Abstract
Overexpression of pruritogens and their precursors may contribute to the sensitization of histamine-dependent and –independent itch-signaling pathways in chronic itch. We presently investigated self- and cross-sensitization of scratching behavior elicited by various pruritogens, and their effects on primary sensory neurons. The MrgprC11 agonist BAM8–22 exhibited self- and reciprocal cross-sensitization of scratching evoked by the protease-activated receptor-2 (PAR-2) agonist SLIGRL. The MrgprA3 agonist chloroquine unidirectionally cross-sensitized BAM8–22-evoked scratching. Histamine unidirectionally cross-sensitized scratching evoked by chloroquine and BAM8–22. SLIGRL unidirectionally cross-sensitized scratching evoked by chloroquine. Dorsal root ganglion (DRG) cells responded to various combinations of pruritogens and algogens. Neither chloroquine, BAM8–22 nor histamine had any effect on responses of DRG cell responses to subsequently applied pruritogens, implying that their behavioral self- and cross-sensitization effects are mediated indirectly. SLIGRL unilaterally cross-sensitized responses of DRG cells to chloroquine and BAM8–22, consistent with the behavioral data. These results indicate that unidirectional cross-sensitization of histamine-independent itch-signaling pathways might occur at a peripheral site through PAR-2. PAR-2 expressed in pruriceptive nerve endings is a potential target to reduce sensitization associated with chronic itch.
Chronic itch is a burdensome clinical problem that decreases the quality of life (Weisshaar et al. 2006). The mechanisms underlying chronic itch appear to involve sensitization of itch signaling pathways, as manifested by hyperknesis (enhanced itch) and alloknesis (touch-evoked itch) (Akiyama et al. 2011; 2010; Akiyama et al. 2012; Ikoma et al. 2003; Simone et al. 1991). These features of sensitization are observed for both histamine-dependent and –independent types of itch-related scratching behavior in animal studies (Akiyama et al. 2010; Akiyama et al. 2012), but the precise mechanisms are still unknown.
Histamine is a well-known itch mediator and is elevated in the skin of atopic dermatitis patients (Johnson et al. 1960), although its primary role in chronic itch is suspect (Heyer et al. 1989; Heyer et al. 1998). Recent studies reveal two distinct histamine-independent itch pathways that are mediated by Mas-related G protein-coupled receptors (Mrgprs) and protease-activated receptor type-2 (PAR-2) (Liu et al. 2009; Steinhoff et al. 2003). Mrgprs consist of over 50 members that include MrgprAs, MrgprB4–5, MrgprC11 and MrgprD and are restricted to small diameter DRG neurons in mice (Dong et al. 2001). Chloroquine and the bovine adrenal medulla peptide 8–22 (BAM8–22) elicit itch-related scratching through MrgprA3 and MrgprC11, respectively, in mice (Liu et al. 2009) and BAM8–22 elicits itch in humans (Sikand et al., 2011). Expression of the precursor of BAM8–22, proenkephalin A, in skin is increased under pathological conditions including psoriasis (Slominski et al. 2011). Another histamine-independent itch pathway is PAR-2, which is a member of the protease-activated receptor family. Both endogenous and exogenous agonists of PAR-2, tryptase and SLIGRL-NH2, respectively, elicit scratching in a dose-dependent manner (Akiyama et al. 2009; Shimada et al. 2006; Ui et al. 2006). The level of PAR-2 expression in nerves is increased in the skin of atopic dermatitis patients (Steinhoff et al. 2003). Furthermore, the expression levels of endogenous PAR-2 agonists including tryptase and kallikreins are elevated in the skin of atopic dermatitis patients (Komatsu et al. 2007; Steinhoff et al. 2003). The aim of this study was to test if histamine and various non-histamine itch mediators interact and contribute to sensitization of itch signaling.
MATERIALS AND METHODS
Behavioral scratching studies
Experiments were conducted using 42 adult male C57BL/6 mice (Simonsen, Gilroy, CA; 19–26 g) under a protocol approved by the UC Davis Animal Care and Use Committee. The fur on the rostral back was shaved and mice were habituated to the Plexiglas recording arena one week prior to testing. On the test day, the animal received an intradermal (id) microinjection of 10 µl of one of the following: nothing (pretreatment none), histamine (271 nmol; Sigma-Aldrich, St. Louis MO), PAR-2 agonist SLIGRL-NH2 (76 nmol; Quality Controlled Biochemicals, Hopkinton, MA), chloroquine (193 nmol; Sigma) or BAM8–22 (50 nmol; Genemed Synthesis Inc., San Antonio, TX). Microinjections were made id in the nape of the neck using a 30 G needle attached to a Hamilton microsyringe by PE-50 tubing. The injection site was marked so a second injection could be made at the same location in experiments testing for self-sensitization and cross-sensitization (Table 1). Immediately after the injection the mouse was placed into the arena and videotaped from above for 40–60 min. Generally 3–4 mice were injected and videotaped simultaneously. Immediately after commencing videotaping all investigators left the room.
Table 1.
Experimental groups to investigate self-sensitization, cross-sensitization in scratching behavior. Column denoted “first” indicates which drug was applied first, and column denoted “second” indicates which agent (if any) was applied next in the same session. After the first agent was injected, mice were videotaped for 40 min, at which time a second injection of the same or a different mediator was made at the same site and mice were videotaped for another 40 min. All treatments are intradermal.
| Group | First | Dose/ Route | Second | Dose/ Route |
|---|---|---|---|---|
| 1 | Chloroquine | 193 nmol/ 10 µl | Chloroquine | 193 nmol/ 10 µl |
| 2 | Chloroquine | 193 nmol/ 10 µl | BAM8–22 | 50 nmol/ 10 µl |
| 3 | Chloroquine | 193 nmol/ 10 µl | Histamine | 271 nmol/ 10 µl |
| 4 | Chloroquine | 193 nmol/ 10 µl | SLIGRL-NH2 | 76 nmol/ 10 µl |
| 5 | BAM8–22 | 50 nmol/ 10 µl | Chloroquine | 193 nmol/ 10 µl |
| 6 | BAM8–22 | 50 nmol/ 10 µl | BAM8–22 | 50 nmol/ 10 µl |
| 7 | BAM8–22 | 50 nmol/ 10 µl | Histamine | 271 nmol/ 10 µl |
| 8 | BAM8–22 | 50 nmol/ 10 µl | SLIGRL-NH2 | 76 nmol/ 10 µl |
| 9 | Histamine | 271 nmol/ 10 µl | Chloroquine | 193 nmol/ 10 µl |
| 10 | Histamine | 271 nmol/ 10 µl | BAM8–22 | 50 nmol/ 10 µl |
| 11 | SLIGRL-NH2 | 76 nmol/ 10 µl | Chloroquine | 193 nmol/ 10 µl |
| 12 | SLIGRL-NH2 | 76 nmol/ 10 µl | BAM8–22 | 50 nmol/ 10 µl |
Videotapes were reviewed by investigators blinded to the treatment, and the number of scratch bouts was counted at 5-min intervals. A scratch bout was defined as one or more rapid back-and-fort hind paw motions directed toward and contacting the injection site, and ending with licking or biting of the toes and/or placement of the hind paw on the floor. Hind paw movements directed away from the injection site (e.g., ear-scratching) and grooming movements were not counted. For analysis of the time course of scratching, two-way repeated-measures ANOVA followed by Student-Newman-Keuls test was used to compare the mean number of scratch bouts/ 5 min interval after pruritogen injection without vs. with pretreatment. One-way ANOVA followed by the Bonferroni post-test was used to compare the total number of scratch bouts across pretreatment groups. In all cases p<0.05 was considered to be significant.
