is interesting to note that the stimulus-evoked BOLD response in STG (Figure 5C) rises earlier and is more similar to the predicted canonical BOLD time course. The delayed response in vmPFC relative to STG is in line with our tinnitus model (Leaver et al., 2011), according to which MGN activation reaches vmPFC indirectly via amygdala, nucleus accumbens, ventral pallidum, and the medial dorsal nucleus of the thalamus, whereas neural activity is passed directly to auditory cortex from MGN.
Within the group of tinnitus patients, the average BOLD response in vmPFC was strongly correlated with general tinnitus loudness ratings and tinnitus awareness (Table 3A, Figure 3). These correlations were particularly strong on trials with stimulation at the tinnitus frequency (“tinn-stim”), but also present on trials with stimulation at non-tinnitus frequencies (“other-stim”) and even on trials without auditory stimulation (“no-stim”). Importantly, correlations with noise sensitivity, negative mood, hearing loss, and age were much smaller or even negative (Table 3A). Interestingly, when assessing correlations between the tinnitus percept and activation differences between the conditions, we found strong positive correlations between tinnitus loudness ratings and contrast “tinn-stim > no-stim” and contrast “tinn-stim > other-stim”, but not for contrast “other-stim > no-stim” (Supplementary Figure S3).
Since tinnitus loudness and tinnitus awareness ratings were highly correlated (r = 0.55), we also performed partial correlation analyses to assess the unique contributions of the two variables to the observed vmPFC signal while controlling for the influence of the other variable. These analyses (reported in Supplementary Table S2) showed that for contrasts “other-stim” and “no-stim”, the unique contributions of both variables were relatively small and not significant. However, for contrast “tinn-stim”, the predictive power of tinnitus loudness ratings was large (r = 0.62) and highly significant (p < 0.005) even after partialling out the influence of tinnitus awareness ratings.
2.3.2. Superior temporal gyrus (STG)
As would be expected of a brain region directly involved in auditory sensation, STG showed significant BOLD responses on trials with auditory stimulation (“tinn-stim” and “other-stim”) bilaterally in both groups, whereas the BOLD responses on trials without auditory stimulation (“no-stim”) were weaker. (A “stim > no-stim” contrast based on a random-effects analysis including all participants is illustrated in Supplementary Figure S4; Supplementary Figure S5 shows the BOLD responses in the separate conditions.) The same held for the ROI in right STG displaying a significant group difference for contrast “tinn-stim > no-stim” (Figure 5, B and C). Interestingly, the group difference was mostly driven by the fact that the BOLD response on “no-stim” trials in this ROI was still significant for controls, but not for tinnitus patients. As can be seen in Figure 5C, the BOLD response on “no-stim” trials also started rising later than the BOLD responses on trials with auditory stimulation. The BOLD responses on trials with auditory stimulation were already near their peak during the second volume acquisition (4.5 to 6 seconds after trial onset), which is consistent with the known time course of the BOLD response to auditory stimulation in auditory cortex (Hall et al., 2000). In contrast, the BOLD response on “no-stim” trials was still near baseline during the second volume acquisition, and only rose above baseline during the third (7.5 to 9 seconds after trial onset). No strong correlations were found between BOLD responses and tinnitus variables for any stimulus condition in this ROI (Table 3A).
2.4. Brain areas displaying correlations with tinnitus characteristics
In addition to group differences between patients and controls, correlations between the average BOLD response and perceptual tinnitus characteristics can also reveal brain areas relevant to tinnitus perception. We thus searched, across the entire brain, for clusters of voxels whose BOLD response on trials with stimulation at the tinnitus frequency (“tinn-stim”) was correlated with patients’ tinnitus loudness ratings (both regarding how loud they perceived their tinnitus on average – “general loudness” and how loud they perceived it immediately after the scan – “postscan loudness”), their tinnitus awareness ratings, and their THI scores (a measure of tinnitus distress).
This analysis identified strong positive correlations (r > 0.60) between patients’ BOLD response on “tinn-stim” trials and postscan tinnitus loudness ratings bilaterally in anterior inferior frontal gyrus (IFG) in the lowermost portion of Brodmann area (BA) 46 (bordering BA 45). Both clusters are illustrated in Figure 6A, along with scatter plots illustrating the correlations for which they were identified. The cluster in right IFG (CoG Talairach coordinates 41, 32, 0) encompassed 82 functional voxels (2214 mm3), and the one in left IFG (CoG Talairach coordinates -41, 34, 7) encompassed 46 functional voxels (1242 mm3). Aside from the correlations for which they were identified (i.e. of contrast “tinn-stim” and post-scan tinnitus loudness ratings), both clusters also showed related correlations between post-scan tinnitus loudness ratings and contrast “other-stim” and between tinnitus awareness ratings and contrasts “tinn-stim” and “no-stim” (Table 3B). All these correlations exceeded 0.30; the two exceeding 0.50 are illustrated in Figure 6B. It should be noted that, unlike in vmPFC, these correlations are not independent of how the ROI was identified, since tinnitus awareness and tinnitus loudness ratings are correlated (see Table S1); i.e., an ROI whose activation is correlated with tinnitus loudness is likely to also show a correlation with tinnitus awareness. Furthermore, an ROI showing a correlation between a certain behavioral variable and the BOLD response in one experimental condition is likely to also show a correlation with the same variable in another, similar condition. Therefore, the correlations depicted in Figure 6B are potentially overestimated (Kriegeskorte et al., 2009; Vul et al., 2009).
Figure 6.
Tinnitus correlations in left and right inferior frontal gyrus (IFG). (A) The bilateral clusters were identified in a whole-brain correlation analysis for displaying a significant (r > 0.60) correlation between the BOLD response on “tinn-stim” trials and tinnitus loudness as rated immediately after the scan. The scatterplots below illustrate the correlation, averaged across all voxels of each cluster. As in Figures 2 and 5, these scatter plots are merely shown to demonstrate that the correlations were not driven by a few outliers; however, note that the depicted correlations overestimate the true effect size (of which we can only say that it exceeds r > 0.60), since the voxels over which we averaged were selected for displaying a significant correlation in the whole brain analysis (Vul et al., 2009). (B) In addition to the correlations for which the clusters were identified, both clusters also displayed positive correlations between the BOLD response on “no-stim” trials and tinnitus awareness ratings (shown here is the correlation for left IFG, which exceeded 0.50). Note, however, that this correlation may at least partly be caused by the correlation between tinnitus loudness ratings and tinnitus awareness ratings (see Table S1). Both clusters also showed correlations between the BOLD response on “other-stim” trials and post-scan tinnitus loudness ratings (shown here is the correlation for right IFG, which exceeded 0.50).
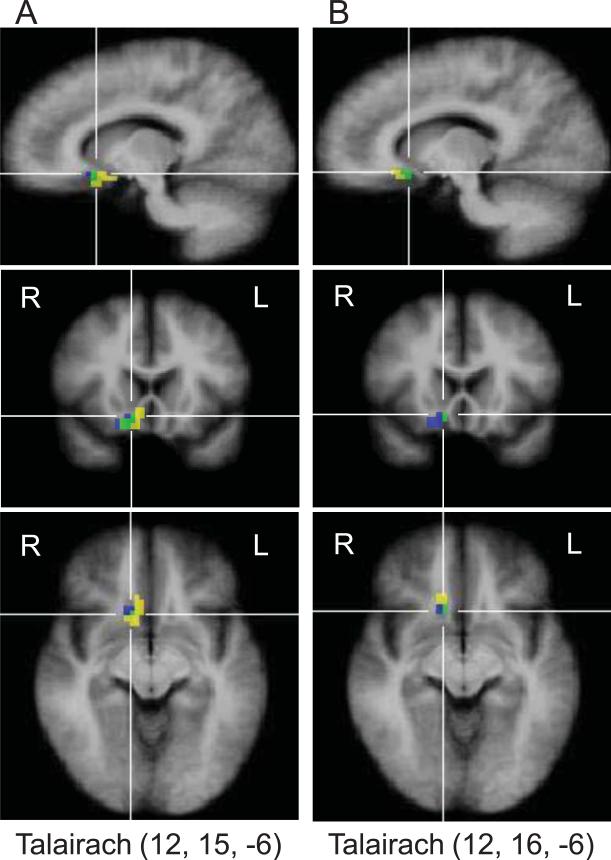
The whole-brain correlation analysis also identified a cluster of 40 voxels (1080 mm3) in right vmPFC (CoG Talairach coordinates 10, 14, -8) whose BOLD response on “tinn-stim” trials was strongly correlated with patients’ general tinnitus loudness ratings, as well as a nearby, partly overlapping cluster (21 voxels, 567 mm3, CoG Talairach coordinates 14, 23, -9) whose BOLD response on “tinn-stim” trials was strongly correlated with patients’ tinnitus awareness ratings. As can be seen in Figure 4, these clusters overlapped with the one identified for displaying group differences for contrast “tinn-stim > no-stim” (as illustrated in Figure 2 and discussed above), thus providing independent confirmation that right vmPFC is tied to tinnitus perception.
