Abstract
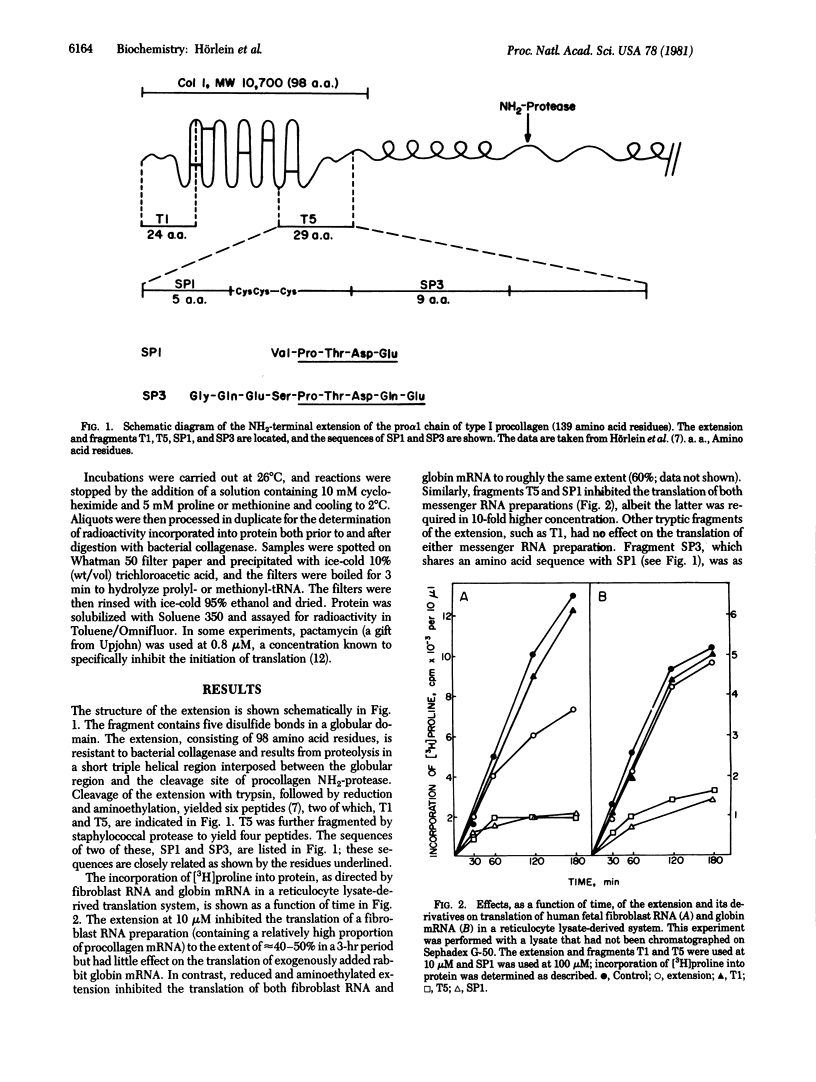
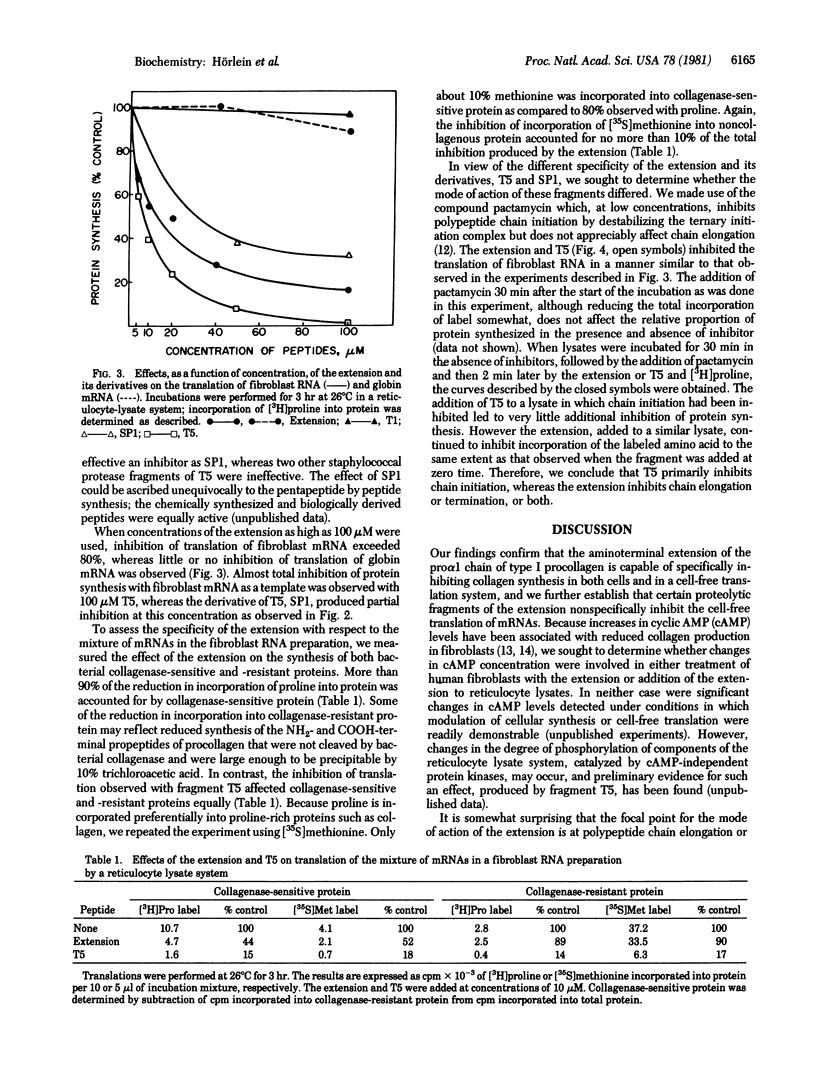
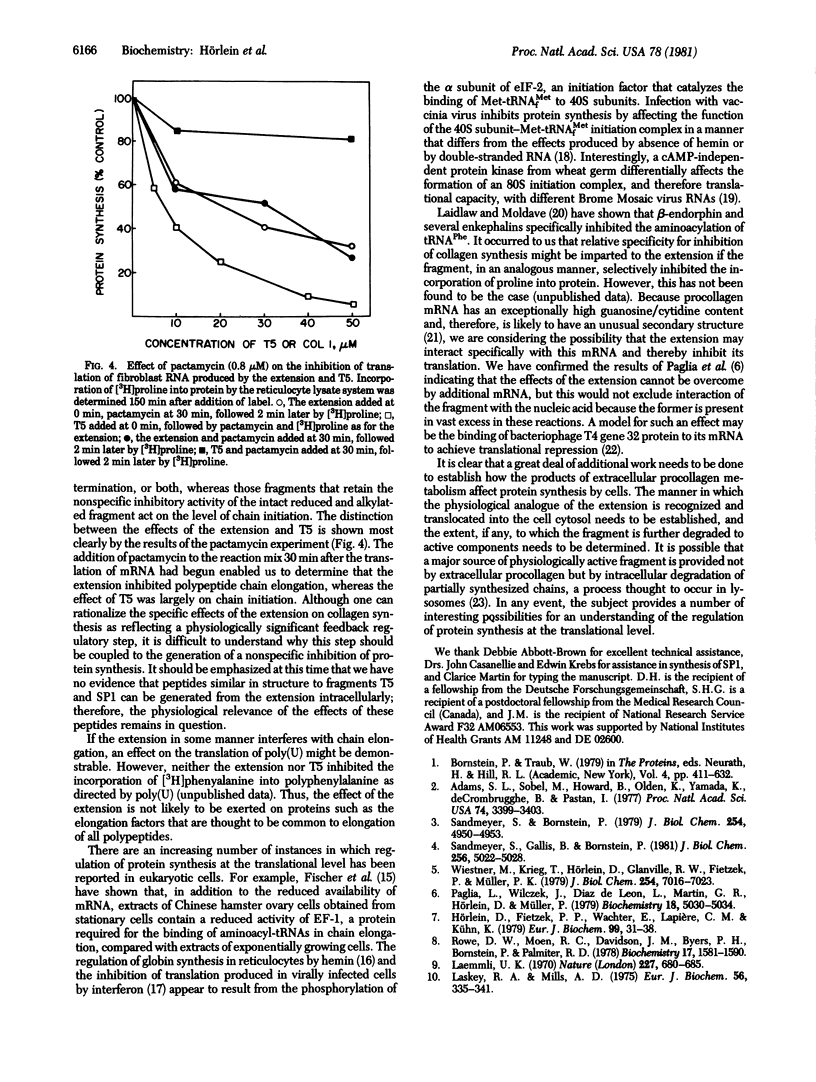
Previous studies have shown that a type I procollagen-derived peptide, called pN alpha 1(I)-Col 1, selectively inhibits collagen synthesis by fibroblasts in culture and the translation of procollagen mRNA in a rabbit reticulocyte lysate system. We prepared the 10,700-dalton peptide from dermatosparactic calf skin, which contained high levels of incompletely processed type I procollagen, by collagenase digestion. Time-course and dose-response studies showed that the peptide specifically inhibited the translation of procollagen mRNA in preparations of human fibroblast RNA while not affecting the translation of globin mRNA or of other messenger RNAs in fibroblast RNA. After reduction and alkylation, the peptide lost its specificity but became a nonspecific inhibitor of translation. Enzymatic cleavage enabled us to localize the nonspecific activity to a short sequence -Pro-Thr-Asp-Glu, an assignment confirmed by peptide synthesis. Using pactamycin, a specific inhibitor of translational initiation, we showed that the intact peptide acts on procollagen mRNA translation by inhibition of polypeptide chain elongation or termination, or both, whereas the nonspecific inhibitory activity of the unfolded peptide and its derivatives can be attributed to an inhibition of chain initiation. Although the native peptide may function in feedback regulation of collagen synthesis, the physiological role of the lower molecular weight fragments, if any, is uncertain.