Abstract
Purpose
Survival analysis is increasingly being used in perinatal epidemiology to assess time-varying risk factors for various pregnancy outcomes. Here we show how quantitative correction for exposure misclassification can be applied to a Cox regression model with a time-varying dichotomous exposure.
Methods
We evaluated influenza vaccination during pregnancy in relation to preterm birth among 2,267 non-malformed infants whose mothers were interviewed as part of the Slone Birth Defects Study during 2006–2011. The hazard of preterm birth was modeled using a time-varying exposure Cox regression model with gestational age as the time-scale. The effect of exposure misclassification was then modeled using a probabilistic bias analysis that incorporated vaccination date assignment. The parameters for the bias analysis were derived from both internal and external validation data.
Results
Correction for misclassification of prenatal influenza vaccination resulted in an adjusted hazard ratio (AHR) slightly higher and less precise than the conventional analysis: bias corrected AHR 1.04 [95% simulation interval 0.70, 1.52]; conventional AHR 1.00 [95% confidence interval 0.71, 1.41].
Conclusion
Probabilistic bias analysis allows epidemiologists to assess quantitatively the possible confounder-adjusted effect of misclassification of a time-varying exposure, in contrast to a speculative approach to understanding information bias.
Keywords: survival analysis, bias, preterm birth, vaccination, pregnancy, Cox regression
INTRODUCTION
In observational epidemiology, bias resulting from exposure misclassification is frequently mentioned as a study limitation, but the effect of that bias, in terms of direction and magnitude, is often not quantified (1, 2). While random error is usually depicted with confidence intervals and/or p-values, bias due to systematic errors, such as exposure misclassification, is rarely incorporated into the quantitative presentation of results. This is despite the fact that systematic error can often substantially distort estimates of association. Recall of exposures during pregnancy may be particularly prone to misclassification as exposures often occurred months or years before data collection and recall may be clouded by significant intervening events, such as the birth of a child with serious medical conditions. In fact, it has long been suspected that mothers of children with health problems recall and report exposures during pregnancy differently than mothers of children without these conditions, a bias known as “maternal recall bias” (3, 4, 5). Therefore, quantifying the error introduced by misclassification of prenatal exposure is an important pursuit in observational retrospective studies that rely on maternal recall.
Commonly, bias analysis is performed using a single set of parameter estimates, such as the sensitivity of exposure recall in cases vs. controls (2). These parameters can be derived from internal validation data or from a related external validation study (6). Alternatively, multiple sets of parameter estimates can be used, creating an array of bias-corrected measures of association (2). An extension of these kinds of sensitivity analyses is probabilistic bias analysis (7, 8, 9). Here we applied probabilistic bias analysis, with Monte Carlo sampling techniques, to a type of regression model that is increasingly used in perinatal epidemiology—Cox regression with a time-varying dichotomous exposure (10, 11, 12, 13, 14, 15). We investigated the association between influenza vaccination during pregnancy and preterm birth; previous studies on this topic have not taken into consideration the effect of inaccurate classification of influenza vaccination (16, 17, 18, 19).
METHODS
Study Population
The Slone Birth Defects Study (BDS) is an on-going case-control study conducted by the Slone Epidemiology Center since 1976. Cases include fetuses/infants diagnosed with at least one major structural malformation. Controls include live-born infants without any malformations. For the study years included in the current analysis (see below), malformed infants and a random sample of non-malformed infants were selected each month from study hospitals serving the areas surrounding Philadelphia and San Diego, as well as New Hampshire, Rhode Island, and (via the New York State Congenital Malformations Registry) parts of New York State. In Massachusetts, cases were identified through its statewide birth defect registry and controls from a population-based random sample of births. The BDS has been approved by the Institutional Review Boards of Boston University and relevant participating hospitals and centers.
Data Collection
Mothers of cases and controls were interviewed by telephone within 6 months of pregnancy completion about demographic, reproductive, medical and behavioral factors before and during pregnancy. All participants were asked to release their obstetric medical record so that key pregnancy variables, such as gestational age, could be verified.
Beginning in September 2006, mothers were asked if they received any vaccines “such as tetanus, pertussis, whooping cough, meningitis, flu shot or any other vaccine” during the period two months before the start of their last menstrual period (LMP) through the end of their pregnancy. The type and date of vaccination were ascertained, as well the setting where the vaccine was administered. If the exact date of vaccination was not known, the respondent was asked if it was the beginning, middle or end of the month and the 5th, 15th or 25th day of that month was recorded, respectively. Women who were only able to specify a more general range of dates were assigned the mid-point of these dates as their date of vaccination. Data collection beginning in September 2009 was conducted as part of the Vaccines and Medications in Pregnancy Surveillance System (VAMPSS) program (20).
Women who reported receiving an influenza vaccination during pregnancy were asked to release their vaccination record, allowing study staff to contact the vaccine provider for more details, such as the date of vaccination.
Variable Definition
Preterm birth was defined as delivery at a gestational age less than 37 weeks (<259 days). Gestational age was determined using the difference between the actual delivery date and the estimated due date based on the mother’s report. Almost all (94%) of mothers provided due dates as determined by ultrasound; the remaining due dates were calculated by adding 280 days to the start of their reported LMP.
Any influenza vaccination during pregnancy was determined by first examining the vaccination record dates available and then, if those were not available, the self-reported vaccination dates. Women were classified as exposed if their influenza vaccination date fell between their LMP and 37 weeks’ gestation. Women reporting vaccinations after 37 weeks, when there was no longer a risk of preterm birth, were classified as unexposed. Only reports of seasonal influenza vaccination were considered in exposure determination.
Variables that were included as potential confounders included 21 maternal demographic, medical, and behavioral factors previously associated with either preterm birth and/or prenatal influenza vaccination (21, 22, 23, 24, 25). Additionally, any report of other vaccinations, including Influenza A H1N1 vaccination, was considered as a possible confounder.
Study Restrictions
Interviews with mothers of both malformed and non-malformed infants were reviewed for the purpose of estimating parameters necessary for bias analysis. However, for the analysis of the association between influenza vaccination and the hazard of preterm birth, study inclusion was restricted to non-malformed infants (i.e. the “controls”) to eliminate possible confounding by the presence of a malformation. Mothers of infants with implausible gestational ages (<25 or >43 gestational weeks; n=2), those missing maternal race or age (n=11), or those for whom vaccination occurred before their LMP (n=53) were excluded from the analysis.