Figure 4.
Overlap (green) between the vmPFC voxels displaying a significant group difference for contrast “tinn-stim > no-stim” (blue) and the vmPFC voxels displaying a significant positive correlation (yellow, r > 0.60) between the BOLD response on “tinn-stim” trials and general tinnitus loudness ratings (A) and tinnitus awareness ratings (B). Talairach coordinates indicate the location of the crosshairs intersection, which was chosen to best show the overlap.
The reported clusters were the only clusters surviving our combined single-voxel and cluster size thresholds for their respective statistical maps. No significant correlations were observed between the BOLD response on “tinn-stim” trials and THI scores.
3. Discussion
3.1. Confirmation of the involvement of vmPFC in tinnitus
According to our tinnitus model (Rauschecker et al., 2010), vmPFC plays a crucial role for determining to what extent aberrant auditory neural activity becomes a conscious tinnitus percept. This model has so far been backed by three MRI studies using voxel-based morphometry (VBM), which identified gray-matter decreases in tinnitus patients compared to controls in a subcallosal region (Mühlau et al., 2006) and, more specifically, vmPFC (Leaver et al., 2011; Leaver et al., 2012). Additional support came from an MEG study comparing Partial Directed Coherence in resting-state cortical networks of tinnitus patients and controls (Schlee et al., 2009). Schlee and colleagues demonstrated that voxels in orbitofrontal cortex (likely including what we refer to as vmPFC) showed decreased outflow but increased inflow in tinnitus patients, meaning that in tinnitus patients, medial prefrontal cortex activity was more strongly influenced by activity in other regions of the brain, and had less influence on activity in other regions of the brain. This finding is consistent with our assumption of vmPFC as part of a gating mechanism whose failure to regulate activity in other brain regions can lead to tinnitus.
The present study complements these findings in new samples of age-matched tinnitus patients and controls by providing fMRI evidence for functional group differences in vmPFC, which has been lacking so far. The BOLD response in right vmPFC exhibited both a group difference for contrast “tinn-stim > no-stim” (mostly driven by the fact that tinnitus patients showed a stronger BOLD response on “no-stim” trials) and strong positive correlations between the BOLD response in all conditions (“tinn-stim”, “no-stim”, and “other-stim”) and the patients’ tinnitus awareness and tinnitus loudness ratings. Importantly, correlations with other variables that could cause differential responses in tinnitus patients and controls, such as depression and anxiety, were small and non-significant, ruling out this alternative explanation. Taken together, the present results demonstrate that tinnitus patients engage ventral prefrontal cortex during an auditory task differently than control participants. This yields further support to the hypothesis that the anatomical differences observed in limbic-related medial prefrontal brain regions of tinnitus patients in previous studies (Mühlau et al., 2006; Leaver et al, 2011) are indeed related to tinnitus.
3.2. The role of ventromedial prefrontal cortex (vmPFC) in tinnitus
In our recent tinnitus model (Rauschecker et al., 2010) we proposed that vmPFC provides the driving input for a thalamic auditory gating mechanism that can suppress the tinnitus signal. In the context of the current study, we assume that the vmPFC activity observed in tinnitus patients reflects the engagement of this tinnitus suppression mechanism. In order to focus on the experimental task and stimuli, all tinnitus patients were required to use their inhibitory gating mechanism to “tune out” the tinnitus signal to their best ability. Control participants without tinnitus were unlikely to have need for employing this gating system. This idea is supported by the presence of vmPFC BOLD responses in tinnitus patients, and their relative absence in controls (Figure 2, B and C).
Please note that these group differences argue against a potential alternative explanation for the observed vmPFC BOLD responses. One might suspect that they reflect the preparation and/or execution of the motor response required on each trial, especially considering that in patients, vmPFC BOLD responses were observed even on trials without auditory stimulation. However, if the responses were indeed related to motor control, one would expect similarly strong BOLD responses in controls, who also made motor responses on each trial. Reaction time analyses revealed no differences between groups that could explain the difference in BOLD responses (data not shown). There was also no correlation between reaction time and vmPFC BOLD response. Taken together, this makes it unlikely that the vmPFC BOLD responses in patients reflect motor preparation.
In addition to the group differences, the observed correlations between the vmPFC BOLD response and tinnitus characteristics (general tinnitus loudness and awareness) further corroborate our hypothesis that vmPFC BOLD responses are directly related to the tinnitus percept. When interpreting these correlations, it is important to bear in mind that higher tinnitus loudness and awareness ratings could occur for different reasons, and probably do so in different patients. Louder tinnitus may result simply from stronger aberrant activity in lower auditory areas (e.g. as a result of stronger damage to the auditory periphery). But even if the “tinnitus signal” itself were constant across participants, the perception could still differ depending on how successful the “noise cancellation mechanism” proposed by us works. This, in turn, could also depend on different things in different patients. Obviously, anatomical damage to parts of the noise cancellation mechanisms (such as the vmPFC gray-matter reductions observed by Leaver et al., 2011) would compromise its functioning. Similarly, even if all parts were intact, functioning would still be compromised if the connections between them were weakened or missing (e.g. due to a lack of relevant neurotransmitters or anatomical connections). Lastly, in some patients, the noise cancellation mechanism could be anatomically intact but nevertheless not suppress the tinnitus, simply because the tinnitus is interpreted as a threatening and relevant stimulus, rather than as noise (De Ridder et al., 2011; Jastreboff, 1990). Below, we outline three potential, not mutually exclusive, interpretations for the observed correlations between vmPFC activity and tinnitus loudness and awareness within the framework of our tinnitus model. Please note that since these interpretations are post-hoc and driven by the present data, future research will have to test whether and under which circumstances each of them holds in an independent data set.
Interpretation 1
Larger vmPFC responses in patients with louder and more intrusive tinnitus may reflect the gating system's stronger (albeit unsuccessful) efforts to suppress a stronger tinnitus signal during the task. Note that this interpretation is well in line with the observation of reduced influence of vmPFC over the activity of other brain areas in tinnitus patients (Schlee et al., 2009). Essentially, vmPFC “goes into overdrive” in an attempt to suppress the tinnitus signal, but its activity does not achieve the desired regulating influence, possibly due to reduced connectivity with other (especially auditory) brain regions. This interpretation suggests vmPFC connectivity as an area of high interest for future tinnitus research. It also raises the question whether the previously observed gray-matter reductions in vmPFC may be the results of excitotoxicity (i.e. damaging of neurons due to excessive and constant excitation).