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams S. L., Sobel M. E., Howard B. H., Olden K., Yamada K. M., de Crombrugghe B., Pastan I. Levels of translatable mRNAs for cell surface protein, collagen precursors, and two membrane proteins are altered in Rous sarcoma virus-transformed chick embryo fibroblasts. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3399–3403. doi: 10.1073/pnas.74.8.3399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baglioni C. Interferon-induced enzymatic activities and their role in the antriviral state. Cell. 1979 Jun;17(2):255–264. doi: 10.1016/0092-8674(79)90151-x. [DOI] [PubMed] [Google Scholar]
- Baum B. J., Moss J., Breul S. D., Berg R. A., Crystal R. G. Effect of cyclic AMP on the intracellular degradation of newly synthesized collagen. J Biol Chem. 1980 Apr 10;255(7):2843–2847. [PubMed] [Google Scholar]
- Baum B. J., Moss J., Breul S. D., Crystal R. G. Association in normal human fibroblasts of elevated levels of adenosine 3':5'-monophosphate with a selective decrease in collagen production. J Biol Chem. 1978 May 25;253(10):3391–3394. [PubMed] [Google Scholar]
- Berg R. A., Schwartz M. L., Crystal R. G. Regulation of the production of secretory proteins: intracellular degradation of newly synthesized "defective" collagen. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4746–4750. doi: 10.1073/pnas.77.8.4746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell P. J., Balkow K., Hunt T., Jackson R. J., Trachsel H. Phosphorylation of initiation factor elF-2 and the control of reticulocyte protein synthesis. Cell. 1977 May;11(1):187–200. doi: 10.1016/0092-8674(77)90330-0. [DOI] [PubMed] [Google Scholar]
- Fischer I., Arfin S. M., Moldave K. Regulation of translation. Analysis of intermediary reactions in protein synthesis in exponentially growing and stationary phase Chinese hamster ovary cells in culture. Biochemistry. 1980 Apr 1;19(7):1417–1425. doi: 10.1021/bi00548a024. [DOI] [PubMed] [Google Scholar]