Calcium Imaging
A total of 43 adult male C57/BL6 mice (7–9 weeks old, 18–21 g) was used under a protocol approved by the UC Davis Institutional Animal Care and Use Committee. In one set of experiments, 13 juvenile mice (3–4 weeks old, 8–12 g) were used. The animal was euthanized under sodium pentobarbital anesthesia and upper- to mid-cervical DRGs were acutely dissected and enzymatically digested at 37°C for 10 min in Hanks’s balanced salt solution (HBSS; Invitrogen, Carlsbad, CA) containing 20 units/ml papain (Worthington Biochemical, Lakewood, NJ) and 6.7 mg/ml L-cysteine (Sigma), followed by 10 min at 37° C in HBSS containing 3 mg/ml collagenase (Worthington Biochemical). The ganglia were then mechanically triturated using fire-polished glass pipettes. DRG cells were pelleted, suspended in MEM Eagle’s with Earle’s BSS (Gibco) containing 100 U/ml penicillin, 100 µg/ml streptomycin (Gibco), 1×vitamin (Gibco) and 10% horse serum (Quad Five, Ryegate, MT), plated on poly-D-lysine-coated glass coverslips, and cultured for 16–24 hr.
DRG cells were incubated in Ringers solution (pH7.4; 140 mM NaCl, 4 mM KCl, 2 mM CaCl2, 1 mM MgCl2, 10 mM HEPES and 4.54 mM NaOH) with 10 µM of Fura-2 AM and 0.05% of Pluronic F-127 (Invitrogen). Coverslips were mounted on a custom made aluminum perfusion block and viewed through an inverted microscope (Nikon TS100, Technical Instruments, San Francisco CA). Fluorescence was excited by UV light at 340 nm and 380 nm alternately and emitted light was collected via a CoolSnap camera attached to a Lambda LS lamp and a Lambda optical filter changer (Sutter Instrument Company, Novato, CA). Ratiometric measurements were made using Simple PCI software (Compix Inc, Cranberry Township, PA) every 3 sec.
Solutions were delivered by a solenoid-controlled 8-channel perfusion system (ValveLink, AutoM8). Two of the following agents were delivered in randomized order: chloroquine (300 µM), BAM8–22 (10 µM), histamine (100 µM) and the PAR-2 agonist SLIGRL-NH2 (100 µM). This was then followed by AITC (200 µM) or capsaicin (1 µM). The concentrations selected for histamine and the PAR-2 agonist were the same as used in prior studies (histamine: Akiyama et al. 2010; Han et al. 2006; Nicolson et al. 2007; Shim et al. 2007; PAR-2 agonist: Akiyama et al. 2010; Amadesi et al. 2004; Steinhoff et al. 2000). Mrgpr agonist concentrations were selected based on pilot experiments to determine the concentration that activated the highest percentage of DRG cells. For chloroquine, the percentages of DRG cells activated were as follows: 1 mM: 8.1% (34/420), 300 µM: 9.8% (189/742), 100 µM: 1.8% (7/386). For BAM8–22, the percentages were: 10 µM: 2.1% (4/184), 3 µM: 0.1% (1/138). In the experiments testing self-sensitization, chloroquine or BAM8–22 was delivered twice at a 3-min interstimulus interval. Potassium at a concentration of 144 mM was always delivered at the end of each experiment. Stimulus duration was 30 sec (10 sec for capsaicin). Ratios were normalized to baseline. Cells were judged to be sensitive if the ratio value increased by more than 10% of the resting level following chemical application. Only cells responsive to high-K+ were included for analysis. Between-group differences were compared by one-way ANOVA followed by Bonferroni’s post-hoc test.
RESULTS
Self- and cross-sensitization of pruritogen-evoked scratching behavior
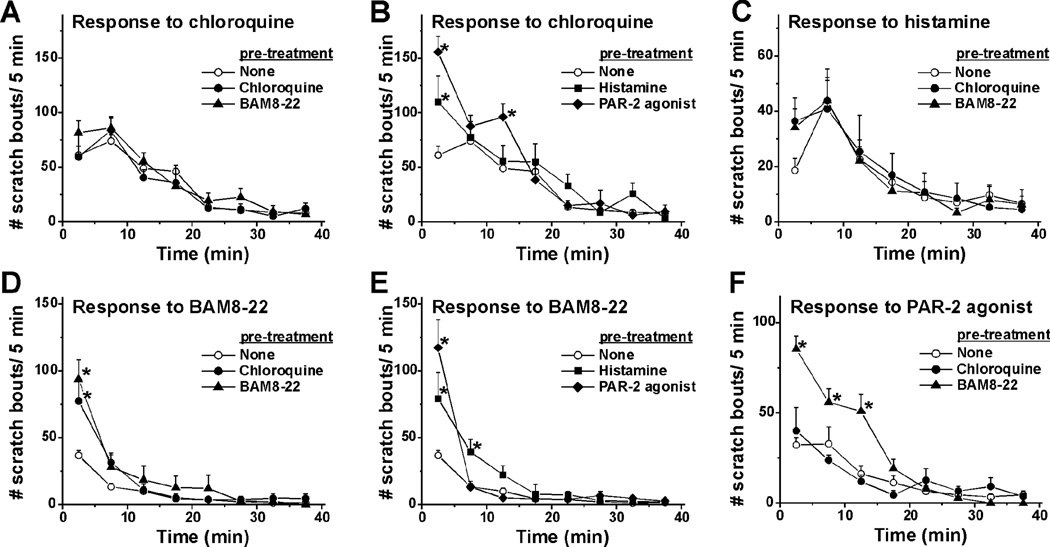
The time course of scratching elicited by intradermal injection of each pruritogen is shown in Fig. 1A–F for chloroquine, histamine, BAM8–22, and a PAR-2 agonist, respectively (○). In all cases, maximal scratching occurred within the initial 5–10 min post- injection and decreased within 20 to 30 min.
Fig. 1.
Time course of self- and cross-sensitization of pruritogen-evoked scratching behavior. A. Mean number of scratch bouts/5-min after chloroquine alone (none; ○) or when preceded by chloroquine (●) or BAM8–22 (▲).Error bars: SEM. n=6–24/group. B. As in A; chloroquine preceded by histamine (■) or PAR-2 agonist (solid diamonds). *, significantly different from chloroquine alone (p < 0.05, two-way repeated-measures ANOVA, Student-Newman-Keuls test, F (47, 336) =3.57). C. Histamine alone (○) or preceded by chloroquine (●) or BAM8–22 (▲). Note the different scale for the y-axis. D. BAM8–22 alone (none; ○) or preceded by chloroquine (●) or BAM8–22 (▲). *, significantly different from BAM8–22 alone (p < 0.0001, F (47, 336) =10). E. BAM8–22 preceded by histamine (■) or PAR-2 agonist (solid diamonds). *, significantly different from first trial of BAM8–22 (p < 0.0001, , F (47, 336) =10). F. PAR-2 agonist alone (none; ○) or when preceded by chloroquine (●) or BAM8–22 (▲). *, significantly different from PAR-2 agonist alone (p < 0.05, F (23, 168) =4.95). Note the different scale for the y-axis.