Conventional Statistical Analysis
We considered our study participants to constitute a retrospective cohort of pregnancies (i.e., exposure status was assessed after preterm birth outcome was known) resulting in a live-born, non-malformed infant (13, 26). Thus it was possible to model influenza vaccination anytime during pregnancy and the hazard of preterm birth using time-varying exposure Cox regression with influenza vaccination modeled as a step function, changing from 0 to 1 on the day of vaccination. Gestational age, in days beginning at LMP, was the time-scale; analyses were also conducted using gestational weeks as the time-scale for comparison. Full-term pregnancies were censored at 37 weeks. Time-varying exposure Cox regression modeling has been advocated for time-varying exposures, like influenza vaccination, and time-dependent outcomes, like preterm birth (10, 14, 27, 28). These models are effectively non-proportional-hazards models, so tests for proportionality were not performed. As a confirmatory analysis, an unadjusted odds ratio (OR) and an unadjusted hazard ratio (HR) were generated from logistic regression and time-invariant exposure Cox regression, respectively, to compare with our primary time-varying exposure unadjusted hazard ratio. Multivariable modeling only included factors associated with both preterm birth and prenatal influenza vaccination in our data.
Bias modeling parameter estimation
Positive predictive value
Positive predictive values (PPVs), the percent of self-reported influenza vaccinations during pregnancy confirmed by vaccination record, were calculated separately for mothers of preterm and full-term infants. In this analysis, confirmation of vaccine receipt was restricted to traditional vaccine providers (e.g., primary care physician, obstetrician, mid-wife) where patient-level record keeping is presumed to be available.
Among -participants with vaccination records available, 3 groups were identified: women whose vaccination record 1) confirmed influenza vaccination occurred during pregnancy; 2) confirmed influenza vaccination occurred but the administration date was outside of pregnancy; and 3) did not indicate the influenza vaccine was administered. We calculated PPV in two different ways: a more conservative (lower PPV) and a less conservative (upper PPV) approach. For the lower PPV calculation, all 3 groups constituted the denominator and group 1 constituted the numerator. Recognizing that some women may have been vaccinated but the vaccination was not recorded in the available record, upper PPVs were calculated by restricting the denominator to groups 1 and 2 with group 1 still constituting the numerator. For the upper PPV, we gave the women the benefit of the doubt and assumed they had been vaccinated by the provider they named, but that vaccination could have occurred either during their pregnancy (LMP- 37 weeks) or outside of it. The lower and upper PPV estimates provided minimums and maximums, respectively, for our parameter distributions and were averaged to calculate the mean PPVs (29).
Negative predictive value
Negative predictive values (NPVs) could not be estimated with our internal validation data as medical record confirmation of not receiving an influenza vaccination was not performed. Therefore, we calculated NPVs separately for preterm and full-term infants using the prevalence of influenza vaccination during pregnancy observed in our study and external sensitivity and specificity estimates (7, 30). These estimates were obtained from a 2007 study of 354 elderly patients in the United Kingdom (31): of those vaccinated for influenza by their health care provider in the preceding 12 months, 190/201 (95%) recalled being vaccinated (sensitivity); of those not-vaccinated by their health care provider, 138/153 (90%) recalled not being vaccinated (specificity).
Probability distributions
Beta distributions were used to model the probability density functions for the following bias parameters using previously described methods (29): preterm and full-term PPVs, sensitivity, and specificity. The 2.5th and 97.5th percentiles of the beta distributions for the PPVs were derived after calculating the distribution parameters (29).
Bias modeling data simulation
The observed dataset was simulated 100,000 times, with each simulation selecting values for the bias parameters using Monte Carlo sampling techniques to draw from the assigned density functions (7, 9, 29, 32). NPVs were calculated for each simulation using the selected sensitivity and specificity values and the observed exposure prevalence. Based upon the selected PPVs and NPVs in each simulated dataset, exposure status could change from that reported in the interview for women who only provided self-reported vaccination information; for women with confirmed vaccination dates, their exposure status was left unchanged. Women reporting no vaccination during pregnancy with LMP dates in December, January and February were exempt from exposure assignment in data simulations because the probability of assigning a vaccination date within the span of their pregnancy was lower than that for the other LMP months. Therefore, we assumed that the original unexposed status among these women was correct.
Unadjusted and adjusted hazard ratios (AHR) were modeled with the resulting simulated datasets, and, after incorporation of sampling error (Rothman, Greenland and Lash, Modern Epidemiology III, page 366 equation 19-17) (2), the 2.5th, 50th (median) and 97.5th percentile values of all simulations were reported (29). The mean prevalence of influenza vaccination in the simulated datasets, stratified by preterm birth status, was also calculated.
Assignment of influenza vaccination date
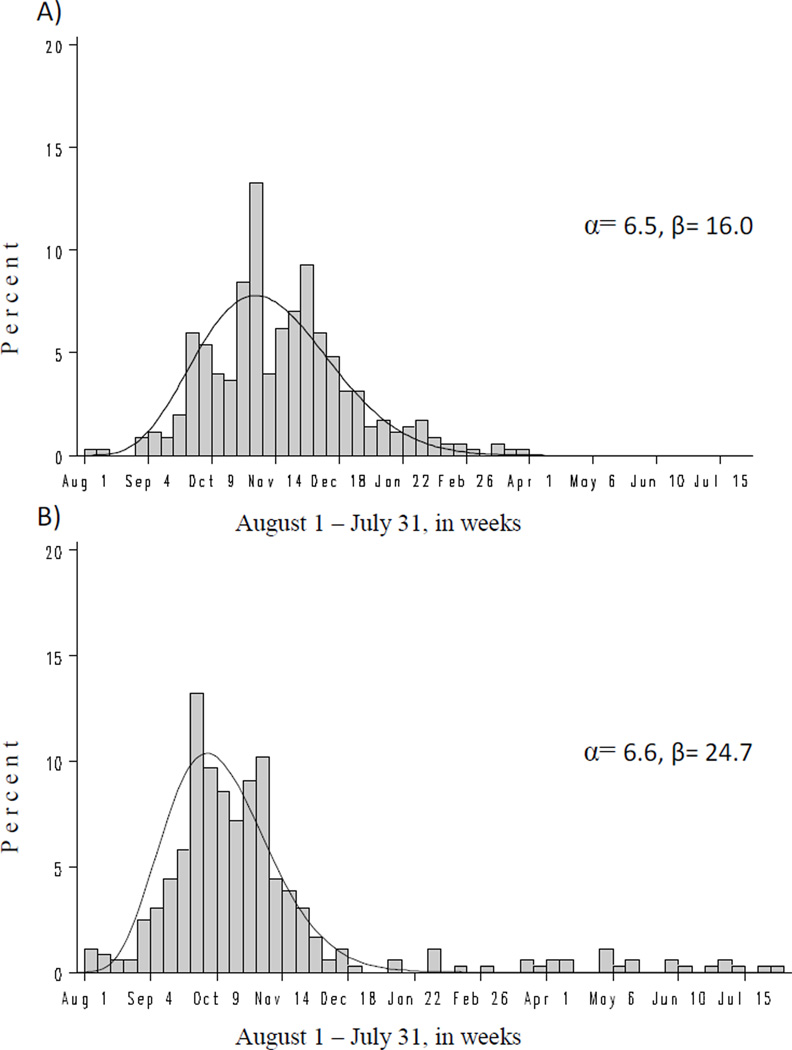
Cox regression with a time-varying exposure requires each influenza vaccination report be accompanied by a date of receipt. For the records that switched from unexposed to exposed in the data simulations, a date of vaccination was randomly selected based on the distribution of observed vaccination dates and retained if the date fell within the LMP to 37 weeks gestational age window. We anticipated the distribution of influenza vaccination dates would depend on the timing of the pregnancy because of the seasonal availability of the influenza vaccine. In fact, vaccination dates reported by women whose first prenatal visit occurred during the months August to January displayed a different pattern than those among women whose first prenatal visit occurred during the months February to July (for women without information on first prenatal visit [n=35], we assumed it occurred at 7 weeks’ gestation). Therefore, separate beta distributions were fit to these two groups of women using the least squares method (Figure 1) with the probability density covering 365 days from the pregnancy’s most proximal August 1 to the following July 31. Given the sparse data, fit of the beta distributions were determined to be adequate by study authors; however, goodness of fit tests indicated poor fit (p-values < 0.02 using the Kolmogorov-Smirnov test). Additional models further stratified by maternal age (< 30 vs. 30 plus years) and race (white vs. non-white), separately, were considered too sparse for an adequate beta distribution fit. For comparison, we also modeled the vaccination date distribution based on the empirical distribution of vaccinations.