- Fuller F., Boedtker H. Sequence determination and analysis of the 3' region of chicken pro-alpha 1(I) and pro-alpha 2(I) collagen messenger ribonucleic acids including the carboxy-terminal propeptide sequences. Biochemistry. 1981 Feb 17;20(4):996–1006. doi: 10.1021/bi00507a054. [DOI] [PubMed] [Google Scholar]
- Hörlein D., Fietzek P. P., Wachter E., Lapière C. M., Kühn K. Amino acid sequence of the aminoterminal segment of dermatosparactic calf-skin procollagen type I. Eur J Biochem. 1979 Aug 15;99(1):31–38. doi: 10.1111/j.1432-1033.1979.tb13227.x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laidlaw S. A., Moldave K. The effects of beta-endorphin and enkephalins on protein biosynthesis in a eukaryotic cell-free system. Inhibition of phenylalanyl-tRNA synthetase. J Biol Chem. 1980 Dec 25;255(24):11908–11913. [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Lemaire G., Gold L., Yarus M. Autogenous translational repression of bacteriophage T4 gene 32 expression in vitro. J Mol Biol. 1978 Nov 25;126(1):73–90. doi: 10.1016/0022-2836(78)90280-2. [DOI] [PubMed] [Google Scholar]
- Lodish H. G., Housman D., Jacobsen M. Initiation of hemoglobin synthesis. Specific inhibition by antibiotics and bacteriophage ribonucleic acid. Biochemistry. 1971 Jun 8;10(12):2348–2356. doi: 10.1021/bi00788a027. [DOI] [PubMed] [Google Scholar]
- Paglia L., Wilczek J., de Leon L. D., Martin G. R., Hörlein D., Müller P. Inhibition of procollagen cell-free synthesis by amino-terminal extension peptides. Biochemistry. 1979 Oct 30;18(22):5030–5034. doi: 10.1021/bi00589a034. [DOI] [PubMed] [Google Scholar]