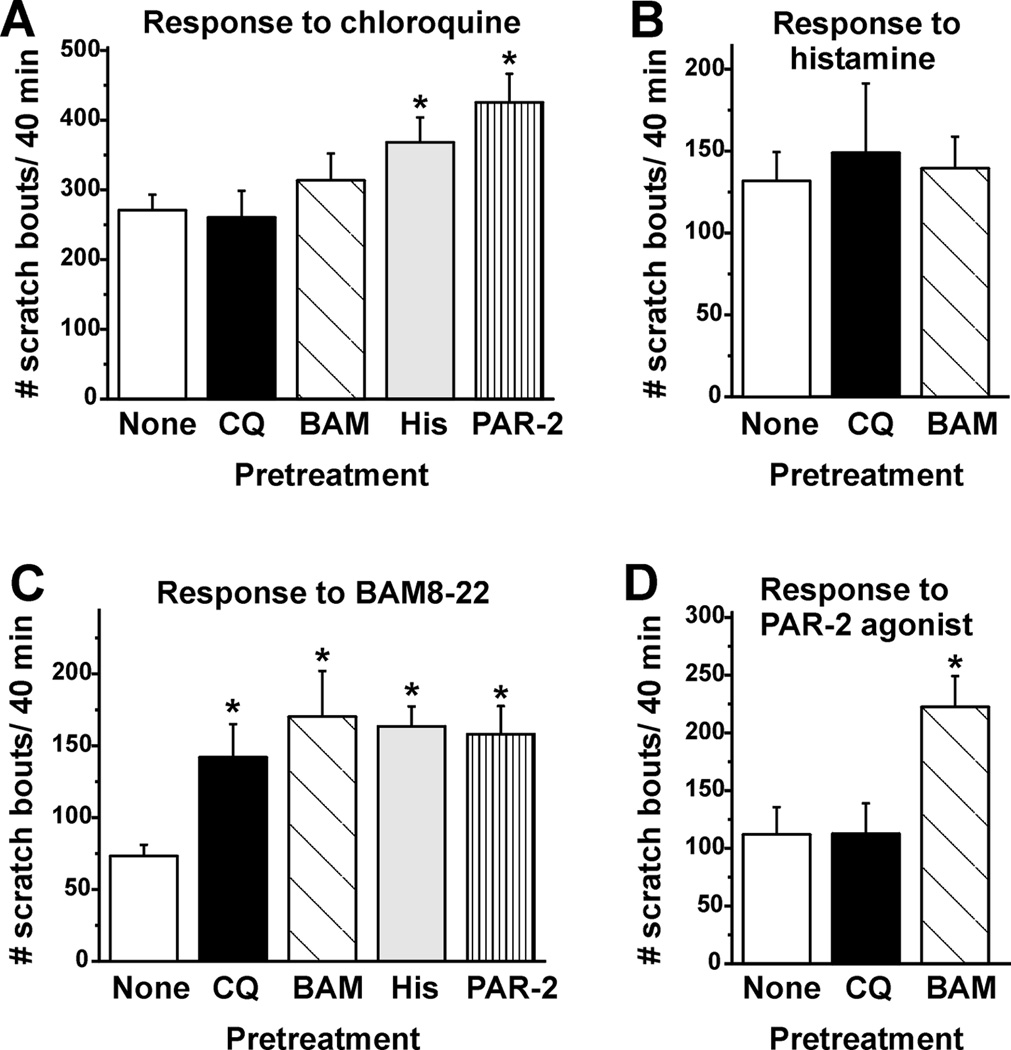
To test for self- or cross-interactions, either the same or a different agent was injected first, followed by injection of the test agent at the identical site 40 min later. Fig. 1A, B show results for chloroquine-evoked scratching. When preceded by an injection of chloroquine (●) or BAM8–22 (▲), subsequent injection of chloroquine elicited equivalent scratching compared to the first chloroquine injection, indicating lack of self- or cross- sensitization. Fig. 1B shows that, when preceded by histamine (■) or the PAR-2 agonist (solid diamonds), subsequent injection of chloroquine elicited significantly more scratch bouts during the initial 5 min (and at 15 min for the PAR-2 agonist), indicative of cross-sensitization. The summary data in Fig. 2A show that the total number of chloroquine-evoked scratch bouts/ 40 min was significantly greater when preceded by histamine or the PAR-2 agonist, but was not by prior BAM8–22 or chloroquine.
Fig. 2.
Summary of self- and cross-sensitization of pruritogen-evoked scratching behavior. A. Mean number of scratch bouts/40 min elicited by chloroquine alone (none; open bar) and when preceded by chloroquine (black bar), BAM8–22 (dark gray bar), histamine (light gray bar) or PAR-2 agonist (hatched bar). Error bars: SEM. *, significantly different from first trial of chloroquine(p < 0.05, One-way ANOVA, Bonferroni post-test, n=6–24/group). B. As in A for histamine. Note the different scale for the y-axis here and in C, D. C. As in A for BAM8–22. *, significantly different from BAM8–22 alone (p < 0.05, n=6/group). D. As in A for PAR-2 agonist. *, significantly different from first trial of PAR-2 agonist (p < 0.05, n=6/group).
Neither chloroquine nor BAM8–22 significantly affected histamine-evoked scratching (Fig. 1C, Fig. 2B), indicating lack of cross-sensitization.
Scratching elicited by BAM8–22 was significantly enhanced at 5 min (and 10 min for the PAR-2 agonist and histamine) when preceded by prior injection of BAM8–22 (Fig. 1D, ▲), chloroquine (Fig. 1D, ●), histamine (Fig. 1E, ■) or the PAR-2 agonist (Fig. 1E, filled diamonds), indicative of self- and cross-sensitization. Fig. 2C shows that both BAM8–22 and each of the other agents tested elicited significantly more scratch bouts/ 40 min.
BAM8–22, but not chloroquine, significantly cross-sensitized scratching elicited by the PAR-2 agonist (Fig. 1F, Fig. 2D).