Figure 1.
Distribution of influenza vaccinations during pregnancy by calendar week alongside modeled beta distribution (curved line) among women with first prenatal visit A) August through January and B) February through July.
RESULTS
A total of 3,346 mothers of non-malformed infants were contacted by phone between September 2006 and July 2011 and 2,333 (70%) agreed to be interviewed and their interviews had completed quality control procedures. Of these, 2,267 (97%) mothers met the study inclusion criteria. Study participants were mostly white and at least 25 years old at the time of conception (Table 1).
Table 1.
Maternal demographic, medical and behavioral factors, risk of preterm birth and prevalence of influenza vaccination during pregnancy. Slone Birth Defect Study 2006–2011 (N=2267).
| All | Preterm birth |
Influenza vaccination |
||||
|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |
| TOTAL | 2267 | 100 | 163 | 7.2 | 718 | 31.7 |
| Race/ethnicity | ||||||
| White | 1424 | 62.8 | 83 | 5.8 | 527 | 37.0 |
| Black | 219 | 9.7 | 18 | 8.2 | 43 | 19.6 |
| Hispanic or Latina | 428 | 18.9 | 47 | 11.0 | 106 | 24.8 |
| Other | 196 | 8.6 | 15 | 7.7 | 42 | 21.4 |
| Age (years) | ||||||
| <20 | 150 | 6.6 | 11 | 7.3 | 24 | 16.0 |
| 20–24 | 383 | 16.9 | 23 | 6.0 | 89 | 23.2 |
| 25–29 | 617 | 27.2 | 41 | 6.6 | 208 | 33.7 |
| 30–34 | 704 | 31.1 | 53 | 7.5 | 262 | 37.2 |
| ≥35 | 413 | 18.2 | 35 | 8.5 | 135 | 32.7 |
| Educational attainment | ||||||
| Less than grammar school | 53 | 2.3 | 4 | 7.5 | 12 | 22.6 |
| Grammar school | 178 | 7.9 | 15 | 8.4 | 39 | 21.9 |
| High school | 856 | 37.8 | 68 | 7.9 | 209 | 24.4 |
| College | 1177 | 51.9 | 76 | 6.5 | 457 | 38.8 |
| Missing | 3 | 0.1 | 0 | 0.0 | 1 | 33.3 |
| Family income | ||||||
| Less than $10,000 | 179 | 7.9 | 11 | 6.1 | 41 | 22.9 |
| $10,000 to 45,000 | 521 | 23.0 | 45 | 8.6 | 130 | 25.0 |
| $45,000 or more | 1367 | 60.3 | 91 | 6.7 | 506 | 37.0 |
| Missing | 200 | 8.8 | 16 | 8.0 | 41 | 20.5 |
| Multifetal gestation | ||||||
| Single | 2215 | 97.7 | 134 | 6.0 | 696 | 31.4 |
| Twins | 46 | 2.0 | 27 | 58.7 | 20 | 43.5 |
| Twins with miscarriage | 6 | 0.3 | 2 | 33.3 | 2 | 33.3 |
| Study center | ||||||
| Boston | 725 | 32.0 | 64 | 8.8 | 273 | 37.7 |
| New York | 332 | 14.6 | 20 | 6.0 | 120 | 36.1 |
| Philadelphia | 692 | 30.5 | 46 | 6.6 | 194 | 28.0 |
| San Diego | 518 | 22.8 | 33 | 6.4 | 131 | 25.3 |
| Infertility medication | ||||||
| Yes | 153 | 6.7 | 29 | 19.0 | 69 | 45.1 |
| No | 2114 | 93.3 | 134 | 6.3 | 649 | 30.7 |
| Illicit drug use | ||||||
| Yes | 43 | 1.9 | 6 | 14.0 | 9 | 20.9 |
| No | 2224 | 98.1 | 157 | 7.1 | 709 | 31.9 |
Conventional results
Approximately one third of women (n=718) reported receiving an influenza vaccination during pregnancy; 336 women had vaccination dates confirmed by vaccination record and the remaining 382 provided self-reported vaccination dates only. The correlation between vaccination record and self-reported vaccination dates, in terms of gestational week of exposure, was high (correlation coefficient = 0.81, p-value < 0.001). The risk of preterm birth was similar in unvaccinated and vaccinated women (7.1% [110/1549] vs. 7.4% [53/718]). Of the medical records available for review, agreement between self-reported and medical record gestational age within 1 week was 95% (773/811).
The conventional analysis AHR was 1.0 (95% confidence interval [CI] 0.71, 1.41), showing a null association between influenza vaccination during pregnancy and preterm birth; when gestational weeks were used as the time-scale instead of gestational days the AHR was nearly the same (0.98 [0.69, 1.38]). The adjusted model included the following covariates: maternal race, age, infertility treatment, illicit drug use during pregnancy, study center, and multifetal gestation (singleton or twin). The unadjusted time-varying HR of 1.11 (0.80, 1.54) was slightly higher than both the unadjusted OR of 1.04 (0.74, 1.47) and time-invariant HR of 1.03 (0.75, 1.44).