Interpretation 2
It is possible that the experimental stimuli interacted with the tinnitus and altered the tinnitus percept, thus modulating the need for gating and, in consequence, vmPFC activity during the task. In patients with soft tinnitus, an experimental stimulus (especially one that is well matched to the tinnitus frequency) may partly mask the tinnitus, or even induce residual inhibition (Roberts et al., 2008), thus reducing the need for employing the gating mechanism. This could explain why the BOLD response on “tinn-stim” trials was much smaller than on “no-stim” trials without auditory stimulation that could mask the tinnitus (Figure 2B), and also smaller than that on trials with stimulation at “other” frequencies distant from the tinnitus (which are less potent tinnitus maskers). Similarly, this interpretation can explain the negative values in the correlation plots for “tinn-stim” (Figure 3). Since in these plots activation is expressed relative to the baseline term of the GLM (i.e., activation levels “between” trials), negative values indicate that activation in the respective condition is lower than during baseline. This is exactly what one would expect if stimulation (especially at the tinnitus frequency) masks the tinnitus and thus reduces the need for gating relative to baseline. In contrast, for other patients, especially for those with loud and intrusive tinnitus (i.e. high tinnitus loudness and tinnitus awareness ratings), an auditory stimulus near the tinnitus frequency might actually increase the perceived loudness of the tinnitus rather than mask it, thus requiring particularly strong activation of the gating system and leading to particularly large BOLD responses in this condition.
This interaction hypothesis would explain why the correlations between the tinnitus percept and vmPFC signal changes were strongest for condition “tinn-stim”, in which the interaction between the experimental stimulus and the patients’ tinnitus would be maximal (Figure 3). Furthermore, tinnitus loudness was positively correlated with vmPFC activation differences between condition “tinn-stim” and both other conditions (constrasts “tinn-stim > other-stim” and “tinn-stim > no-stim”), but not with activation differences between conditions “no-stim” and “other-stim” (Supplementary Figure S3). This, too, suggests that the “tinn-stim” condition was in some way “special”, likely due to the interaction between the stimulus at the tinnitus frequency and the tinnitus itself. A last piece of evidence in favor of the interaction hypothesis is provided by partial correlation analyses (Supplementary Table S2); only in condition “tinn-stim” were tinnitus loudness ratings highly correlated with vmPFC activation after controlling for tinnitus awareness ratings. This indicates a unique relationship between tinnitus loudness and vmPFC activation in the “tinn-stim” condition, suggesting that tinnitus loudness determines whether stimulation at the tinnitus frequency results in either: A) masking, reduced need for gating, and thus reduced vmPFC activation, or B) amplification of the tinnitus percept, increased need for gating, and thus increased activation in vmPFC during the task.
Interpretation 3
It is also possible that the observed correlations at least partly reflect baseline differences. If vmPFC activity at baseline influences to what extent the tinnitus signal is perceived, patients with fairly high vmPFC baseline activity would report lower tinnitus awareness and loudness than patients whose vmPFC is less active at baseline (all other things equal). In those patients with soft tinnitus, the elevated vmPFC baseline activity would then leave less room for activation increases during the experimental task. In contrast, patients with lower vmPFC baseline activity (and thus louder and more intrusive tinnitus) could show larger increases during a task that requires tuning out the tinnitus. Please note that interpretation 3 in no way contradicts, but rather complements interpretation 1. Interpretation 1 predicts larger vmPFC activity in patients with higher tinnitus awareness during the task, whereas interpretation 3 predicts higher tinnitus awareness in patients with lower vmPFC activity at baseline (which, in turn, leaves room for larger increases during the task).
Importantly, the fact that tinnitus patients activated their gating system during our experimental task does not necessarily mean that this activity was sufficient to completely suppress the tinnitus. As noted above, vmPFC gray-matter reductions and/or compromised connectivity between vmPFC and other parts of the gating system may make it impossible to achieve complete tinnitus suppression. Additional “distractor control” may thus be needed, and lateral PFC is a highly plausible candidate for this function.
3.3. The role of lateral prefrontal cortex (lPFC)
The role of lateral prefrontal cortex in attention and inhibitory control (Watanabe 1986; Sasaki et al., 1989; MacDonald et al., 2000; Ploner et al., 2005) and even, specifically, in auditory gating (Skinner & Yingling, 1977; Woods & Knight, 1986; Knight et al., 1989) is well known. BOLD activity in lateral PFC increases with task difficulty and with distractor salience (Tomasi et al., 2005). It is also needed for suppressing internal distractions such as intrusive thoughts and emotions (Lévesque et al., 2003; Anderson et al., 2004). We thus assume that the lPFC activation observed in the present study reflects the effort involved in focusing on performing the experimental task while ignoring distractions.
While distractions, for example in form of scanner noise, somatosensory sensations, and motor impulses, were certainly present for all participants, the tinnitus percept (and, quite possibly, associated emotions and thoughts) constituted an additional internal distractor for the tinnitus patients. Consistent with this interpretation, activation in the two lateral prefrontal ROIs (Figure 6) did not differ significantly between groups, but was nevertheless strongly correlated with tinnitus loudness ratings given immediately after the scan. We hypothesize that these correlations arose because the patients with the loudest postscan tinnitus ratings experienced a more potent distractor during the scan and thus had to exert more effort to perform the task. This was, however, not reflected in the error rates, since the oddball detection task was simple and all participants achieved near-optimal scores.
3.4. Auditory cortex activation in tinnitus
Many have argued that aberrant activity in the auditory system is what generates the tinnitus signal. This is well supported by empirical evidence (for a recent review, see Roberts et al., 2010), especially from animal studies indicating increased spontaneous and sound-evoked neuronal activity at various stations along the auditory pathways; however, studies disagree at which level of the auditory system (cochlear nuclei, inferior colliculi, thalamus, or auditory cortex) the aberrant activity is generated, and whether it is directly related to tinnitus or rather to hyperacusis, a hypersensitivity to sound observed in many tinnitus patients (Gu et al., 2010). Unfortunately, since fMRI cannot measure absolute blood oxygenation, but only compare relative blood oxygenation between conditions (Logothetis 2008; Gusnard & Raichle 2001), it cannot detect the neuronal signature of a tinnitus signal that is constantly present and affects all measurements alike. Nevertheless, the present study picked up on an effect in auditory cortex that is likely related to tinnitus, albeit indirectly, perhaps via the modulating influence of top-down attention (as was previously suggested by Gu et al., 2010).
As shown in Figure 5A, tinnitus patients showed a larger signal change for contrast “tinn-stim > no-stim” than controls in right STG. This effect was mostly due to the fact that controls showed a significantly stronger BOLD response on “no-stim” trials than tinnitus patients (Figure 5B). We attribute the auditory response on “no-stim” trials to “top-down” feedback perhaps due to auditory attention, which is known to enhance auditory cortex activity (Grady et al., 1997), even in the absence of stimulation (Voisin et al., 2006). Since auditory stimuli occurred at the same time during all trials except “no-stim”, and since the “no-stim” trials were randomly interleaved with stimulation trials, participants would have expected auditory stimuli to occur even on “no-stim” trials. A violation of this expectation likely triggered the allocation of additional top-down attention. Consistent with this feedback interpretation, the BOLD response on silent trials started to rise later than that on trials with auditory stimulation (Figure 5C). We hypothesize that the feedback-related BOLD response looks smaller in tinnitus patients because their tinnitus draws a certain amount of auditory attention (and thus feedback activation into auditory cortex) at all times. This results in an elevated baseline compared to which there is less room for task-related feedback BOLD responses. A way of testing this hypothesis in a future dataset would be to assess whether tinnitus patients indeed show enhanced functional connectivity between brain areas associated with top-down attentional control and auditory cortex at rest (i.e. in the absence of task and stimulation).
3.5. Summary and conclusion
The present fMRI study revealed functional differences between tinnitus patients and controls matched for age and sex in right vmPFC and right STG. In addition, it identified two lateral prefrontal brain regions (left and right IFG, BA 46) whose BOLD response to stimulation at the tinnitus frequency was strongly correlated with tinnitus loudness ratings given immediately after the scan. We attribute these latter correlations to the fact that the tinnitus constituted a distractor and thus increased task difficulty proportional to its perceived loudness during the scan. In agreement with previous results (Gu et al., 2010), we assume that the group differences observed in STG were likely due to tinnitus-related effects on auditory attention, but not to the tinnitus percept itself. Ventromedial prefrontal cortex was the only brain region that displayed both a significant group difference between tinnitus patients and controls and strong correlations between its activation and the tinnitus percept itself. Its BOLD response in all conditions, but especially on trials with stimulation at the tinnitus frequency, was strongly correlated with tinnitus loudness and tinnitus awareness. Importantly, correlations with variables capturing factors often associated with tinnitus, such as hearing loss, depression, or anxiety, were considerably smaller.
