This study addresses the switch from pluripotency to commitment during embryonic development. The authors identify a novel role for Notch—distinct from its role in specifying cell fates—in promoting the transition from plasticity to commitment in C. elegans. Removal of Notch in cells programmed to produce ectoderm prolongs their plasticity, allowing reprogramming into endoderm fate well after they normally commit. This new signaling program involves a novel, secreted Notch ligand, DSL-3, required for commitment of ectodermal cells but not for their specification or differentiation.
Keywords: Notch, reprogramming, embryo culture, commitment, plasticity
Abstract
We report that Notch signaling is essential for the switch from developmental plasticity to commitment during Caenorhabditis elegans embryogenesis. The GLP-1 and LIN-12 Notch receptors act to set a memory state that affects commitment of cells arising from the major ectodermal progenitor (AB blastomere) several cell divisions later, thereby preventing their forced reprogramming by an endoderm-determining transcription factor. In contrast to Notch-dependent cell fate induction, this activity is autonomous to the AB lineage, is independent of the known cell fate-inducing Notch ligands, and requires a putative secreted Notch ligand, Delta Serrate Lag-3 (DSL-3). Thus, Notch signaling promotes developmental commitment by a mechanism that is distinct from that involved in specifying cell fates.
Fundamental to our understanding of developmental and stem cell biology is how cells switch from a pluripotent to a developmentally committed state. Methods for growing and engineering tissues and organs in vitro depend critically on the ability to manipulate this process. All somatic cells in early Caenorhabditis elegans embryos have been shown to be pluripotent, as evidenced by their capacity to be reprogrammed into cells of all three germ layers in response to forced expression of cell fate-regulating transcription factors (Horner et al. 1998; Zhu et al. 1998; Gilleard and McGhee 2001; Fukushige and Krause 2005). Later in embryogenesis, at about the time that the endoderm progenitor, or E cell, has progressed beyond three rounds of division (the 8E stage) (Joshi et al. 2010), cells become refractory to reprogramming by these factors, marking a major transition from plasticity to restriction in developmental potential (e.g., as can be observed by challenging normally nonendodermal precursor cells to undergo endoderm development) (Supplemental Fig. S1A). While cell-autonomous mechanisms in this critical developmental transition (e.g., Joshi et al. 2010) are known, the action of cell-extrinsic signaling in this process has not been well characterized.
The contact-dependent signal transduction mechanism known as Notch signaling is broadly deployed to direct cell identity throughout metazoan development (Artavanis-Tsakonas and Muskavitch 2010). In C. elegans, Notch signaling is known primarily from its role in specifying cell identities and regulating cell behavior in the embryo, larvae, and adult germline (Kodoyianni et al. 1992; Berry et al. 1997; Chen and Greenwald 2004; Shaye and Greenwald 2005; McGovern et al. 2009). Here we show that Notch pathway components are essential for a completely distinct function independent of their action in specifying cellular differentiation: regulating the ability of embryonic cells to be reprogrammed. We show that the Notch receptor GLP-1 restricts the lineage of AB, the major embryonic ectoblast, from being reprogrammed into endoderm by a mechanism that is autonomous to the AB lineage and therefore distinct from the known early embryonic inductions. Furthermore, we implicate a presumptive Notch ligand, Delta Serrate Lag-3 (DSL-3), in the process that prevents cells in the AB lineage from becoming reprogrammed but not in specifying cell identity in this lineage per se. These findings reveal that Notch controls the transition from plasticity to committed differentiation independent of its previously known action in cell fate specification.