Bias parameter estimation
Most (73%) influenza vaccinations were received from traditional medical providers. Because influenza vaccination PPV estimates were similar (+/− 4%) between non-malformed and malformed infants, they were combined for bias analysis parameterization to increase precision (Table 2). The average PPVs did not differ appreciably between mothers of preterm (mean 0.88, 95% CI 0.82, 93) and full-term infants (0.85, [0.79, 0.90]). The NPVs produced by the simulated data showed nearly the same distribution for preterm (median 0.97, 95% simulation interval [SI] 0.94, 0.98) vs. full-term infants (0.97 [0.95, 0.98]).
Table 2.
Calculation of upper and lower positive predictive values (PPVs) for self-reported influenza vaccination during pregnancy in preterm and full-term infants separately and stratified by malformation status. The PPV parameter distributions provided for the bias model, along with their 95% confidence intervals (CIs), and produced by the bias model, along with their 95% simulation intervals (SIs), are also shown.
| Positive predictive values (PPV) | PPV parameter distribution provided for bias model |
PPV parameter distribution produced by bias model |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Uppera | Lowerb | Mean | Median | |||||||
| Confirmed | All | PPV | All | PPV | PPV | 95% CIc | PPV | 95% SId | ||
| Preterm | Malformed | 99 | 103 | 0.96 | 125 | 0.79 | ||||
| Non-malformed | 15 | 15 | 1.00 | 18 | 0.83 | |||||
| Both | 114 | 118 | 0.97 | 143 | 0.80 | 0.88 | 0.82,0.93 | 0.88 | 0.82, 0.93 | |
| Full-term | Malformed | 448 | 480 | 0.93 | 585 | 0.77 | ||||
| Non-malformed | 244 | 266 | 0.92 | 313 | 0.78 | |||||
| Both | 692 | 746 | 0.93 | 898 | 0.77 | 0.85 | 0.79,0.90 | 0.85 | 0.79, 0.90 | |
Upper PPV: the proportion of self-reported influenza vaccinations during pregnancy that were confirmed, among medical records with documented influenza vaccination.
Lower PPV: the proportion of self-reported influenza vaccinations during pregnancy that were confirmed, among medical records located.
95% confidence interval for beta distribution derived after calculating distribution parameters: alpha and beta. See Lash, Fox, and Fink 2009.
95% simulation interval determined from 2.5th and 97.5th percentile values in simulated data
Bias analysis results
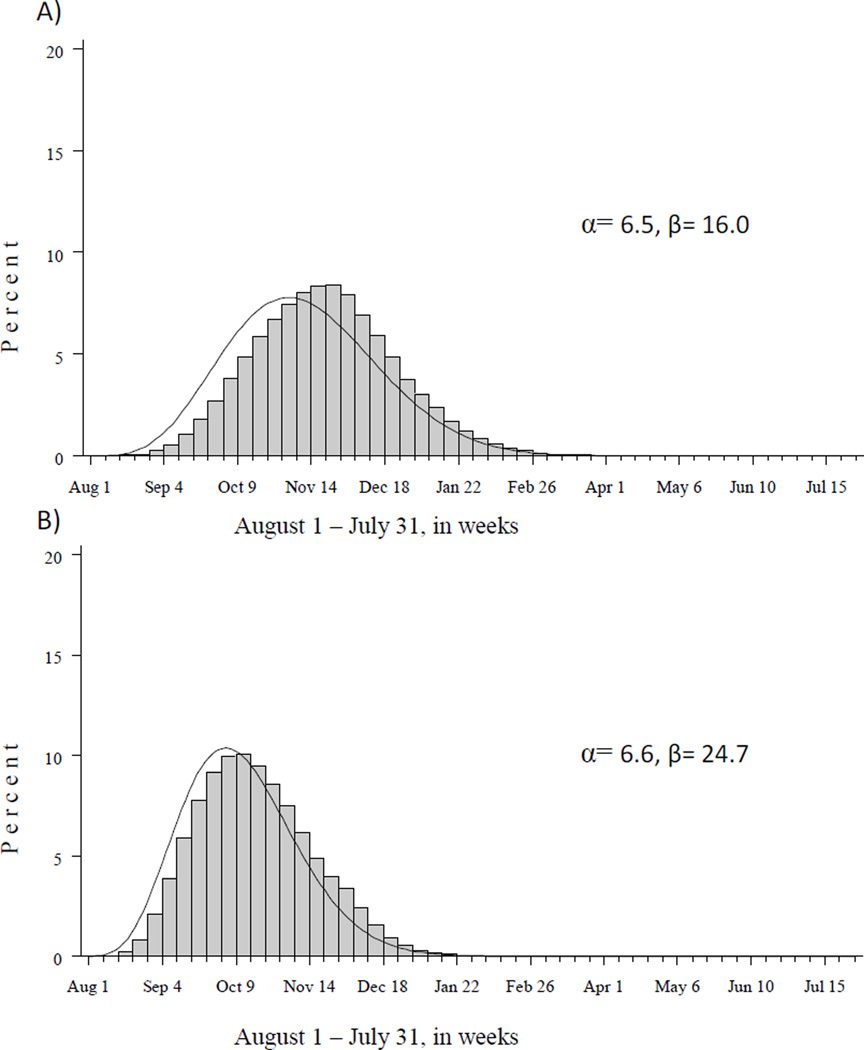
The distribution of assigned dates of influenza vaccination fell reasonably well within the beta distributions chosen for the model simulations (Figure 2), given the LMP to 37 weeks gestational age constraint. The average influenza vaccination prevalence in the simulated data was slightly lower than the observed prevalence (30.6% simulated vs. 31.6% observed).
Figure 2.
Distribution of influenza vaccination dates, by calendar week, assigned during data simulation alongside modeled beta distribution (curved line) among women with first prenatal visit A) August through January and B) February through July.