Calcium imaging of DRG cells
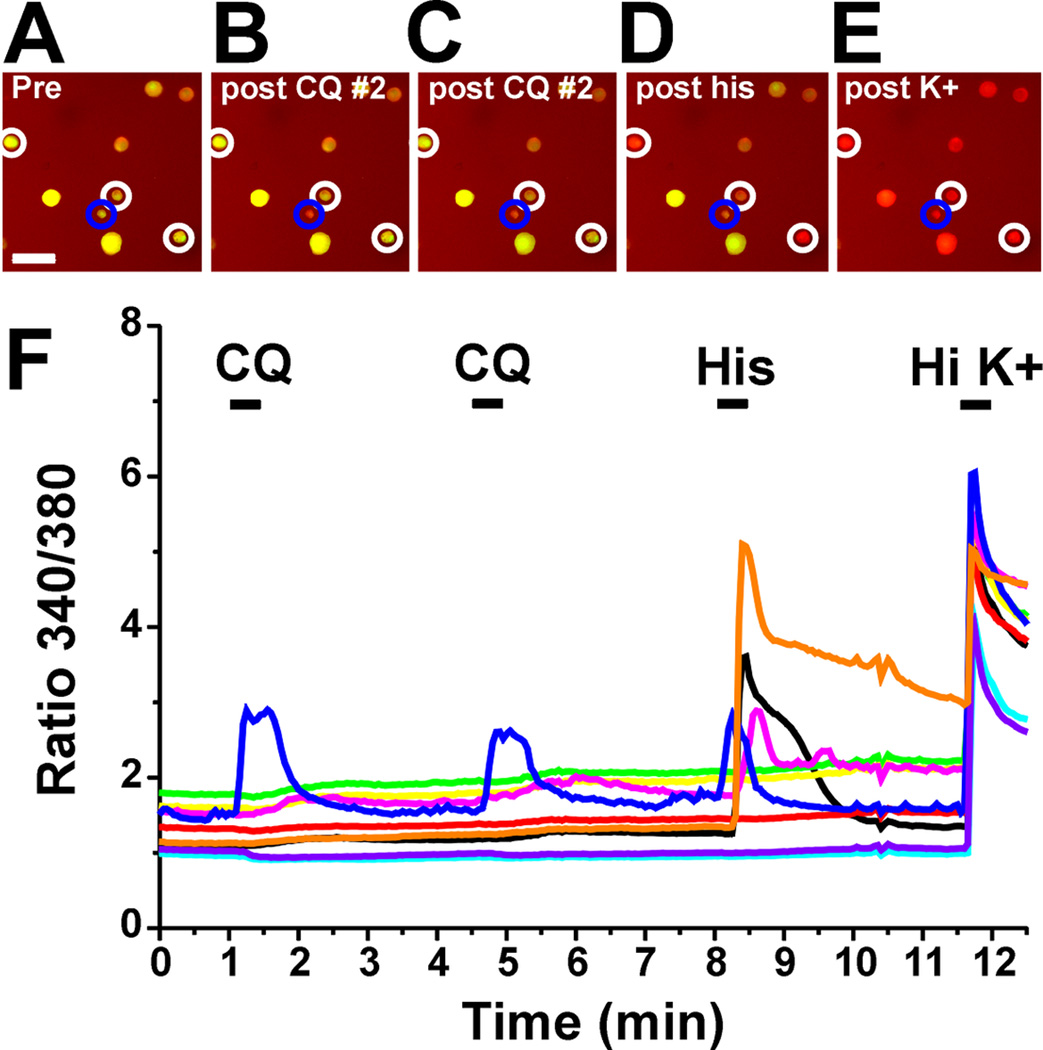
A total of 10,366 DRG cells was imaged for responsiveness to chloroquine, BAM8–22, histamine, SLIGRL, AITC and capsaicin. After the initial application of a given agent, either the same or another agent was applied to the DRG cells 3 min later to test for self- or cross-sensitization. Examples of DRG cell responses are shown in Fig. 3A–F. One cell (encircled in blue; blue trace in Fig. 3A) responded to chloroquine twice and histamine. Other cells (encircled in white; orange, black and yellow traces) responded to histamine, but not chloroquine.
Fig. 3.
Pruritogen activation of DRG cells. A: Graph plots 340/380 nm ratio as a function of time. Black bars indicate time of application of each indicated chemical. One cell (blue trace; encircled in blue in B-F) responded to CQ twice followed by His. Other cells (orange, black and magenta traces; encircled in white in B-F) responded to His, but not CQ. All cells responded high K+ (HiK). B-F: Fluorescence images of DRG cells. Scale bar in B is 50 µm and applies to B-F. B: Before application. C: post first application of chloroquine (CQ; 300 µM). D: post second application of CQ. E: post- histamine (His; 100 µM). F: post application of high potassium solution (K+).
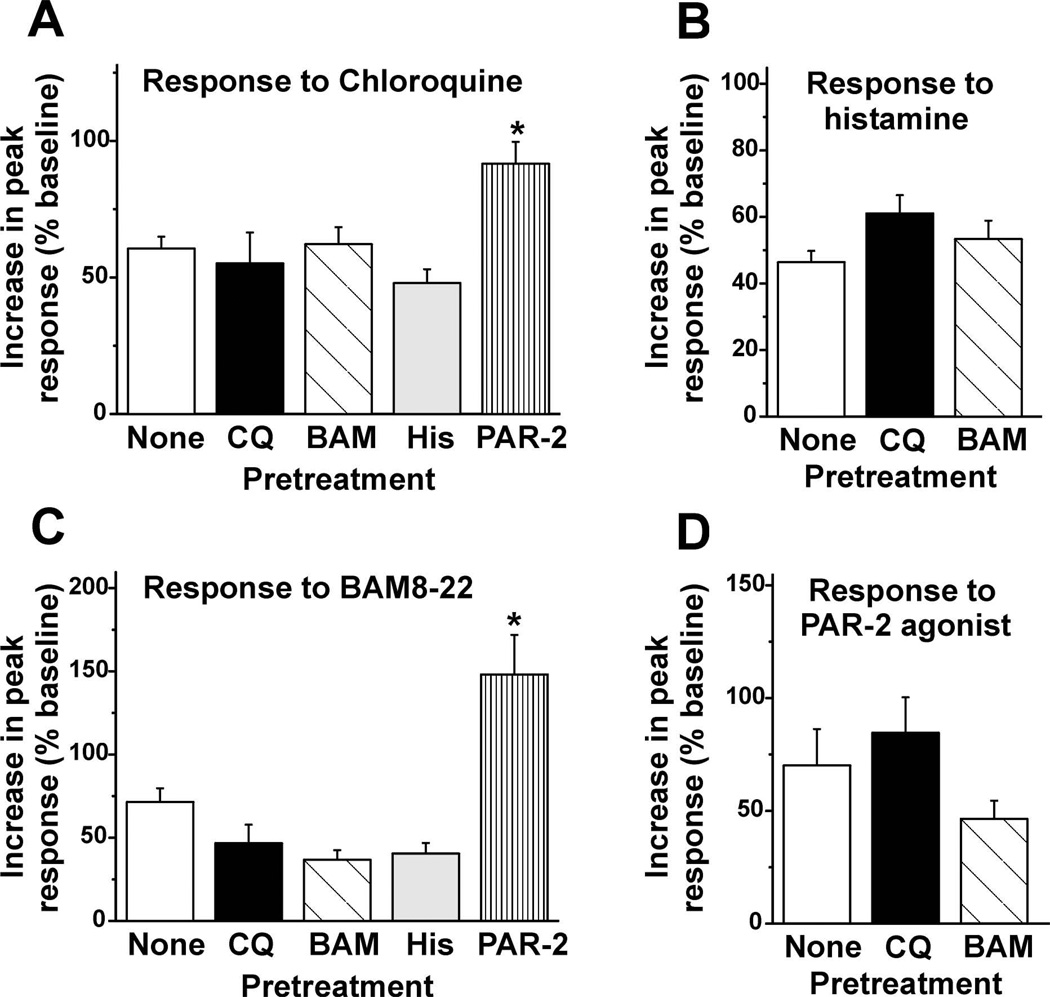
Fig. 4A–D shows mean peak response of DRG cells to each pruritogen alone (None) or when preceded by the same or another pruritogen. Mean peak responses of DRG cells to histamine (Fig. 4B) or the PAR-2 agonist (Fig. 4D) were not affected by preceding application of either chloroquine or BAM8–22. Moreover, the proportions of cells responding to histamine (21.5% ± 2.1 [SEM]) were not significantly affected by prior application of chloroquine or BAM8–22 (16.3 ± 2.5 and 16.1 ± 1.7 %, respectively). Similarly, the proportion of PAR-2 agonist-responsive DRG cells (2.0 ± 0.5 %) was not significantly affected by prior application of chloroquine or BAM8–22 (4.0 ± 1.4 % and 2.3 ± 0.5 %, respectively).