Results and Discussion
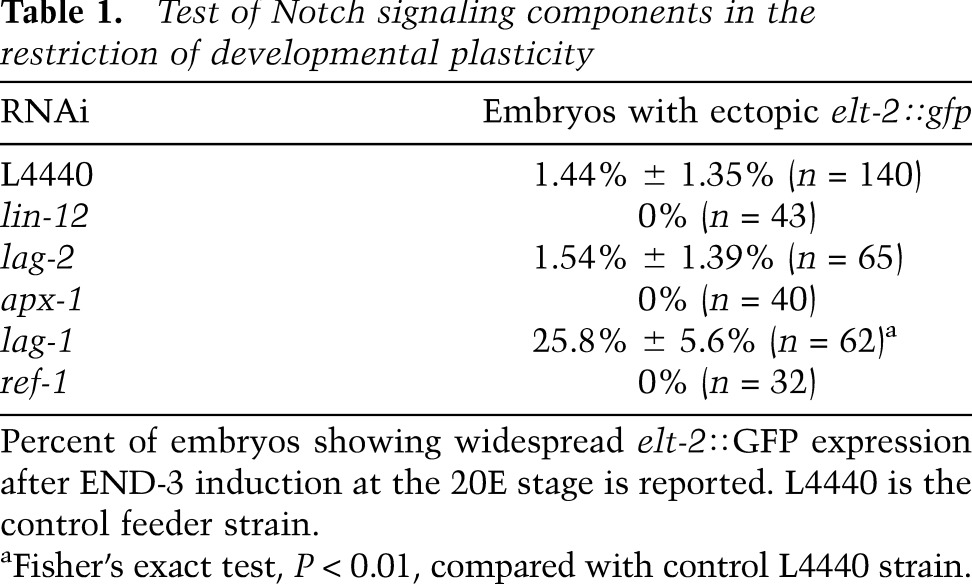
Contact-dependent signaling through Notch-type receptors is recursively and widely deployed to specify the identity of many descendants of AB, the anterior blastomere of the two-cell C. elegans embryo, which generates most of the ectoderm (Priess et al. 1987; Moskowitz and Rothman 1996; Schnabel and Priess 1997; Priess 2005). As Notch signaling functions most prominently prior to the plasticity → commitment transition, we wondered whether it might also act in restricting developmental plasticity. Indeed, we found that eliminating the function of the GLP-1 Notch receptor greatly extends the period during which nonendodermal cells can be reprogrammed into endoderm in response to the endoderm-promoting END-3 GATA transcription factor (Fig. 1A). In the absence of GLP-1 function, lineage reprogramming, evident by expression of several endodermal markers, can occur as late as the 20E stage, well after developmental plasticity is normally lost in wild-type embryos (Fig. 1B,C; Supplemental Fig. S3). This period of extended plasticity does not continue indefinitely, however, as cells do not respond to ectopic END-3 when its expression is induced late in morphogenesis (i.e., at approximately the “twofold stage”) (Fig. 1A). This ability of late embryos to undergo ectopic endoderm development is not the result of a general defect in differentiation, as glp-1(−) embryos undergo timely development and differentiation (Hutter and Schnabel 1994; Moskowitz et al. 1994; Schnabel and Priess 1997; data not shown), albeit with altered cell lineage patterns and fates, owing to misspecification of cells within the AB lineage. GLP-1 appears to act at least in part through canonical Notch signaling in this process: We found that the LAG-1 transcription factor, which transduces Notch inductive signals (Christensen et al. 1996), is also required for the temporal restriction in developmental plasticity (Table 1).
Figure 1.
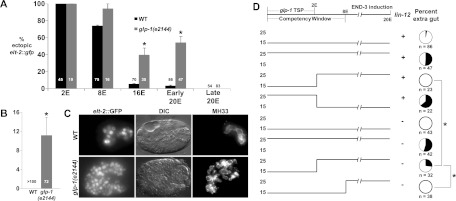
Extended period of developmental plasticity in embryos lacking GLP-1/Notch function, and temporal requirement for GLP-1. (A) Proportion of terminal embryos with ectopic elt-2∷GFP in wild-type (WT) and glp-1(e2144) embryos following induction of END-3 at the indicated stage. “Early 20E” indicates that END-3 was induced in embryos shortly after the last E descendants were born (approximately “bean” to “comma” stage). “Late 20E” indicates that END-3 was induced at approximately the “twofold” stage of morphogenesis, based on time from the two- to four-cell stage. Numbers in bars indicate total embryos carrying the hs-end-3 array scored. (B) Proportion of terminal embryos with ectopic expression of the late gut differentiation marker IFB-2, detected with antibody MH33 (Zhu et al. 1998) following induction of END-3 at the 20E stage. (C) Expression of intestinal differentiation markers following late activation of END-3 in embryos of the indicated genotype. (D) Timelines of temperature shift experiments. Embryos were held at the indicated temperature [permissive (15°C) or nonpermissive (25°C) for the glp-1(e2144) mutation] and shifted at the indicated developmental stage. (Top) The known temperature-sensitive period for GLP-1 lethality (Priess et al. 1987) and the normal period of developmental plasticity (“competency window”) are shown on the developmental time line. lin-12(−) indicates that the gene was knocked down by RNAi in the mothers of the embryos where indicated. Pie diagrams show the proportion of embryos with ectopic endoderm; the total number of embryos carrying the END-3 transgene is indicated below each. In B–D, hs-end-3 was activated at a stage equivalent to the 20E stage in wild-type embryos. Error bars represent standard error. (*) Fisher's exact test, P < 0.01.