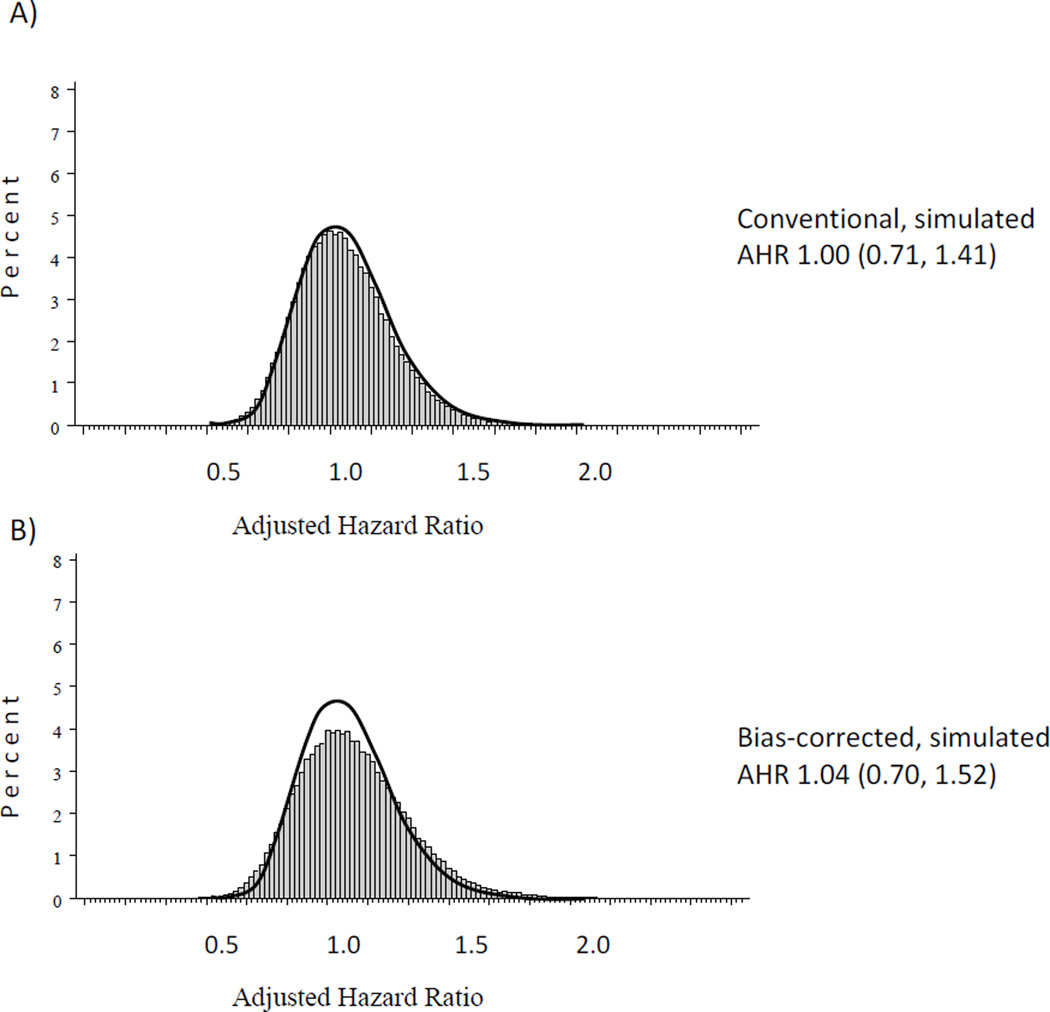
After correction for exposure misclassification, the AHR for the association between influenza vaccination during pregnancy and preterm birth increased 4.0% to 1.04 [95% SI 0.70, 1.52] (Table 3; Figure 3). In addition, 95% simulation intervals for AHRs corrected for misclassification were wider than the 95% confidence intervals from the conventional analysis (ratio of upper to lower limits were 2.2 and 2.0, respectively). When the empirical distribution of vaccination dates was used as an alternative to the modeled beta distribution, the bias-corrected AHR was the same (1.04 [0.70, 1.52]).
Table 3.
Hazard ratio (HR) of influenza vaccination during pregnancy on the risk of preterm birth in conventional analysis (with 95% confidence interval [CI]) and bias-corrected analysis (with 95% simulation interval [SI]).
| Preterm | Unadjusted | 95% CI/SI | Adjusted | 95% CI/SI | |||||
|---|---|---|---|---|---|---|---|---|---|
| Model | Exposure | n | % | n | % | HR | HRa | ||
| Whole population | 2267 | 100.0 | 163 | 7.2 | |||||
| Conventional | No influenza vaccination during pregnancy | 1549 | 68.3 | 110 | 7.1 | Reference | |||
| Any influenza vaccination during pregnancy | 718 | 31.7 | 53 | 7.4 | 1.11b | 0.80, 1.54 | 1.00 | 0.71, 1.41 | |
| Bias-corrected | No influenza vaccination during pregnancy | 1574 | 69.4 | 111 | 7.1 | Reference | |||
| Any influenza vaccination during pregnancy | 693 | 30.6 | 52 | 7.5 | 1.12 | 0.78, 1.61 | 1.04 | 0.70, 1.52 | |
Adjusted for maternal race, age, infertility treatment, illicit drug use, study center and multifetal gestation
Unadjusted odds ratio: 1.04 [0.74, 1.47]; unadjusted time-invariant HR: 1.03 [0.75, 1.44]
Figure 3.
Histograms of simulated adjusted hazard ratios (AHR) and median AHRs and 95% simulation intervals for the A) conventional and B) bias-corrected analyses of the association between influenza vaccination during pregnancy and preterm birth. The thick black line outlines the simulated conventional analysis AHR distribution. Models were adjusted for maternal race, age, infertility treatment, study center and multifetal gestation.
DISCUSSION
Correction for exposure misclassification in a confounder-adjusted Cox regression model with a time-varying dichotomous exposure can be implemented with relative ease. In our example of prenatal influenza vaccination and the risk of preterm birth, the bias-corrected AHR was slightly higher and less precise than the AHR obtained using conventional analysis. While in this instance both AHR estimates were essentially null findings, correction for this bias could result in a much greater change in effect estimates depending on the magnitude and pattern of exposure misclassification.
A variety of sensitivity analyses of maternal recall of exposures during pregnancy have been implemented as exposure misclassification is recognized as a potentially large source of error. Particularly, the misclassifications of prenatal exposures such as environmental toxicants (33, 34, 35), smoking (36, 37, 38), vasoactive exposures (39), antibiotic use (40), vitamin use (41, 42), genital tract infections (43) and prepregnancy body mass index (BMI) (44) have been examined. Various methods for evaluating bias have been employed, including assessing the statistical significance of adding validity data to multiple linear regression models (36) or stratification of study results by length of recall period (39, 43), interview method (35), or level of knowledge regarding the hypothesis under study (41). Effect estimates have also been presented after correcting for inaccurate self-reports using single parameter estimates from internal and/or external validation study data (34, 37). Some studies have estimated an unadjusted measure of association after performing simulation trials assuming different misclassification scenarios (33, 40, 42) or an adjusted one using Bayesian methods (38). The perinatal epidemiology study that comes closest to the one we present here is a confounder-adjusted probabilistic bias analysis of prepregnancy BMI on adverse pregnancy outcomes (44). However, that study evaluated pregnancy outcomes using logistic regression; the study we present is the first, to our knowledge, to extend this type of bias analysis to survival analysis with a time-varying exposure. While regression calibration remains a popular method for correcting for continuous covariates measured with error, even in time-varying exposure Cox regression models, its applicability to categorical time-varying exposures is limited (45, 46) and it has not been implemented in studies of pregnancy outcomes.