Fig. 4.
Summary of self- and cross-sensitization of pruritogen-evoked responses in DRG cells. A: Peak ratio was normalized to baseline. Bar graph plots mean increase in peak response from baseline of DRG cells elicited by chloroquine when it was applied first (open bar) and when it was applied 3 min after a prior application of chloroquine (black bar), BAM8–22 (dark gray bar), histamine (light gray bar) or the PAR-2 agonist (dotted bar). Error bars: SEM. *, significantly different from chloroquine alone (none, p < 0.05, One-way ANOVA, Bonferroni post-test, n=46–189/group).
B: As in A for histamine. (n= 45–173/group). Error bars: SEM. C: As in A for BAM8–22. *, significantly different from BAM8–22 alone (p < 0.05, One-way ANOVA, Bonferroni post-test, n=13–87/group). Note the different scale for the y-axis here and in D. D: As in A for the PAR-2 agonist (n=13–24/group).
Conversely, application of the PAR-2 agonist (but no other pruritogen) significantly enhanced the mean peak response to subsequent application of chloroquine (Fig. 4A). However, the percentage of chloroquine-responsive cells (9.8 ± 1.4 %) was not significantly different following application of the PAR-2 agonist (12.7±3.6 %) or any other pruritogen (chloroquine: 9.6 ± 2.7 %; BAM8–22: 9.4 ± 1.4 %; histamine: 16.2 ± 2.9 %; the PAR-2 agonist: 12.7 ± 3.6 %).
The mean peak response to BAM8–22 was also significantly greater following prior application of the PAR-2 agonist (Fig. 4C), but no other pruritogen. This was accompanied by a significant increase in the percentage of BAM8–22-responsive cells (8.4 ± 1.6 %; p < 0.05; one-way ANOVA, Bonferroni post-test) compared to the percentages of cells responsive to BAM8–22 alone (3.6 ± 0.6 %) or when preceded by other pruritogens (chloroquine: 2.2 ± 0.5 %; BAM8–22: 2.0 ± 0.5 %; histamine: 4.6 ± 0.8 %).
Most DRG cells were tested with multiple stimuli, including each of the 4 pruritogens plus capsaicin and AITC. Pruritogens were delivered in randomized order. However, data were excluded from cells tested first with the PAR-2 agonist followed by BAM8–22, due to the observed cross-sensitization and increased percentage of BAM8–22-responsive cells. Also, capsaicin and AITC were tested after the pruritogens to avoid any potential cross-desensitizing effects. Otherwise, no order effects were observed.
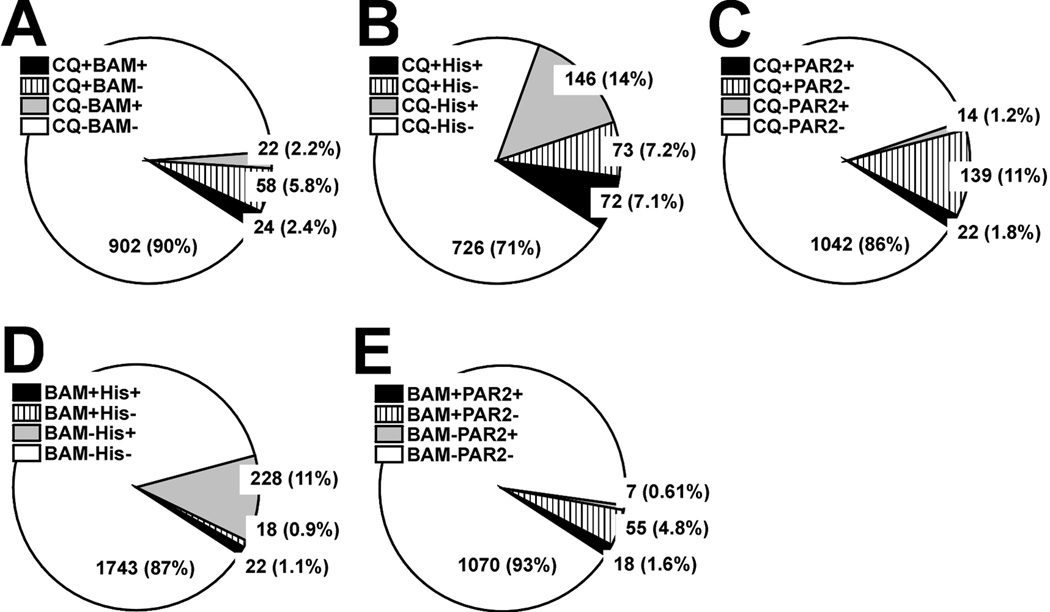
The pie charts in Fig. 5 show the percentages of DRG cells that responded to the first two pruritogens tested in a given experimental sequence. Overall, the percentages of responsive DRG cells were as follows: chloroquine 9.8%, BAM8–22 3.4%, histamine 19.1%, the PAR-2 agonist 2.5%, capsaicin 44.7%, and AITC 29.3%. These values are consistent with previous studies (Akiyama et al. 2010; Liu et al. 2009; Wilson et al. 2011). Fifty two percent of BAM8–22 responsive cells also responded to chloroquine (Fig. 5A). Fifty percent of chloroquine-responsive cells responded to histamine (Fig. 5B). Fifty-five percent of BAM8–22-responsive cells responded to histamine (Fig. 5D). These results are unexpected, since it was previously report that BAM8–22-responsive DRG cells from juvenile mice are a subset of chloroquine-responsive cells which are a subset of histamine-responsive cells (Liu et al. 2009).
Fig. 5.
Proportion of DRG cells responsive to various pruritogens. Each pie chart shows the percentages of DRG cells that responded to either or both of the first two pruritogens tested in a given experimental sequence. A: Chloroquine (CQ; 300 µM) followed by BAM8–22 (BAM; 10 µM). + indicates that the cell responded and − indicates lack of response. The number in each slice is the number of DRG cells in the corresponding group (percentage in parentheses). B: Chloroquine (CQ) followed by histamine (His; 100 µM). C: CQ followed by PAR-2 agonist (PAR2; 100 µM). D: As in BAM8–22 (BAM; 10 µM) followed by histamine (His; 100 µM). E: BAM followed by PAR-2 agonist (PAR2; 100 µM).