These results confirm and extend previous findings of functional and anatomical differences in limbic and limbic-related brain regions of tinnitus patients. The observed group differences and correlations are not suited for testing causality or directionality of influence between auditory and limbic brain regions. However, it is notable that the vmPFC BOLD response was correlated most strongly with how often patients were aware of their tinnitus and how loud they perceived it in general, and much less correlated with how loud they perceived it immediately after the scan. This suggests that the functional changes in vmPFC reported here, just like the anatomical changes reported previously (Leaver et al., 2011; Leaver et al., 2012), are related to the long-term characteristics (or consequences) of the tinnitus percept, rather than to short-term variations in the tinnitus percept or its possible emotional consequences.
4. Experimental Procedures
4.1. Participants
Forty-four participants (24 patients and 20 controls) who met standard MRI inclusion criteria participated in this study. All experimental procedures were approved by the Institutional Review Board at Georgetown University and fully disclosed to the participants, who gave written informed consent. Of the tinnitus patients, four were excluded from the analysis (and no control was matched to them) for the following reasons: One patient reported unpleasant physical sensations during the anatomical scan so that we decided not to acquire functional data, one patient moved excessively during the scan, and in two patients, high-frequency hearing loss made it impossible to play all auditory stimuli at a level audible to the participants without introducing noticeable sound distortions. Controls were recruited to match the patients by age and sex. Each group consisted of eleven women and nine men, and the groups did not differ significantly regarding age (patients ranged from 23 to 66 years of age, with a mean of 46.7 and a standard deviation of 13.4 years; controls ranged from 29 to 67 years, with a mean of 48.5 and a standard deviation of 12.3 years).
4.2. Behavioral data acquisition
4.2.1. Audiometry and tinnitus match
Each participant received an extended examination at Georgetown University's Department of Otolaryngology to ensure that no audiological disease (other than subjective tinnitus and sensorineural hearing loss) was present. In addition to a standard clinical audiogram (assessing hearing thresholds for pure tones from 250 to 8000 Hz), hearing thresholds were tested up to 20 kHz. Loudness discomfort levels (LDLs) were assessed by gradually increasing the intensity of a 1 kHz pure tone (up to 100 dB HL) until the participant rated the sound as uncomfortably loud. Tinnitus patients were asked to adjust a test sound to match their tinnitus as well as possible using software written for MATLAB (The MathWorks, Inc.). Patients could vary frequency (in 1/12 octave steps), intensity (in 3 dB steps), and bandwidth (in 1/6 octave steps) of the test tone, as well as whether it was played to the left, right, or both ears. We also told participants that we could make the steps even finer if they determined that the best match was somewhere in between these steps, but no participant ever requested that. Even though only 13 of the 20 patients described their tinnitus as tonal, after trying different bandwidth settings, all patients ultimately determined a pure tone as the best match. They were then encouraged to try stimuli an octave above and below to ameliorate the problem of octave confusion. Without being encouraged to do so, many patients spontaneously reported using a masking criterion for the match, determining that tone as the best match which blended with their tinnitus so that they could no longer distinguish between the two. Overall, participants described the resulting matches as “good” to “excellent”.
4.2.2. Questionnaires
All participants completed an MRI screening form (assessing, among other things, history of neurological diseases, head injuries, and use of neuromodulatory medications), as well as the Patient Health Questionnaire (PHQ9, Kroenke et al., 2001) to assess depressive symptoms, the Generalized Anxiety Disorder questionnaire (GAD7, Spitzer et al., 2006), the Hospital Anxiety and Depression Scale (HADS, Zigmond & Snaith, 1983), and non-tinnitus specific questions of the Tinnitus Sample Case History Questionnaire (TSCHQ, Langguth et al., 2007). Tinnitus patients also completed the remaining items of the TSCHQ, as well as the Tinnitus Handicap Inventory (THI, Newman et al., 1996).
4.3. Use of behavioral data
Group differences for the different behavioral variables were investigated using non-directional t-tests assuming unequal variance between groups. The reported p-values are not corrected for multiple tests, which is a conservative test under the present circumstances since it facilitates the detection of significant group differences regarding variables other than tinnitus, which are undesired. Hearing thresholds, averaged across the entire audiogram and both ears, did not differ significantly between patient and control groups (for average audiograms, see Supplementary Figure S2). There was only a non-significant trend (p = 0.064) for tinnitus patients to have higher average thresholds (mean M = 31 dB HL, standard error SE = 3 dB HL) than controls (M = 23 dB HL, SE = 3 dB HL). However, paired t-tests of hearing thresholds for the stimulus frequencies used in the experiment revealed that tinnitus patients had higher hearing thresholds than their stimulus-matched controls for the higher frequencies (see Table 2). While these differences would not have been deemed significant at an error level corrected for multiple comparisons using the Bonferroni method, they nevertheless indicate suboptimal matching between the groups. We thus performed additional fMRI analyses to check whether this contributed to the group differences observed in the fMRI analysis (see section 4.5.4.).
To obtain a general noise sensitivity measure, we combined LDLs with a question from the TSCHQ worded as follows: “Do you have a problem tolerating sounds because they often seem much too loud? That is, do you often experience sounds which other people around you find quite comfortable as too loud or hurtful? (0=never; 1=rarely; 2=sometimes; 3=usually; 4=always).” After normalizing LDLs to the maximum intensity tested (100 dB HL), we added the result to the normalized TSCHQ noise sensitivity rating. The resulting noise sensitivity score thus ranges from 0 (for participants for whom even 100 dB HL are not uncomfortably loud and who never experience sounds as louder or more hurtful than other people) to 2 (for participants for whom even the softest sounds are uncomfortably loud and who always experience sounds as more loud or hurtful than other people). Noise sensitivity differed significantly (p = 0.000002) between patients (M = 0.61, SE = 0.07) and controls (M = 0.14, SE = 0.04). We thus performed additional fMRI analyses to check whether this contributed to the group differences observed in the fMRI analysis (see section 4.5.4.). Please note that it is likely that the measures of noise sensitivity used here are influenced by both anxiety and hearing loss. Participants with high anxiety levels will likely experience the gradual increase in loudness during the LDL assessment as threatening and stop the procedure before truly uncomfortable levels have been reached. This would lead to an overestimation of noise sensitivity. On the other hand, participants with higher hearing thresholds will perceive sounds as less loud, which could lead to an underestimation of noise sensitivity. The noise sensitivity measure used here should thus be interpreted with caution.
Depression and anxiety self-ratings were highly correlated across the different scales (all r > 0.71). For this reason, we combined PHQ9, GAD7, and HADS scores into a single “negative mood” score by dividing, for each scale, each participant's score by the maximum possible score (so that normalized scores for each scale ranged from 0 to 1), and then summing the normalized scores across the three scales (so that the resulting “negative mood” scores ranged from 0 to 3). As with hearing loss, there was no significant difference between patients and controls, but a trend (p = 0.073) for patients to have higher negative mood scores (M = 0.55, SE = 0.10) than controls (M = 0.34, SE = 0.06).
The following two questions from the TSCHQ were used to assess tinnitus characteristics: “Describe the loudness of your tinnitus using a scale from 1 to 100 (1 = very faint; 100 = very loud)” and “What percentage of your total awake time, over the past month, have you been aware of your tinnitus? For example, 100 % would indicate that you were aware of your tinnitus all the time, and 25 % would indicate that you were aware of your tinnitus ¼ of the time”. In addition, we also asked patients to rate the perceived loudness of their tinnitus immediately before and after the scan, using the question “Please rate the current loudness of your tinnitus, as you experience it right now” followed by a visual analog scale from one to ten. There were no systematic differences between pre- and post-scan tinnitus loudness ratings (they did not differ at all for half of the tinnitus patients, and deviated by up to 2 points in either direction for the remaining patients), indicating that the scanning procedure neither worsened nor improved the tinnitus. We thus decided to use only post-scan tinnitus loudness ratings as an estimate of tinnitus loudness at scan time. Note that while post-scan tinnitus loudness ratings did not differ significantly from general tinnitus loudness ratings (paired t-test, p = 0.50), the two measures were not perfectly correlated, either (see Supplementary Table S1). This indicates that tinnitus loudness as perceived on the day of the scan did not necessarily represent patients’ general tinnitus loudness ratings. To assess tinnitus impact, we used the total THI score.