- Palmiter R. D., Davidson J. M., Gagnon J., Rowe D. W., Bornstein P. NH2-terminal sequence of the chick proalpha1(I) chain synthesized in the reticulocyte lysate system. Evidence for a transient hydrophobic leader sequence. J Biol Chem. 1979 Mar 10;254(5):1433–1436. [PubMed] [Google Scholar]
- Person A., Ben-Hamida F., Beaud G. Inhibition of 40S--Met--tRNAfMet ribosomal initiation complex formation by vaccinia virus. Nature. 1980 Sep 25;287(5780):355–357. doi: 10.1038/287355a0. [DOI] [PubMed] [Google Scholar]
- Rowe D. W., Moen R. C., Davidson J. M., Byers P. H., Bornstein P., Palmiter R. D. Correlation of procollagen mRNA levels in normal and transformed chick embryo fibroblasts with different rates of procollagen synthesis. Biochemistry. 1978 May 2;17(9):1581–1590. doi: 10.1021/bi00602a001. [DOI] [PubMed] [Google Scholar]
- Rychlik W., Kupidlowska E., Nowak E., Zagórski W. Wheat germ protein kinase affects the translation of Brome Mosaic virus ribonucleic acid in vitro. Biochemistry. 1980 Nov 11;19(23):5249–5255. doi: 10.1021/bi00564a015. [DOI] [PubMed] [Google Scholar]
- Sandmeyer S., Bornstein P. Declining procollagen mRNA sequences in chick embryo fibroblasts infected with rous sarcoma virus. Correlation with procollagen synthesis. J Biol Chem. 1979 Jun 25;254(12):4950–4953. [PubMed] [Google Scholar]
- Sandmeyer S., Gallis B., Bornstein P. Coordinate transcriptional regulation of type I procollagen genes by Rous sarcoma virus. J Biol Chem. 1981 May 25;256(10):5022–5028. [PubMed] [Google Scholar]
- Wiestner M., Krieg T., Hörlein D., Glanville R. W., Fietzek P., Müller P. K. Inhibiting effect of procollagen peptides on collagen biosynthesis in fibroblast cultures. J Biol Chem. 1979 Aug 10;254(15):7016–7023. [PubMed] [Google Scholar]