When inferring etiologic relations between prenatal exposures and pregnancy outcomes, Cox regression may be more appropriate than logistic regression (10, 14). For many adverse pregnancy outcomes, like preterm birth, low birth weight, and stillbirth, average pregnancy length is shorter in cases than in the controls. Survival analysis methods, like Cox regression, can account for that discrepancy in pregnancy length and the reduced opportunity for exposure among the cases. Further, for exposures that can change over time, time-varying exposure analysis more accurately measures the actual time exposed than simply assuming the exposure occurred for the duration of the pregnancy. In our analysis, we found the OR and time-invariant HR were approximately 7% lower than the time-varying HR, which was not a meaningful difference. However, others have shown the potential for substantial bias resulting from incorrect model selection (27, 28). This may partly explain the highly protective effect of influenza vaccination on preterm birth observed in a recent population-based Pregnancy Risk Assessment Monitoring System study that used logistic regression analysis (16, 28).
Our study imputed vaccination dates for records in the simulated datasets that were assigned to prenatal influenza vaccination. Date assignment has been performed in epidemiological studies before, particularly for longitudinal studies where outcomes may be missing (47, 48) or for infectious disease models where dates of serological events, such as seroconversion or immunological progression to more severe disease, are not observed (49, 50, 51). Our method of date assignment followed a similar approach, basing the probability distribution for date assignment on the distribution of observed data. Our assumption that the vaccination date distribution for women we observed to have been vaccinated was the same as that for women who falsely reported not receiving a vaccination may have been in error and is a limitation of our analysis.
There were several other limitations to our bias analysis. First, we used an external study conducted among elderly persons in the United Kingdom to estimate sensitivity and specificity of self-reported influenza vaccination (31). Ideally, estimates of these classification parameters from an internal validation study would have been preferred, but that was infeasible, as determination of true influenza vaccination status for all subjects was not possible in our study population. Alternatively, an external validation study conducted among pregnant women in the United States would have been preferable, but this was not available. That being said, calculation of the PPV using our study’s influenza vaccination exposure prevalence and the 2007 UK study’s sensitivity and specificity estimates produced a value (0.83 [95% CI 0.79, 0.85]) (7, 30) that was similar to the overall PPV we found (0.85 [0.83, 0.86]), helping to justify the transportability of the UK study. Second, we modeled influenza vaccination over time as a step function, changing from 0 to 1 on the day of vaccination; alternative functions could have been applied to model the change in influenza vaccination over time (52). Third, our choice of beta distributions to model the observed vaccination date distributions may be questioned given the statistical lack of fit. A larger data set, collected by self-report during Behavioral Risk Factor Surveillance System interviews in 2008–2010, showed a clearer right-skewed unimodal distribution, with vaccinations beginning in August, peaking in late fall, and diminishing by January (53). Despite our sparse data, we still felt it was appropriate to create separate beta distributions based on timing of first prenatal visit because of the differential availability of vaccinations by calendar month. Modeling the influenza vaccination dates using beta distributions resulted in the same bias-corrected AHR as we found using an empirical distribution, leading us to believe that choice of vaccination date distribution probably had little effect on the final results. Indeed, on average, only 1.4% of records in the simulated data contained assigned vaccination dates. However, the effect of the exposure distribution model choice could be much greater in analyses involving a higher proportion of imputed data.
Additional bias study limitations include the use of the observed prevalence of exposure in the NPV calculations, even when it was that very prevalence that we were evaluating in the bias analysis, and calculating PPV estimates solely using preterm vs. full-term status, rather than a more complicated model that incorporated other maternal factors. Although our study included over 2000 mothers of non-malformed infants, we were limited in power by the small number of preterm births and were concerned about over-specifying our models.
In conclusion, our study is the first to implement probabilistic bias analysis to address exposure misclassification in a Cox regression model with a time-varying exposure. Other studies examining exposures during pregnancy assessed by retrospective maternal recall could consider using this approach to evaluate the quantitative impact of exposure misclassification. In particular, our methods are applicable to one-time or intermittent exposures during pregnancy and pregnancy outcomes that differ in average gestational length, such as preterm birth, low birth weight or stillbirth.
Acknowledgements
We thank Dawn Jacobs, Paula Wilder, Rita Krolak, Fiona Rice, Lindsay Andrus, Kathleen Sheehan, Clare Coughlin, Moira Quinn, Laurie Cincotta, Mary Thibeault, Nancy Rodriguez, Laine Catlin, Ileana Gatica, and Nastia Dynkin for their assistance in data collection and computer programming. We also thank all the women who participated in the study. During the drafting of this manuscript, Katherine Ahrens was a pre-doctoral trainee supported by NIH T32 HD052458 (Boston University Reproductive, Perinatal and Pediatric Epidemiology training program). This project has been funded in whole or in part with Federal funds from the Office of the Assistant Secretary for Preparedness and Response, Biomedical Advanced Research and Development Authority, Department of Health and Human Services, under Contract No. HHSO100201000038C. Data collection was also supported by the following grants: AHRQ 1R18HS018463-01, NICHD 1R01 HD059861, and NICHD 2 R01 HD46595.
List of abbreviations
- AHR
Adjusted hazard ratio
- AOR
Adjusted odds ratio
- BDS
Slone Birth Defects Study
- CI
Confidence interval
- H1N1
Influenza A H1N1
- HR
Hazard ratio
- LMP
Last menstrual period
- NPV
Negative predictive value
- OR
Odds ratio
- PPV
Positive predictive value
- SI
Simulation interval
- VAMPSS
Vaccines and Medications in Pregnancy Surveillance System
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
A preliminary version of this analysis was presented at the Advanced Methods Workshop during the 2011 meeting of the Society for Pediatric and Perinatal Epidemiologic Research (SPER) in Montreal, Canada.