4.4. MRI data acquisition
4.4.1. Scanning parameters
Imaging was performed on a 3.0-Tesla Siemens TIM Trio scanner with a 12-channel head coil. High-resolution anatomical images of the whole brain were acquired with the following parameters: TR = 2,530 ms, TE = 3.5 ms, inversion time = 1,100 ms, flip angle = 7°, 176 sagittal slices, 1 × 1 × 1 mm3 resolution. For the collection of functional images we chose a sparse-sampling design in which subsequent volume acquisitions were separated by a short period of silence (1.5 s) during which auditory stimuli could be presented in the absence of scanner noise. As can be seen in Figure 1A (lowermost panel), these frequent intermittent scans are expected to evoke BOLD responses, creating a continuously elevated level of auditory activation that can be expected to affect all volume acquisitions and conditions alike. However, because the BOLD response to experimental stimuli or the task will be measured relative to this continuously elevated baseline, the elevated level of auditory activation should neither affect comparisons across conditions nor across time points,.
To reduce signal loss due to susceptibility artifacts in vmPFC, the acquisition box was tilted 30° from the ACPC line so as to avoid intersection of MRI slices and the sinuses (Figure 1B). The parameters were: TR = 3,000 ms, TR delay = 1,500 ms, TE = 30 ms, flip angle = 90°, FOV = 192 mm, 64 × 64 matrix, 28 transversal slices of 3.5 mm thickness, resulting in functional voxels of 3 × 3 × 3.5 mm3. Note that the TR chosen for this study is shorter than what is normally used in sparse-sampling paradigms for auditory fMRI (e.g. Hall et al., 1999). This shorter TR allows us to sample the BOLD time course at multiple time points while at the same time allowing us to present auditory stimuli in silent intervals uncorrupted by scanner noise, and has the reduced benefit of requiring less scan time than stroboscopic designs with longer TRs (Belin et al., 1999).
4.4.2. Stimuli
Auditory stimuli were presented binaurally through electrostatic headphones (STAX), constructed to have a relatively flat frequency response up to 20 kHz and mounted in ear defenders (Bilsom) to provide shielding from scanner noise. Stimuli were trains of four “chirps” (band-passed noise bursts, 1/6 octave bandwidth, duration 1/6 s, with amplitude linearly increasing from 0 for the first 5 ms of the stimulus and linearly decreasing to 0 over the last 5 ms so as to avoid distortions associated with sudden onset and offset, followed by 1/6 s of silence each). Standard center frequencies were 375 Hz, 1,500 Hz, and 6,000 Hz, and, for each patient and his or her stimulus-matched control, the standard frequency closest to the patient's tinnitus frequency was replaced by a stimulus centered at the tinnitus frequency. To ensure that patient and control participant perceived the stimuli at similar levels despite possible differences in the audiogram, hearing thresholds for each stimulus were assessed immediately prior to the functional scan while the participants were already in the scanner. Stimuli were then played at a constant level above threshold (15 to 30 dB SL, depending on which intensity allowed presentation of the highest frequency without inducing noticeable sound artifacts). Two tinnitus patients were excluded from the study because significant hearing loss prevented them from hearing the highest stimulus frequency at the maximum distortion-free intensity.
4.4.3. Experimental task
To keep participants awake and attentive throughout the scan, they were given a simple oddball task. To mark each stimulation period (even those during which no auditory stimulus was played), the fixation cross changed to a circle. When the fixation cross returned at the end of the stimulation period (accompanied by scanner noise marking the next image acquisition), participants pressed a button in their right hand to indicate that they had heard either four chirps or nothing at all. On oddball trials (which made up less than 10% of the trials and were excluded from the analysis), the third of the four chirps was missing, creating a clearly audible gap, to which participants responded by pressing a button in their left hand. Each stimulation period (containing either chirps or silence) was followed by four volume acquisitions to sample the hemodynamic response at different time points. The time course of a trial is illustrated in Figure 1A. Participants completed three functional runs of about 10 minutes each, for a total of 36 trials per condition (silence, two standard frequencies, and the tinnitus patient's tinnitus frequency).
4.5. MRI data analysis
4.5.1. Preprocessing and design modeling
MRI data were analyzed using BrainVoyager QX (Brain Innovation, Inc.). Functional images were corrected for image inhomogeneities, motion-corrected to the first image of the second run, relieved of linear trend, and high-pass filtered at 3 Hz. After alignment with the anatomical images and interpolation into Talairach space (Talairach & Tournoux, 1988) at 3 × 3 × 3 mm3 resolution, they were smoothed in space using a 6-mm full-width-at-half-maximum (FWHM) Gaussian kernel.
Because of the tilted volume acquisition box (see Figure 1B) and differences in head size, the amount of brain tissue included in the functional data set differed across participants, including the entire brain for some and excluding parts of occipital and parietal cortex for others. We thus constrained our analyses to those voxels (in Talairach space) for which functional data were available for all participants. In addition, we also excluded voxels for which functional data were available, but for which the signal intensity was low (less than 100 on a scale from 0 – corresponding to black – to 255 – corresponding to white in the functional images), and functional voxels coinciding with voxels of the average anatomical image that were classified as white matter or CSF.
Statistical analyses were based on a general linear model (GLM) with separate predictors for trials with stimulation at the patient's tinnitus frequency (“tinn-stim”), trials with stimulation at the remaining two standard frequencies (“other-stim”), and trials without auditory stimulation (“no-stim”). The “no-stim” condition was modeled with a separate predictor, rather than including it in the baseline, because we expected BOLD responses related to the expectation of sound even on trials without experimental stimulation (Voisin et al., 2006). In addition, we included several “predictors of no interest” to capture the influence of oddball trials, error trials (in which participants did not respond or responded incorrectly), and between-subject variance. Each predictor (with the exception of those modeling between-subject variance) was convolved with a standard hemodynamic response function. This way, the “baseline” term of the GLM was mostly determined by the signal measured during the last volume acquisition of each trial, at which the BOLD response can be assumed to have returned to near-zero values, and by the first volume acquisition of each trial, at which the BOLD response has not started rising significantly. Unless stated otherwise, the results of contrasts “tinn-stim”, “other-stim”, and “no-stim” are reported relative to this baseline.
4.5.2. Whole-brain analyses
To identify functional ROIs potentially related to tinnitus, we performed “whole-brain analyses” including all voxels for which functional data from all participants were available. First, we tested each voxel for significant group differences regarding the BOLD responses to the two auditory stimulation conditions compared to the condition without auditory stimulation (i.e. for significant group differences regarding contrasts “tinn-stim > no-stim” and “other-stim > no-stim”) using random-effects (RFX) analyses based on the GLM described above (section 4.5.1.). This approach first uses the GLM to estimate, for each functional voxel, “beta weights” separately for each participant and predictor. These beta weights reflect how well the time course of each voxel matches the predictor's time course (which is based on the knowledge of when a certain condition was present and on the assumed time course of the hemodynamic response function). In a second step, these “summary statistics” are then compared between groups while treating the different participants as random samples from the population, which allows us to generalize our results from this particular sample of participants to the general populations from which they were drawn.
A single-voxel threshold of p < 0.005 was applied to the resulting statistical maps. We then determined a cluster size threshold using the “Cluster level statistical threshold estimator” plugin to BrainVoyager, which is based on an extension to 3D space of the approach described by Forman and colleagues (1995). This approach first estimates the inherent smoothness of the data and then uses Monte Carlo simulations to determine with which likelihood clusters of a certain size appear by chance. Only clusters whose size has a likelihood smaller than p < 0.05 of appearing by chance are deemed significant.