To determine if the responsiveness of DRG cells to multiple itch mediators decreases with age, we compared the percentages of chloroquine- and BAM8–22-responsive cells that also responded to histamine in DRG cells from juvenile (3–4 wk old) vs. adult (7–9 wk old) mice. The percentage of chloroquine-responsive DRG cells that also responded to histamine was greater in juvenile vs. adult mice (59.6 vs. 49.7%; n=91 and 145 cells, respectively). Similarly, the percentage of BAM8–22-responsive DRG cells that also responded to histamine was higher in juveniles vs. adults (70% vs. 55%; n=10 and 40 cells, respectively). In addition, most of the PAR-2 agonist-responsive cells responded to chloroquine (61.1%, Fig. 5C) or BAM8–22 (72.0%, Fig. 5E), consistent with a recent study (Liu et al. 2011).
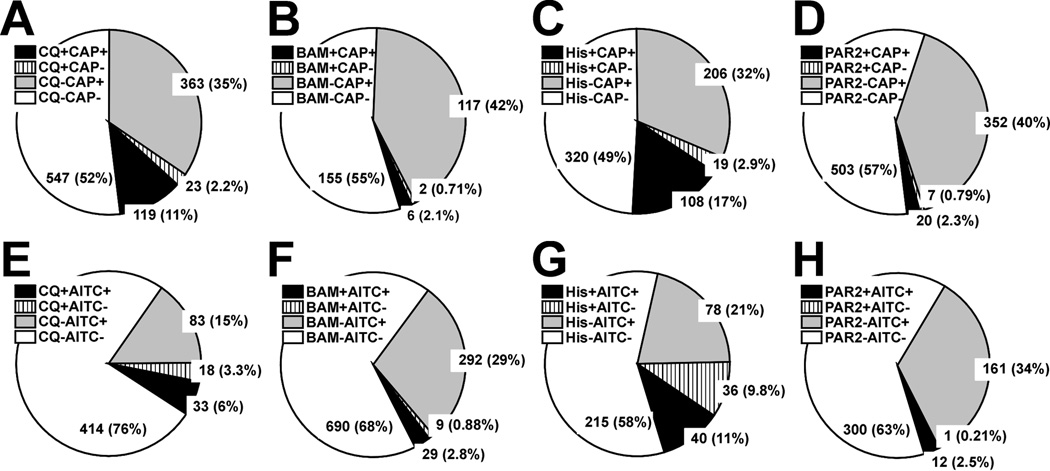
The pie charts in Fig. 6 show the percentages of DRG cells that responded to the pruritogen and algogen tested in a given experimental sequence. Most chloroquine-responsive cells responded to capsaicin (83.2%, Fig. 6A) and AITC (64.7%, Fig. 6E). Similarly, most BAM8–22-responsive cells also responded to capsaicin (75.0%, Fig. 6B) and AITC (76.3%, Fig. 6F). These percentages are consistent with recent studies (Liu et al. 2009; Wilson et al. 2011). Most histamine-responsive cells also responded to capsaicin (85.0%, Fig. 6C), also consistent with previous studies (Akiyama et al. 2010; Shim et al. 2007). On the other hand, about half of histamine-responsive cells did not respond to AITC (52.6%, Fig. 6G). Most PAR-2 agonist-responsive cells responded to capsaicin (74.1%, Fig. 6D) and AITC (92.3%, Fig. 6H), which is consistent with a previous study (Akiyama et al. 2010).
Fig. 6.
Proportion of DRG cells responsive to various pruritogens and algogens. Each pie chart shows the percentages of DRG cells that responded to either or both of pruritogens and algogens tested in a given experimental sequence. A, B, C and D: Chloroquine (CQ; 300 µM), BAM8–22 (BAM; 10 µM), histamine (His; 100 µM) or PAR-2 agonist (PAR2; 100 µM) followed by capsaicin (CAP; 1 µM), respectively. + indicates that the cell responded and – indicates lack of response. The number in each slice is the number of DRG cells in the corresponding group (percentage in parentheses). E, F, G and H: As in A for CQ, BAM, His or PAR2 followed by allyl isothiocyanate (AITC; 100 µM), respectively.
DISCUSSION
Histamine and the PAR-2 agonist SLIGRL-NH2 enhanced scratching elicited by agonists of MrgprA3 and MrgprC11 (chloroquine and BAM8–22, respectively). Moreover, the PAR-2 agonist enhanced the response of DRG cells to Mrgpr agonists, possibly reflecting a peripheral sensitization of pruriceptors that signal histamine-independent itch. PAR-2 expressed in keratinocytes or pruriceptive nerve endings thus represents a potential target for treating conditions of chronic itch by reducing sensitization of itch signaling.
BAM8–22, but not chloroquine exhibited self-sensitization of scratching. In contrast, scratching elicited by histamine, the PAR-4 agonist, and 5-HT exhibited self-desensitization (Akiyama et al. 2009). These results imply that BAM8–22, which elicits itch (Sikand et al., 2011), also enhances itch. MrgprC11 is specifically expressed in DRG neurons (Dong et al. 2001), yet BAM8–22 did not cross-sensitize DRG neuronal responses. Moreover, BAM8–22 cross-sensitized scratching evoked by the PAR-2 agonist SLIGRL-NH2, which was recently reported to have agonistic activity at MrgprC11 (Liu et al. 2011), but did not cross-sensitize responses of DRG cells to SLIGRL-NH2. These observations imply that the scratch-sensitizing effect of BAM8–22 might be mediated via a mechanism independent of MrgprC11 expressed in DRG cells. This is consistent with lack of self-sensitization of scratching evoked by the PAR-2 agonist (Akiyama et al., 2009). One possibility is that BAM8–22 might elicit self-sensitization indirectly via the activation of non-neuronal cells such as keratinocytes. Keratinocytes produce a variety of mediators that induce or enhance itch, such as leukotriene B4, endothelin-1 and NO (Andoh et al. 2001; Andoh and Kuraishi 2003; Andoh et al. 2012). BAM8–22 is unlikely to act at mast cells, since a recent human study reported that intradermal injection of BAM8–22 elicited itch that was not significantly affected by an antihistamine, and was not accompanied by flare (Sikand et al., 2011).
The PAR-2 agonist enhanced scratching evoked by Mrgpr agonists (Fig. 2A, C), but reduced 5-HT-evoked scratching and had no effect on scratching evoked by histamine or a PAR-4 agonist (Akiyama et al. 2009). The PAR-2 agonist also cross-sensitized responses of DRG cells to Mrgpr agonists. This enhancement appeared not to depend on the activation of MrgprC11, since BAM8–22 failed to enhance the response of DRG cells to itself or chloroquine (Fig. 4A, C). These findings imply that the activation of PAR-2 expressed by primary sensory neurons contributes to the sensitization of histamine-independent itch signaling through Mrgprs. Small sub-populations of DRG cells responded to both the PAR-2 agonist and chloroquine (Fig. 5C) or BAM8–22 (Fig. 5E), supporting the possibility that activation of PAR-2 might enhance the sensitivity of Mrgprs co-expressed in the same DRG cell.