Table 1.
Test of Notch signaling components in the restriction of developmental plasticity
We found that when GLP-1 is inactivated prior to the 28-cell (2E) stage, embryos remain competent to respond to late (up to the 20E stage) ectopic expression of END-3 (Fig. 1A,D); reactivating GLP-1 function after the 28-cell stage does not reverse this effect (Fig. 1D). In contrast, when GLP-1 is inactivated only after the 28-cell stage, embryos become developmentally committed at the normal time (approximately 8E stage) (Fig. 1D). These results suggest that GLP-1/Notch sets up a memory state in the early embryo that activates the plasticity → commitment transition later in embryogenesis.
After the early inductive interactions in the AB lineage, mediated by maternal GLP-1, zygotically expressed GLP-1 and its paralog, LIN-12, act redundantly to induce the fates of a small subset of AB-derived cells (Hutter and Schnabel 1994; Moskowitz and Rothman 1996; Henderson et al. 1997). Thus, LIN-12 might compensate for the absence of GLP-1 in regulating developmental plasticity after the 28-cell stage. We found that while embryos lacking only LIN-12 function become developmentally committed at the same time (approximately 8E stage) as in wild type, embryos lacking both GLP-1 and LIN-12 after the 28-cell stage can be provoked to produce ectopic endoderm late in development (Fig. 1D). Consistent with the known requirement of early GLP-1 activity for LIN-12 expression (Moskowitz and Rothman 1996), we observed no significant difference in the ability of embryos to be developmentally reprogrammed when GLP-1 activity was absent throughout embryogenesis in lin-12(RNAi) embryos compared with lin-12(+), embryos (Fig. 1D). It is possible that Notch signaling is necessary only to the 8E stage to restrict plasticity. Indeed, we found that lin-12(−) embryos in which GLP-1 activity was maintained until the 8E stage and then subsequently inactivated were resistant to END-3-mediated reprogramming (Fig. 1D). These results define an apparently continuous requirement for Notch function in the transition from a plastic to a committed state, including an essential early (<28-cell) requirement mediated by GLP-1 alone and a later (>28-cell) requirement in which either GLP-1 or LIN-12 appears to be sufficient.