In addition to group differences, we also looked for correlations between tinnitus-related behavioral variables and the BOLD signal changes observed in the three stimulus conditions. Again, the analysis was performed for each voxel in the brain, and the resulting statistical maps were corrected for multiple comparisons by applying a single-voxel threshold of p < 0.005 (corresponding to correlations of r > 0.60 or r < -0.60 for the 20 tinnitus patients) and a cluster size threshold given which only clusters whose size had a likelihood smaller than p < 0.05 of appearing by chance were considered significant.
4.5.3. ROI analyses
For each ROI identified in either whole brain analysis, we then extracted average BOLD responses for the three stimulus conditions, separately for each participant. For those ROIs identified for group differences, we performed post-hoc t-tests to identify which stimulus conditions contributed to the group differences. For all ROIs, we computed the correlations between tinnitus-related behavioral variables and the tinnitus patients’ BOLD responses in all conditions. These correlations are reported in Table 3. Since a Bonferroni-correction for the large number of correlations computed here would yield even the largest correlations statistically insignificant, the reported correlations should be considered exploratory. As a criterion for which correlations should be discussed, we chose Cohen's (1988) criterion of r > 0.50 for identifying “large” correlations.
In addition, we also extracted the time-course of the BOLD response following trial start separately for the two groups and all three experimental conditions (see Figures 2C and 5C). Note that in this case, the baseline relative to which the BOLD signal changes are measured is not the same as in the GLM. Instead, the BOLD time course plots show signal changes relative to the signal measured during the first volume acquisition of the same trial, and then averaged across trials of the same condition. Because the first volume acquisition occurred 1.5 s after trial start (and, on trials with auditory stimulation, after the stimulation period), the reference signal is measured at a point in time where the BOLD response has already started to rise. Thus, the amplitude of the illustrated BOLD responses may be slightly underestimated.
4.5.4. Analyses ruling out influences of group differences other than tinnitus
Since paired t-tests revealed differences between patients and controls regarding loudness discomfort levels and hearing loss at certain frequencies, we performed additional analyses to ensure that these differences were not responsible for the observed fMRI group differences. First, we repeated the fMRI random-effects (RFX) group analyses described above (section 4.5.2.) while excluding pairs of participants whose hearing levels were mismatched most strongly (and without whom the pairwise t-tests did not show significant group differences). Second, we performed analyses including all participants’ data while including hearing loss and, in a separate analysis, noise sensitivity, as covariates. Since these additional analyses identified group differences in the same brain areas identified in the analysis including all participants and no covariates, only the results of the analysis including all participants are reported.
Supplementary Material
Figure S1. Comparison of vmPFC signal in functional data acquired in slices oriented parallel to the ACPC line (left column) and slices tilted by 30° (right column). Data were acquired only five minutes apart on the same participant, changing nothing except the slice orientation. Both data sets were transformed into Talairach space, and corresponding views are shown in both columns. Signal improvements in vmPFC are clearly visible in the left images, especially in the area highlighted by the white square. Note that this area was chosen to highlight the differences between the two acquisition schemes. It does not reflect an ROI, and the location at which we found group differences is more superior and lateral than the depicted view.
Figure S2. Average audiograms for tinnitus patients (TPs, black lines) and controls (CTs, gray lines). Hearing thresholds were assessed for each of the frequencies indicated on the x-axis.
Figure S3. Correlations between BOLD signal differences between conditions and tinnitus characteristics in the right vmPFC ROI identified for displaying group differences regarding contrast “tinn-stim > no-stim”.
Figure S4. BOLD signal changes associated with a “stim > no-stim” contrast. The highlighted voxels along the superior temporal gyri show a significantly stronger response on “tinn-stim” and “other-stim” trials than on “no-stim” trials. Note that the single-voxel threshold applied to the map shown in this figure is corrected for multiple comparisons using the Bonferroni method (i.e., dividing the tolerated error level of 0.05 by the number of voxels) and thus much stricter than the one applied to the maps shown in the main text, indicating that the activations shown here are extremely robust.
Figure S5. BOLD signal changes associated with the different experimental conditions. All conditions led to BOLD increases in the posterior medial thalamus (thal), the putamen (put), anterior insula (ins), posterior superior temporal sulcus (postSTS), along the precentral and postcentral gyri (preCG and postCG), in the pre-supplementary motor area (pre-SMA), and in the posterior cingulate gyrus (postCing). The conditions involving auditory stimulation (“other-stim” and “tinn-stim”) also resulted in significant BOLD increases along the superior temporal gyri (STG). These increases were not apparent at the same (strict) threshold in the “no-stim” trials; however, they do appear at more lenient thresholds. Note that, as in Figure S4, the single-voxel threshold applied to the map shown in this figure is corrected for multiple comparisons using the Bonferroni method and is thus much stricter than the one applied to the maps shown in the main text, indicating that the activations shown here are extremely robust.
Acknowledgments
We wish to thank Sylke-Monina Chowdhury for her technical assistance. This work was funded by the National Institutes of Health (RC1-DC010720 to JPR). ASG was supported by a Feodor-Lynen fellowship from the Alexander von Humboldt Foundation.
Abbreviations
- BOLD
blood oxygen level dependent
- CoG
center of gravity
- CSF
cerebrospinal fluid
- CT
control participant
- fMRI
functional magnetic resonance imaging
- FOV
field of view
- GAD7
Generalized Anxiety Disorder questionnaire
- GLM
general linear model
- HADS
Hospital Anxiety and Depression Scale
- IFG
inferior frontal gyrus
- LC
locus of caudate
- LDL
loudness discomfort level
- lPFC
lateral prefrontal cortex
- MGN
medial geniculate nucleus
- PHQ9
Patient Health Questionnaire
- RFX
random effects (analysis)
- ROI
region of interest
- STG
superior temporal gyrus
- TE
echo time
- THI
Tinnitus Handicap Inventory
- TP
tinnitus patient
- TR
repetition time
- TRN
thalamic reticular nucleus
- TSCHQ
Tinnitus Sample Case History Questionnaire
- VBM
voxel-based morphometry
- vmPFC
ventromedial prefrontal cortex
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions
ASG, AML, JPR, HJK, and SM designed the experiment; ASG and AML implemented the experiment; ASG, TKT, AML, and SM collected the data; ASG, AML, and TKT analyzed the data; ASG, AML, JPR, and TKT wrote the manuscript.
Appendix
Two supplementary tables and five supplementary figures can be found online.