Histamine cross-sensitized Mrgpr agonist-evoked scratching but not DRG cell responses. Similarly, chloroquine enhanced BAM8–22-evoked scratching, but not responses of DRG cells. Since histamine and chloroquine can trigger mast cell degranulation (He and Xie 2004; Liu et al. 2009), tryptase released from mast cells may contribute to the unidirectional cross-sensitization by histamine and chloroquine via PAR-2 expressed in DRG neurons. The PAR-2 agonist also sensitized TRPA1 in DRG cells through the activation of phospholipase C (PLC) (Dai et al. 2007). TRPA1 is required for scratching elicited by chloroquine and BAM8–22 (Wilson et al. 2011). Sensitization of TRPA1 through PLC may contribute to unidirectional cross-sensitization by the PAR-2 agonist of DRG cell responses to chloroquine and BAM8–22. Accordingly, chloroquine might be expected to induce self-sensitization of scratching, but this was not presently observed. This discrepancy might be explained by the involvement of Gβγ in itch signaling evoked by chloroquine, but not BAM8–22 (Wilson et al. 2011). One target of Gβγ is the G protein-coupled receptor kinase 2 (GRK2), which initiates desensitization of G protein-coupled receptors (GPCRs), such as MrgprA3, by phosphorylating activated GPCRs (Lodowski et al. 2005). This desensitization effect could be competitive with the sensitization occurring via PAR-2.
While the proportion of cells responding to chloroquine was not significantly affected by prior application of the PAR-2 agonist, the proportion of BAM8–22-responsive DRG cells increased significantly after application of the PAR-2 agonist. The activation of PAR-2 might contribute to either increased activity or upregulation of MrgprC11.
The responsiveness of DRG cells to multiple itch mediators decreased with age Thus, the percentage of chloroquine-responsive DRG cells that also responded to histamine was greater in juvenile than adult mice. TRPA1 and TRPV1 are required for itch signaling evoked by Mrgpr agonists and histamine, respectively (Shim et al. 2007; Wilson et al. 2011). TRPA1-positive and TRPV1-negative neurons were absent in mice at developmental stage P14 and only emerged in adulthood (Hjerling-Leffler et al. 2007). This is consistent with our present data showing a greater proportion of histamine-insensitive, Mrgpr agonist-responsive DRG cells from adults compared to juvenile mice.
In conclusion, the present results support a role for PAR-2 in sensitization of non-histaminergic itch via a direct action on primary sensory nerve endings. PAR-2 represents a viable target for the development of treatments to reduce chronic itch by interfering with peripheral sensitization of pruriceptors.
Highlights.
MrgprC11 agonist BAM8–22 cross-sensitized scratching evoked by PAR-2 agonist
MrgprA3 agonist chloroquine cross-sensitized BAM8–22-evoked scratching
Histamine cross-sensitized chloroquine- and BAM8–22-evoked scratching
PAR-2 agonist cross-sensitized chloroquine-evoked scratching and DRG cell responses
PAR-2 is a peripheral target to reduce sensitization associated with chronic itch.
ACKNOWLEDGEMENTS
The work was supported by supported by grants from the National Institutes of Health DE013685 and AR057194. T. Akiyama and M. Tominaga received postdoctoral fellowships from the Japan Society for the Promotion of Science.
ABBREVIATIONS
- 5-HT
5-hydroxytrmptamine (5-HT)
- AITC
allyl isothiocyanate
- ANOVA
analysis of variance
- BAM8–22
bovine adrenal medulla peptide 8–22
- CQ
chloroquine
- DRG
dorsal root ganglion
- Mrgpr
Mas-related G protein-coupled receptor
- PAR
protease-activated receptor
- SEM
standard error of the mean
- SLIGRL-NH2
PAR-2 agonist
- Veh
vehicle
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST
The authors state no conflict of interest.
REFERENCES
- Akiyama T, Carstens MI, Carstens E. Enhanced responses of lumbar superficial dorsal horn neurons to intradermal PAR-2 agonist but not histamine in a mouse hindpaw dry skin itch model. Journal of neurophysiology. 2011;105:2811–2817. doi: 10.1152/jn.01124.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama T, Carstens MI, Carstens E. Enhanced scratching evoked by PAR-2 agonist and 5-HT but not histamine in a mouse model of chronic dry skin itch. Pain. 2010;151:378–383. doi: 10.1016/j.pain.2010.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama T, Carstens MI, Ikoma A, Cevikbas F, Steinhoff M, Carstens E. Mouse model of touch-evoked itch (alloknesis) The Journal of investigative dermatology. 2012;132:1886–1891. doi: 10.1038/jid.2012.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama T, Merrill AW, Zanotto K, Carstens MI, Carstens E. Scratching behavior and Fos expression in superficial dorsal horn elicited by protease-activated receptor agonists and other itch mediators in mice. The Journal of pharmacology and experimental therapeutics. 2009;329:945–951. doi: 10.1124/jpet.109.152256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amadesi S, Nie J, Vergnolle N, Cottrell GS, Grady EF, Trevisani M, Manni C, Geppetti P, McRoberts JA, Ennes H, Davis JB, Mayer EA, Bunnett NW. Protease-activated receptor 2 sensitizes the capsaicin receptor transient receptor potential vanilloid receptor 1 to induce hyperalgesia. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2004;24:4300–4312. doi: 10.1523/JNEUROSCI.5679-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andoh T, Katsube N, Maruyama M, Kuraishi Y. Involvement of leukotriene B(4) in substance P-induced itch-associated response in mice. The Journal of investigative dermatology. 2001;117:1621–1626. doi: 10.1046/j.0022-202x.2001.01585.x. [DOI] [PubMed] [Google Scholar]
- Andoh T, Kuraishi Y. Nitric oxide enhances substance P-induced itch-associated responses in mice. British journal of pharmacology. 2003;138:202–208. doi: 10.1038/sj.bjp.0705004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andoh T, Yoshida T, Lee JB, Kuraishi Y. Cathepsin E induces itch-related response through the production of endothelin-1 in mice. European journal of pharmacology. 2012;686:16–21. doi: 10.1016/j.ejphar.2012.04.024. [DOI] [PubMed] [Google Scholar]
- Dai Y, Wang S, Tominaga M, Yamamoto S, Fukuoka T, Higashi T, Kobayashi K, Obata K, Yamanaka H, Noguchi K. Sensitization of TRPA1 by PAR2 contributes to the sensation of inflammatory pain. The Journal of clinical investigation. 2007;117:1979–1987. doi: 10.1172/JCI30951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong X, Han S, Zylka MJ, Simon MI, Anderson DJ. A diverse family of GPCRs expressed in specific subsets of nociceptive sensory neurons. Cell. 2001;106:619–632. doi: 10.1016/s0092-8674(01)00483-4. [DOI] [PubMed] [Google Scholar]