References
- 1.Fox MP. Creating a demand for bias analysis in epidemiological research. J Epidemiol Community Health. 2009;63:91. doi: 10.1136/jech.2008.082420. [DOI] [PubMed] [Google Scholar]
- 2.Greenland S, Lash T. Chapter 19: Bias Analysis. In: Rothman K, Greenland S, Lash T, editors. Modern Epidemiology III. 2008. pp. 345–381. [Google Scholar]
- 3.MacMahon B, Pugh T, Ipsen J. Epidemiologic Methods. Boston: Little, Brown; 1960. [Google Scholar]
- 4.Mitchell A. Studies of drug-induced birth defects. In: Strom B, editor. Pharmacoepidemiology. Chichester, England: John Wiley & Sons, Ltd; 2012. [Google Scholar]
- 5.Werler MM. Birth Defects. In: Louis GMB, Platt RW, editors. Reproductive and Perinatal Epidemiology. New York: Oxford Univ Press; 2011. pp. 186–203. [Google Scholar]
- 6.Lash T, Fox M, Fink A. Applying Quantitative Bias Analysis to Epidemiologic Data. New York, NY: Springer Science+Business Media; 2009. Data Sources for Bias Analysis; pp. 33–42. [Google Scholar]
- 7.Lash T, Fox M, Fink A. Applying Quantitative Bias Analysis to Epidemiologic Data. New York, NY: Springer Science+Business Media; 2009. [Google Scholar]
- 8.Fox MP, Lash TL, Greenland S. A method to automate probabilistic sensitivity analyses of misclassified binary variables. Int J Epidemiol. 2005;34:1370–1376. doi: 10.1093/ije/dyi184. [DOI] [PubMed] [Google Scholar]
- 9.Lash T, Fink A. Semi-automated sensitivity analysis to assess systematic errors in observational data. Epidemiology. 2003;14:451–458. doi: 10.1097/01.EDE.0000071419.41011.cf. [DOI] [PubMed] [Google Scholar]
- 10.Platt RW. The Fetuses-at-Risk Approach: An Evolving Paradigm. In: Louis GMB, Platt RW, editors. Reproductive and Perinatal Epidemiology. New York: Oxford Univ Press; 2011. pp. 262–275. [Google Scholar]
- 11.Platt RW, Joseph KS, Ananth CV, et al. A Proportional Hazards Model with Time-dependent Covariates and Time-varying Effects for Analysis of Fetal and Infant Death. Am J Epidemiol. 2004;160:199–206. doi: 10.1093/aje/kwh201. [DOI] [PubMed] [Google Scholar]
- 12.Strand LB, Barnett AG, Tong S. Maternal exposure to ambient temperature and the risk of preterm birth and stillbirth in Brisbane, Australia. Am J Epidemiol. 2012;175:99–107. doi: 10.1093/aje/kwr404. [DOI] [PubMed] [Google Scholar]
- 13.Hernandez-Diaz S, Werler M, Louik C, et al. Risk of gestational hypertension in relation to folic acid supplementation during pregnancy. Am J Epidemiol. 2002;156:806–812. doi: 10.1093/aje/kwf129. [DOI] [PubMed] [Google Scholar]
- 14.O'Neill M, Hertz-Picciotto I, Pastore L, et al. Have studies of urinary tract infection and preterm delivery used the most appropriate methods? Paediatr Perinat Epidemiol. 2003;17:226–233. doi: 10.1046/j.1365-3016.2003.00499.x. [DOI] [PubMed] [Google Scholar]
- 15.Suh YJ, Kim H, Seo JH, et al. Different effects of PM 10 exposure on preterm birth by gestational period estimated from time-dependent survival analyses. Int Arch Occup Environ Health. 2009;82:613–621. doi: 10.1007/s00420-008-0380-7. [DOI] [PubMed] [Google Scholar]
- 16.Omer SB, Goodman D, Steinhoff MC, et al. Maternal Influenza Immunization and Reduced Likelihood of Prematurity and Small for Gestational Age Births: A Retrospective Cohort Study. PLoS Medicine. 2011;8 doi: 10.1371/journal.pmed.1000441. e1000441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Black S, Shinefield H, France E, et al. Effectiveness of influenza vaccine during pregnancy in preventing hospitalizations and outpatient visits for respiratory illness in pregnant women and their infants. Am J Perinatol. 2004;21:333–339. doi: 10.1055/s-2004-831888. [DOI] [PubMed] [Google Scholar]
- 18.France E, Smith-Ray R, McClure D, et al. Impact of maternal influenza vaccination during pregnancy on the incidence of acute respiratory illness visits among infants. Arch Pediatr Adolesc Med. 2006;160:1277–1283. doi: 10.1001/archpedi.160.12.1277. [DOI] [PubMed] [Google Scholar]
- 19.Munoz F, Greisinger A, Wehmanen O, et al. Safety of influenza vaccination during pregnancy. Am J Obstet Gynecol. 2005;192:1098–1106. doi: 10.1016/j.ajog.2004.12.019. [DOI] [PubMed] [Google Scholar]
- 20.Schatz M, Chambers CD, Jones KL, et al. Safety of influenza immunizations and treatment during pregnancy: the Vaccines and Medications in Pregnancy Surveillance System. Am J Obstet Gynecol. 2011;204:S64–S68. doi: 10.1016/j.ajog.2011.01.047. [DOI] [PubMed] [Google Scholar]
- 21.Goldenberg R, Culhane J, Iams J, et al. Epidemiology and causes of preterm birth. The Lancet. 2008;371:75–84. doi: 10.1016/S0140-6736(08)60074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Romero R, Espinoza J, Kusanovic J, et al. The preterm parturition syndrome. BJOG: An International Journal of Obstetrics & Gynaecology. 2006;113:17–42. doi: 10.1111/j.1471-0528.2006.01120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ahluwalia I, Harrison L, Jamieson D, et al. Receipt of Influenza Vaccine during Pregnancy among Women with Live Births—Georgia and Rhode Island, 2004–2007. Morbidity and mortality weekly report Recommendations and reports/Centers for Disease Control. 2009;58:972–975. [PubMed] [Google Scholar]
- 24.Kim H, Raymond P, Paine V, et al. Seasonal influenza vaccination coverage among pregnant women in Rhode Island. Medicine & Health/Rhode Island. 2009;92:345–347. [PubMed] [Google Scholar]
- 25.Fridman D, Steinberg E, Azhar E, et al. Predictors of H1N1 vaccination in pregnancy. Am J Obstet Gynecol. 2011;204:S124–S127. doi: 10.1016/j.ajog.2011.04.011. [DOI] [PubMed] [Google Scholar]
- 26.Hernandez R, Mitchell A, Werler M. Decongestant Use During Pregnancy and its Association with Preterm Delivery. Birth Defects Research Part A, Clinical and Molecular Teratology. 2010;88:715–721. doi: 10.1002/bdra.20699. [DOI] [PubMed] [Google Scholar]
- 27.Beyersmann J, Wolkewitz M, Schumacher M. The impact of time-dependent bias in proportional hazards modelling. Stat Med. 2008;27:6439–6454. doi: 10.1002/sim.3437. [DOI] [PubMed] [Google Scholar]
- 28.Nunes A, Gregory A, Houseman E, et al. Time-dependent bias on non-binary exposures: examples in perinatal epidemiology. Presented at Third North American Congress of Epidemiology; June 21–24 2011; Montreal, Canada. 2011. [Google Scholar]
- 29.Lash T, Fox M, Fink A. Applying Quantitative Bias Analysis to Epidemiologic Data. New York, NY: Springer Science+Business Media; 2009. Probabilistic Bias Analysis; pp. 117–150. [Google Scholar]
- 30.Lash T, Fox M, Fink A. Applying Quantitative Bias Analysis to Epidemiologic Data Errata, 2010. ( http://docs.google.com/viewer?a=v&pid=sites&srcid=ZGVmYXVsdGRvbWFpbnxiaW FzYW5hbHlzaXN8Z3g6NTdhOTYzMWE4YzkzNGEx).