References
- Alpini D, Cesarani A. Tinnitus as an alarm bell: stress reaction tinnitus model. Journal for Oto-Rhino-Laryngology, Head and Neck Surgery. 2006;68:31–37. doi: 10.1159/000090488. [DOI] [PubMed] [Google Scholar]
- Anderson MC, Ochsner KN, Kuhl B, Cooper J, Robertson E, Gabrieli SW, Glover GH, Gabrieli JDE. Neural systems underlying the suppression of unwanted memories. Science. 2004;303:232–235. doi: 10.1126/science.1089504. [DOI] [PubMed] [Google Scholar]
- Andersson G, Kaldo-Sandström V, Ström L, Strömgren T. Internet administration of the Hospital Anxiety and Depression Scale in a sample of tinnitus patients. J Psychosom Res. 2003;55:259–262. doi: 10.1016/s0022-3999(02)00575-5. [DOI] [PubMed] [Google Scholar]
- Belin P, Zatorre RJ, Hoge R, Evans AC, Pike B. Event related fMRI of the auditory cortex. NeuroImage. 1999;10:417–429. doi: 10.1006/nimg.1999.0480. [DOI] [PubMed] [Google Scholar]
- Cheung SW, Larson PS. Tinnitus modulation by deep brain stimulation in locus of caudate neurons (area LC). Neuroscience. 2010;169:1768–1778. doi: 10.1016/j.neuroscience.2010.06.007. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. 2nd Ed Lawrence Erlbaum Associates; Hillsdale, NJ: 1988. [Google Scholar]
- Deichmann R, Gottfried JA, Hutton C, Turner R. Optimized EPI for fMRI studies of the orbitofrontal cortex. NeuroImage. 2003;19:430–441. doi: 10.1016/s1053-8119(03)00073-9. [DOI] [PubMed] [Google Scholar]
- De Ridder D, Elgoyhen AB, Romo R, Langguth B. Phantom percepts: Tinnitus and pain as persisting aversive memory networks. Proc Natl Acad Sci USA. 2011;108:8075–8080. doi: 10.1073/pnas.1018466108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich V, Nieschalk M, Stoll W, Rajan R, Pantev C. Cortical reorganization in patients with high frequency cochlear hearing loss. Hear Res. 2001;158:95–101. doi: 10.1016/s0378-5955(01)00282-9. [DOI] [PubMed] [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magn Reson Med. 1995;33:636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Grady CL, Van Meter JW, Maisog JM, Pietrini P, Krasuski J, Rauschecker JP. Attention-related modulation of activity primary and secondary auditory cortex. Neuroreport. 1997;8:2511–2516. doi: 10.1097/00001756-199707280-00019. [DOI] [PubMed] [Google Scholar]
- Gu JW, Halpin CF, Nam EC, Levine RA, Melcher JR. Tinnitus, diminished sound level tolerance, and elevated auditory activity in humans with normal hearing sensitivity. J Neurophysiol. 2010;104:3361–3370. doi: 10.1152/jn.00226.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusnard DA, Raichle ME. Searching for a baseline: functional imaging and the resting human brain. Nat Rev Neurosci. 2001;2:685–694. doi: 10.1038/35094500. [DOI] [PubMed] [Google Scholar]
- Halford JB, Anderson SD. Anxiety and depression in tinnitus sufferers. J Psychosom Res. 1991;35:383–390. doi: 10.1016/0022-3999(91)90033-k. [DOI] [PubMed] [Google Scholar]
- Hall DA, Haggard MP, Akeroyd MA, Palmer AR, Summerfield AQ, Elliott MR, Gurney EM, Bowtell RW. “Sparse” temporal sampling in auditory fMRI. Hum Brain Map. 1999;7:213–223. doi: 10.1002/(SICI)1097-0193(1999)7:3<213::AID-HBM5>3.0.CO;2-N. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall DA, Summerfield AQ, Gonçalves MS, Foster JR, Palmer AR, Bowtell RW. Time-course of the auditory BOLD response to scanner noise. Magn ResonMed. 2000;43:601–606. doi: 10.1002/(sici)1522-2594(200004)43:4<601::aid-mrm16>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Henry JA, Dennis KC, Schechter MA. General review of tinnitus: prevalence, mechanisms, effects, and management. J Speech Lang Hear Res. 2005;48:1204–1235. doi: 10.1044/1092-4388(2005/084). [DOI] [PubMed] [Google Scholar]
- Hoffman HJ, Reed GW. Epidemiology of tinnitus. In: Snow JB, editor. Tinnitus: Theory and Management. BC Decker, Inc.; Hamilton, ON: 2004. [Google Scholar]
- Jastreboff PJ. Phantom auditory perception (tinnitus): mechanisms of generation and perception. Neurosci Res. 1990;8:221–254. doi: 10.1016/0168-0102(90)90031-9. [DOI] [PubMed] [Google Scholar]
- Kaltenbach JA, Rachel JD, Mathog TA, Zhang J, Falzarano PR, Lewandowski M. Cisplatin-induced hyperactivitiy in the dorsal cochlear nucleus and its relation to outer hair cell loss: relevance to tinnitus. J Neurophysiol. 2002;88:699–714. doi: 10.1152/jn.2002.88.2.699. [DOI] [PubMed] [Google Scholar]
- Knight RT, Scabini D, Woods DL. Prefrontal cortex gating of auditory transmission in humans. Brain Res. 1989;504:338–342. doi: 10.1016/0006-8993(89)91381-4. [DOI] [PubMed] [Google Scholar]
- Kriegeskorte N, Simmons WK, Bellgowan PSF, Baker CI. Circular analysis in systems neuroscience: the dangers of double dipping. Nat Neurosci. 2009;12:535–540. doi: 10.1038/nn.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landgrebe M, Langguth B, Rosengarth K, Braun S, Koch A, Kleinjung T, May A, de Ridder D, Hajak G. Structural brain changes in tinnitus: grey matter decrease in auditory and non-auditory brain areas. Neuroimage. 2009;46:213–218. doi: 10.1016/j.neuroimage.2009.01.069. [DOI] [PubMed] [Google Scholar]
- Langguth B, Goodey R, Azevedo A, et al. Consensus for tinnitus patient assessment and treatment outcome measurement (Tinnitus Research Initiative Meeting, Regensburg. 2007 2006 Jul; doi: 10.1016/S0079-6123(07)66050-6. Progress in Brain Research 166: Appendix. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leaver AM, Renier L, Chevillet MA, Morgan S, Kin HJ, Rauschecker JP. Dysregulation of limbic and auditory networks in tinnitus. Neuron. 2011;69:33–43. doi: 10.1016/j.neuron.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leaver AM, Seydell-Greenwald A, Turesky TK, Morgan S, Kim HJ, Rauschecker JP. Cortico-limbic morphology separates tinnitus from tinnitus distress. Front Syst Neurosci. 2012;6:21. doi: 10.3389/fnsys.2012.00021. doi:10.3389/fnsys.2012.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lévesque J, Eugène F, Joanette Y, Paquette V, Mensour B, Beaudoin G, Leroux JM, Bourgouin P, Beauregard M. Neural circuitry underlying voluntary suppression of sadness. Biol Psychiatry. 2003;53:502–510. doi: 10.1016/s0006-3223(02)01817-6. [DOI] [PubMed] [Google Scholar]
- Levine RA. Somatic (craniocervical) tinnitus and the dorsal cochlear nucleus hypothesis. Am J Otolaryngol. 2003;20:351–362. doi: 10.1016/s0196-0709(99)90074-1. [DOI] [PubMed] [Google Scholar]
- Lockwood AH, Salvi RJ, Coad ML, Towsley ML, Wack DS, Murphy BW. The functional neuroanatomy of tinnitus: Evidence for limbic system links and neural plasticity. Neurology. 1998;50:114–120. doi: 10.1212/wnl.50.1.114. [DOI] [PubMed] [Google Scholar]
- Logothetis NK. What we can do and what we cannot do with fMRI. Nature. 2008;453:869–878. doi: 10.1038/nature06976. [DOI] [PubMed] [Google Scholar]
- MacDonald AW, III, Cohen JD, Stenger VA, Carter CS. Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science. 2000;288:1835–1838. doi: 10.1126/science.288.5472.1835. [DOI] [PubMed] [Google Scholar]
- Mirz F, Gjedde A, Ishizu K, Pedersen CB. Cortical networks subserving the perception of tinnitus – a PET study. Acta Otolaryngol Suppl. 2000;543:241–243. doi: 10.1080/000164800454503. [DOI] [PubMed] [Google Scholar]
- Mühlau M, Rauschecker JP, Oestreicher E, Gaser C, Röttinger M, Wohlschläger AM, Simon F, Etgen T, Conrad B, Sander D. Structural brain changes in tinnitus. Cereb Cortex. 2006;16:1283–1288. doi: 10.1093/cercor/bhj070. [DOI] [PubMed] [Google Scholar]
- Mulders WH, Robertson D. Hyperactivity in the auditory midbrain after acoustic trauma: dependence on cochlear activity. Neuroscience. 2009;164:733–746. doi: 10.1016/j.neuroscience.2009.08.036. [DOI] [PubMed] [Google Scholar]
- Newman CW, Jacobson GP, Spitzer JB. Development of the tinnitus handicap inventory. Arch Otolaryngol Head Neck Surg. 1996;122:143–148. doi: 10.1001/archotol.1996.01890140029007. [DOI] [PubMed] [Google Scholar]