- Grazzini E, Puma C, Roy MO, Yu XH, O'Donnell D, Schmidt R, Dautrey S, Ducharme J, Perkins M, Panetta R, Laird JM, Ahmad S, Lembo PM. Sensory neuron-specific receptor activation elicits central and peripheral nociceptive effects in rats. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:7175–7180. doi: 10.1073/pnas.0307185101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han SK, Mancino V, Simon MI. Phospholipase Cbeta 3 mediates the scratching response activated by the histamine H1 receptor on C-fiber nociceptive neurons. Neuron. 2006;52:691–703. doi: 10.1016/j.neuron.2006.09.036. [DOI] [PubMed] [Google Scholar]
- He SH, Xie H. Modulation of tryptase secretion from human colon mast cells by histamine. World journal of gastroenterology : WJG. 2004;10:323–326. doi: 10.3748/wjg.v10.i3.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyer G, Hornstein OP, Handwerker HO. Skin reactions and itch sensation induced by epicutaneous histamine application in atopic dermatitis and controls. The Journal of investigative dermatology. 1989;93:492–496. doi: 10.1111/1523-1747.ep12284051. [DOI] [PubMed] [Google Scholar]
- Heyer G, Koppert W, Martus P, Handwerker HO. Histamine and cutaneous nociception: histamine-induced responses in patients with atopic eczema, psoriasis and urticaria. Acta dermato-venereologica. 1998;78:123–126. doi: 10.1080/000155598433458. [DOI] [PubMed] [Google Scholar]
- Hjerling-Leffler J, Alqatari M, Ernfors P, Koltzenburg M. Emergence of functional sensory subtypes as defined by transient receptor potential channel expression. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27:2435–2443. doi: 10.1523/JNEUROSCI.5614-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong Y, Dai P, Jiang J, Zeng X. Dual effects of intrathecal BAM22 on nociceptive responses in acute and persistent pain--potential function of a novel receptor. British journal of pharmacology. 2004;141:423–430. doi: 10.1038/sj.bjp.0705637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikoma A, Rukwied R, Stander S, Steinhoff M, Miyachi Y, Schmelz M. Neuronal sensitization for histamine-induced itch in lesional skin of patients with atopic dermatitis. Archives of dermatology. 2003;139:1455–1458. doi: 10.1001/archderm.139.11.1455. [DOI] [PubMed] [Google Scholar]
- Johnson HH, Jr, Deoreo GA, Lascheid WP, Mitchell F. Skin histamine levels in chronic atopic dermatitis. The Journal of investigative dermatology. 1960;34:237–238. [PubMed] [Google Scholar]
- Komatsu N, Saijoh K, Kuk C, Liu AC, Khan S, Shirasaki F, Takehara K, Diamandis EP. Human tissue kallikrein expression in the stratum corneum and serum of atopic dermatitis patients. Experimental dermatology. 2007;16:513–519. doi: 10.1111/j.1600-0625.2007.00562.x. [DOI] [PubMed] [Google Scholar]
- Liu Q, Tang Z, Surdenikova L, Kim S, Patel KN, Kim A, Ru F, Guan Y, Weng HJ, Geng Y, Undem BJ, Kollarik M, Chen ZF, Anderson DJ, Dong X. Sensory neuron-specific GPCR Mrgprs are itch receptors mediating chloroquine-induced pruritus. Cell. 2009;139:1353–1365. doi: 10.1016/j.cell.2009.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Weng HJ, Patel KN, Tang Z, Bai H, Steinhoff M, Dong X. The distinct roles of two GPCRs, MrgprC11 and PAR2, in itch and hyperalgesia. Science signaling. 2011;4:ra45. doi: 10.1126/scisignal.2001925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodowski DT, Barnhill JF, Pyskadlo RM, Ghirlando R, Sterne-Marr R, Tesmer JJ. The role of G beta gamma and domain interfaces in the activation of G protein-coupled receptor kinase 2. Biochemistry. 2005;44:6958–6970. doi: 10.1021/bi050119q. [DOI] [PubMed] [Google Scholar]
- Nicolson TA, Foster AF, Bevan S, Richards CD. Prostaglandin E2 sensitizes primary sensory neurons to histamine. Neuroscience. 2007;150:22–30. doi: 10.1016/j.neuroscience.2007.09.003. [DOI] [PubMed] [Google Scholar]
- Shim WS, Tak MH, Lee MH, Kim M, Koo JY, Lee CH, Oh U. TRPV1 mediates histamine-induced itching via the activation of phospholipase A2 and 12-lipoxygenase. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27:2331–2337. doi: 10.1523/JNEUROSCI.4643-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada SG, Shimada KA, Collins JG. Scratching behavior in mice induced by the proteinase-activated receptor-2 agonist, SLIGRL-NH2. European journal of pharmacology. 2006;530:281–283. doi: 10.1016/j.ejphar.2005.11.012. [DOI] [PubMed] [Google Scholar]
- Simone DA, Alreja M, LaMotte RH. Psychophysical studies of the itch sensation and itchy skin ("alloknesis") produced by intracutaneous injection of histamine. Somatosensory & motor research. 1991;8:271–279. doi: 10.3109/08990229109144750. [DOI] [PubMed] [Google Scholar]
- Slominski AT, Zmijewski MA, Zbytek B, Brozyna AA, Granese J, Pisarchik A, Szczesniewski A, Tobin DJ. Regulated proenkephalin expression in human skin and cultured skin cells. The Journal of investigative dermatology. 2011;131:613–622. doi: 10.1038/jid.2010.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhoff M, Neisius U, Ikoma A, Fartasch M, Heyer G, Skov PS, Luger TA, Schmelz M. Proteinase-activated receptor-2 mediates itch: a novel pathway for pruritus in human skin. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2003;23:6176–6180. doi: 10.1523/JNEUROSCI.23-15-06176.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhoff M, Vergnolle N, Young SH, Tognetto M, Amadesi S, Ennes HS, Trevisani M, Hollenberg MD, Wallace JL, Caughey GH, Mitchell SE, Williams LM, Geppetti P, Mayer EA, Bunnett NW. Agonists of proteinase-activated receptor 2 induce inflammation by a neurogenic mechanism. Nature medicine. 2000;6:151–158. doi: 10.1038/72247. [DOI] [PubMed] [Google Scholar]
- Ui H, Andoh T, Lee JB, Nojima H, Kuraishi Y. Potent pruritogenic action of tryptase mediated by PAR-2 receptor and its involvement in anti-pruritic effect of nafamostat mesilate in mice. European journal of pharmacology. 2006;530:172–178. doi: 10.1016/j.ejphar.2005.11.021. [DOI] [PubMed] [Google Scholar]
- Weisshaar E, Apfelbacher C, Jager G, Zimmermann E, Bruckner T, Diepgen TL, Gollnick H. Pruritus as a leading symptom: clinical characteristics and quality of life in German and Ugandan patients. The British journal of dermatology. 2006;155:957–964. doi: 10.1111/j.1365-2133.2006.07430.x. [DOI] [PubMed] [Google Scholar]
- Wilson SR, Gerhold KA, Bifolck-Fisher A, Liu Q, Patel KN, Dong X, Bautista DM. TRPA1 is required for histamine-independent, Mas-related G protein-coupled receptor-mediated itch. Nature neuroscience. 2011;14:595–602. doi: 10.1038/nn.2789. [DOI] [PMC free article] [PubMed] [Google Scholar]