- 31.Mangtani P, Shah A, Roberts J. Validation of influenza and pneumococcal vaccine status in adults based on self-report. Epidemiol Infect. 2007;135:139–143. doi: 10.1017/S0950268806006479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lash T, Schmidt M, Jensen A, et al. Methods to apply probabilistic bias analysis to summary estimates of association. Pharmacoepidemiology and Drug Safety. 2010;19:638–644. doi: 10.1002/pds.1938. [DOI] [PubMed] [Google Scholar]
- 33.Wright JM, Bateson TF. A sensitivity analysis of bias in relative risk estimates due to disinfection by-product exposure misclassification. Journal of Exposure Science and Environmental Epidemiology. 2004;15:212–216. doi: 10.1038/sj.jea.7500389. [DOI] [PubMed] [Google Scholar]
- 34.Rull RP, Ritz B, Shaw GM. Validation of self-reported proximity to agricultural crops in a case–control study of neural tube defects. Journal of Exposure Science and Environmental Epidemiology. 2005;16:147–155. doi: 10.1038/sj.jea.7500444. [DOI] [PubMed] [Google Scholar]
- 35.Hertz-Picciotto I, Swan SH, Neutra RR. Reporting bias and mode of interview in a study of adverse pregnancy outcomes and water consumption. Epidemiology. 1992:104–112. doi: 10.1097/00001648-199203000-00007. [DOI] [PubMed] [Google Scholar]
- 36.Almeida ND, Koren G, Platt RW, et al. Hair Biomarkers as Measures of Maternal Tobacco Smoke Exposure and Predictors of Fetal Growth. Nicotine & Tobacco Research. 2011;13:328–335. doi: 10.1093/ntr/ntq259. [DOI] [PubMed] [Google Scholar]
- 37.England LJ, Grauman A, Qian C, et al. Misclassification of maternal smoking status and its effects on an epidemiologic study of pregnancy outcomes. Nicotine & Tobacco Research. 2007;9:1005–1013. doi: 10.1080/14622200701491255. [DOI] [PubMed] [Google Scholar]
- 38.MacLehose RF, Olshan AF, Herring AH, et al. Bayesian methods for correcting misclassification: an example from birth defects epidemiology. Epidemiology. 2009;20:27–35. doi: 10.1097/EDE.0b013e31818ab3b0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Werler MM, Sheehan JE, Hayes C, et al. Vasoactive exposures, vascular events, and hemifacial microsomia. Birth Defects Research Part A: Clinical and Molecular Teratology. 2004;70:389–395. doi: 10.1002/bdra.20022. [DOI] [PubMed] [Google Scholar]
- 40.Lyles RH, Zhang F, Drews-Botsch C. Combining internal and external validation data to correct for exposure misclassification: a case study. Epidemiology. 2007;18:321–328. doi: 10.1097/01.ede.0000260004.49431.70. [DOI] [PubMed] [Google Scholar]
- 41.Werler MM, Shapiro S, Mitchell AA. Periconceptional folic acid exposure and risk of occurrent neural tube defects. JAMA: the journal of the American Medical Association. 1993;269:1257–1261. [PubMed] [Google Scholar]
- 42.Jurek AM, Maldonado G, Spector L, et al. Periconceptional maternal vitamin supplementation and childhood leukaemia: an uncertainty analysis. J Epidemiol Community Health. 2009;63:168–172. doi: 10.1136/jech.2008.080226. [DOI] [PubMed] [Google Scholar]
- 43.Carter TC, Olney RS, Mitchell AA, et al. Maternal self reported genital tract infections during pregnancy and the risk of selected birth defects. Birth Defects Research Part A: Clinical and Molecular Teratology. 2011 doi: 10.1002/bdra.20749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bodnar LM, Siega-Riz AM, Simhan HN, et al. The impact of exposure misclassification on associations between prepregnancy BMI and adverse pregnancy outcomes. Obesity. 2010 doi: 10.1038/oby.2010.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liao X, Zucker DM, Li Y, et al. Survival Analysis with Error-Prone Time-Varying Covariates: A Risk Set Calibration Approach. Biometrics. 2011;67:50–58. doi: 10.1111/j.1541-0420.2010.01423.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fraser GE, Stram DO. Regression Calibration When Foods (Measured With Error) Are the Variables of Interest: Markedly Non-Gaussian Data With Many Zeroes. Am J Epidemiol. 2012;175:325–331. doi: 10.1093/aje/kwr316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dubois MF, Hebert R. Imputation of missing dates of death or institutionalization for time-to-event analyses in the Canadian Study of Health and Aging. Int Psychogeriatr. 2001;13:91–97. doi: 10.1017/s1041610202008025. [DOI] [PubMed] [Google Scholar]
- 48.Howard G, McClure LA, Moy CS, et al. Imputation of incident events in longitudinal cohort studies. Am J Epidemiol. 2011;174:718–726. doi: 10.1093/aje/kwr155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Taylor JMG, Muñoz A, Bass SM, et al. Estimating the distribution of times from HIV seroconversion to AIDS using multiple imputation. Stat Med. 1990;9:505–514. doi: 10.1002/sim.4780090504. [DOI] [PubMed] [Google Scholar]
- 50.Taffé P, May M. A joint back calculation model for the imputation of the date of HIV infection in a prevalent cohort. Stat Med. 2008;27:4835–4853. doi: 10.1002/sim.3294. [DOI] [PubMed] [Google Scholar]
- 51.Geskus RB. Methods for estimating the AIDS incubation time distribution when date of seroconversion is censored. Stat Med. 2001;20:795–812. doi: 10.1002/sim.700. [DOI] [PubMed] [Google Scholar]
- 52.Fisher L, Lin D. Time-dependent covariates in the Cox proportional-hazards regression model. Annu Rev Public Health. 1999;20:145–157. doi: 10.1146/annurev.publhealth.20.1.145. [DOI] [PubMed] [Google Scholar]
- 53.Centers for Disease Control and Prevention. Interim Results: State-Specific Seasonal Influenza Vaccination Coverage --- United States, August 2009--January 2010. Morbidity and mortality weekly report Recommendations and reports/Centers for Disease Control. 2010;59:477–484. [PubMed] [Google Scholar]