- Noreña AJ, Eggermont JJ. Changes in spontaneous neural activity immediately after an acoustic trauma: implications for neural correlates of tinnitus. Hear Res. 2003;183:137–153. doi: 10.1016/s0378-5955(03)00225-9. [DOI] [PubMed] [Google Scholar]
- Penhune VB, Zatorre RJ, MacDonald JD, Evans AC. Interhemispheric anatomical differences in human primary auditory cortex: probabilistic mapping and volume measurement from magnetic resonance scans. Cereb Cortex. 1996;6:661–672. doi: 10.1093/cercor/6.5.661. [DOI] [PubMed] [Google Scholar]
- Ploner CJ, Gaymard BM, Rivaud Péchoux S, Pierrot-Deseilligny C. The prefrontal substrate of reflexive saccade inhibition in humans. Biol Psychiatry. 2005;57:1159–1165. doi: 10.1016/j.biopsych.2005.02.017. [DOI] [PubMed] [Google Scholar]
- Rajan R. Receptor organ damage causes loss of cortical surround inhibition without topographic map plasticity. Nat Neurosci. 1998;1:138–143. doi: 10.1038/388. [DOI] [PubMed] [Google Scholar]
- Rajan R, Irvine D. Neuronal responses across cortical field A1 in plasticity induced by peripheral auditory damage. Audiol Neurootol. 1998;3:123–144. doi: 10.1159/000013786. [DOI] [PubMed] [Google Scholar]
- Rauschecker JP, Leaver AM, Mühlau M. Tuning out the noise: Limbic auditory interactions in tinnitus. Neuron. 2010;66:819–826. doi: 10.1016/j.neuron.2010.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts LE, Moffat G, Baumann M, Ward LM, Bosnyak DJ. Residual inhibition functions overlap tinnitus spectra and the region of auditory threshold shift. J Assoc Res Otolaryngol. 2008;9:417–435. doi: 10.1007/s10162-008-0136-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts LE, Eggermont JJ, Caspary DM, Shore SE, Melcher JR, Kalternbach JA. Ringing ears: the neuroscience of tinnitus. J Neurosci. 2010;10:14972–14979. doi: 10.1523/JNEUROSCI.4028-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson D, Irvine DRF. Plasticity of frequency organization in auditory cortex of adult guinea pigs with partial unilateral deafness. J Comp Neurol. 1989;282:456–471. doi: 10.1002/cne.902820311. [DOI] [PubMed] [Google Scholar]
- Sasaki K, Gemba H, Tsujimoto T. Suppression of visually initiated hand movement by stimulation of the prefrontal cortex in the monkey. Brain Res. 1989;495:100–107. doi: 10.1016/0006-8993(89)91222-5. [DOI] [PubMed] [Google Scholar]
- Schlee W, Mueller N, Hartmann T, Keil J, Lorenz I, Weisz N. Mapping cortical hubs in tinnitus. BMC Biol. 2009;7:80. doi: 10.1186/1741-7007-7-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt C, Patak M, Kröner-Herwig B. Stress and the onset of sudden hearing loss and tinnitus. Int Tinnitus J. 2000;6:41–49. [PubMed] [Google Scholar]
- Seki S, Eggermont JJ. Changes in spontaneous firing rate and neural synchrony in cat primary auditory cortex after localized tone-induced hearing loss. Hear Res. 2003;180:28–38. doi: 10.1016/s0378-5955(03)00074-1. [DOI] [PubMed] [Google Scholar]
- Shore S, Zhou J, Koehler S. Neural mechanisms underlying somatic tinnitus. Prog Brain Res. 2007;166:107–123. doi: 10.1016/S0079-6123(07)66010-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner JE, Yingling CD. Reconsideration of the cerebral mechanisms underlying selective attention and slow potential shifts. In: Attention, Voluntary Contraction and Event-related Cerebral Potentials. In: Desmedt JE, editor. Progress in Clinical Neurophysiology. Vol. 1. Karger; Basel, CH.: 1977. pp. 30–69. [Google Scholar]
- Spitzer RL, Kroenke K, Williams JBW, Löwe B. A brief measure for assessing generalized anxiety disorder. Arch Intern Med. 2006;166:1092–1097. doi: 10.1001/archinte.166.10.1092. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co planar stereotaxic atlas of the human brain. Thieme; New York: 1988. [Google Scholar]
- Tomasi D, Caparelli EC, Chang L, Ernst T. fMRI-acoustic noise alters brain activation during working memory tasks. NeuroImage. 2005;27:377–386. doi: 10.1016/j.neuroimage.2005.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voisin J, Bidet-Caulet A, Bertrand O, Fonlupt P. Listening in silence activates auditory areas: A functional magnetic resonance imaging study. J Neurosci. 2006;26:273–278. doi: 10.1523/JNEUROSCI.2967-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vul E, Harris C, Winkielman P, Pashler H. Puzzlingly high correlations in fMRI studies of emotion, personality, and social cognition. Perspect Psychol Sci. 2009;4:274–290. doi: 10.1111/j.1745-6924.2009.01125.x. [DOI] [PubMed] [Google Scholar]
- Watanabe M. Prefrontal unit activity during delayed conditional Go/No-go discrimination in the monkey. II. Relation to Go ad No-go responses. Brain Res. 1986;382:15–27. doi: 10.1016/0006-8993(86)90105-8. [DOI] [PubMed] [Google Scholar]
- Woods DL, Knight RT. Electrophysiologic evidence of increased distractability after dorsolateral prefrontal lesions. Neurology. 1986;36:212–216. doi: 10.1212/wnl.36.2.212. [DOI] [PubMed] [Google Scholar]
- Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- Zikopoulos B, Barbas H. Prefrontal projections to the thalamic reticular nucleus form a unique circuit for attentional mechanisms. J Neurosci. 2006;26:7348–7361. doi: 10.1523/JNEUROSCI.5511-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Comparison of vmPFC signal in functional data acquired in slices oriented parallel to the ACPC line (left column) and slices tilted by 30° (right column). Data were acquired only five minutes apart on the same participant, changing nothing except the slice orientation. Both data sets were transformed into Talairach space, and corresponding views are shown in both columns. Signal improvements in vmPFC are clearly visible in the left images, especially in the area highlighted by the white square. Note that this area was chosen to highlight the differences between the two acquisition schemes. It does not reflect an ROI, and the location at which we found group differences is more superior and lateral than the depicted view.
Figure S2. Average audiograms for tinnitus patients (TPs, black lines) and controls (CTs, gray lines). Hearing thresholds were assessed for each of the frequencies indicated on the x-axis.
Figure S3. Correlations between BOLD signal differences between conditions and tinnitus characteristics in the right vmPFC ROI identified for displaying group differences regarding contrast “tinn-stim > no-stim”.
Figure S4. BOLD signal changes associated with a “stim > no-stim” contrast. The highlighted voxels along the superior temporal gyri show a significantly stronger response on “tinn-stim” and “other-stim” trials than on “no-stim” trials. Note that the single-voxel threshold applied to the map shown in this figure is corrected for multiple comparisons using the Bonferroni method (i.e., dividing the tolerated error level of 0.05 by the number of voxels) and thus much stricter than the one applied to the maps shown in the main text, indicating that the activations shown here are extremely robust.
Figure S5. BOLD signal changes associated with the different experimental conditions. All conditions led to BOLD increases in the posterior medial thalamus (thal), the putamen (put), anterior insula (ins), posterior superior temporal sulcus (postSTS), along the precentral and postcentral gyri (preCG and postCG), in the pre-supplementary motor area (pre-SMA), and in the posterior cingulate gyrus (postCing). The conditions involving auditory stimulation (“other-stim” and “tinn-stim”) also resulted in significant BOLD increases along the superior temporal gyri (STG). These increases were not apparent at the same (strict) threshold in the “no-stim” trials; however, they do appear at more lenient thresholds. Note that, as in Figure S4, the single-voxel threshold applied to the map shown in this figure is corrected for multiple comparisons using the Bonferroni method and is thus much stricter than the one applied to the maps shown in the main text, indicating that the activations shown here are extremely robust.