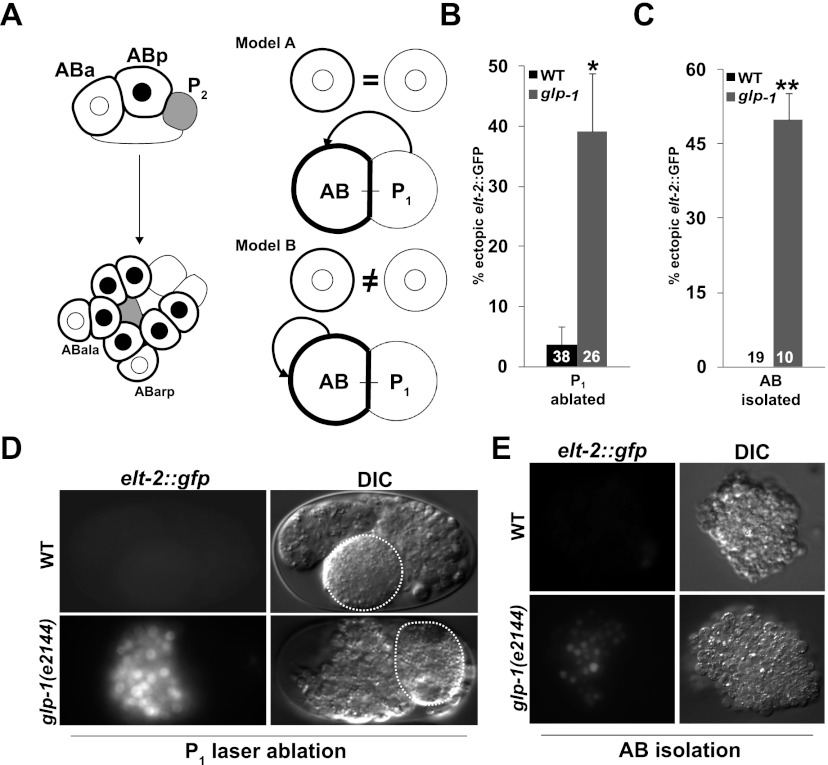
As with the known early Notch-mediated embryonic inductions, we found that GLP-1/Notch function in restricting developmental plasticity is limited to AB descendants. While partial wild-type embryos derived by laser ablation of either AB or its sister, P1, showed the same temporal restriction to developmental reprogramming as in intact embryos (Supplemental Fig. S4A,C), AB-derived partial embryos lacking GLP-1 could be provoked to develop endoderm well after the period in which developmental plasticity is normally lost (Fig. 2B,D). In contrast, this temporal extension of plasticity was not seen in P1-derived glp-1(−) partial embryos (Supplemental Fig. S4B), demonstrating the AB specificity.
Figure 2.
Notch signals regulating developmental plasticity are specific to the AB lineage. (A, left panel). Summary of early known cell fate-inducing Notch signals activated by P1 descendants. Gray-filled cells, derived from P1, signal their AB-derived Notch-expressing neighbors. Black outlines indicate cells expressing the GLP-1/Notch receptor. Cells in which Notch signal transduction has been received by signaling from P1 descendants contain black nuclei. (Right panel) Alternative models for Notch-dependent regulation of developmental plasticity. Circles at the top of each model represent isolated AB cells with (heavy lines) or without (light lines) GLP-1 present. Model A: Inductive signals from P1 descendants both activate cell fates and restrict developmental plasticity. Model B: AB-derived signals, distinct from the cell fate-inducing signals arising from P1 descendants, restrict developmental plasticity. (B) Proportion of P1-ablated embryos from wild-type and glp-1(e2144) embryos that express ectopic elt-2∷GFP following late induction of END-3 expression. (C) Proportion of partial embryos obtained from in vitro culturing of physically isolated AB blastomeres from wild-type and glp-1(e2144) embryos that express ectopic elt-2∷GFP. (D) elt-2∷GFP expression in wild-type and glp-1(e2144) embryos in which AB was isolated by laser ablation followed by late induction of END-3 expression. Ablated blastomeres are denoted by dashed lines. (E) elt-2∷GFP in partial embryos obtained from physically isolated AB blastomeres. In all experiments, END-3 was induced by heat shock when the number of cells corresponded approximately to the 20E stage in intact wild-type embryos. In B and C, numbers in the bars represent the total number of embryos carrying the END-3 transgene. (*) Fisher's exact test, P = 0.01; (**) Fisher's exact test, P < 0.01. Error bars represent standard error.
Given these observations and the known role of P1 descendants as the source of early inductive Notch signals that pattern the AB lineage (summarized in Fig. 2A; Priess et al. 1987; Schnabel and Priess 1997; Priess 2005), it was reasonable to suppose that the signals required to restrict developmental plasticity in AB descendants later in development might also arise from P1 descendants (Model A, Fig. 2A). However, several observations indicated that this is not the case. First, although the entire AB lineage appears to be resistant to developmental reprogramming after the 8E stage in wild-type embryos, two of the AB great-granddaughters, while expressing the GLP-1/Notch receptor, never receive Notch signals and would therefore be expected to show extended developmental plasticity (Hutter and Schnabel 1994; Moskowitz et al. 1994). In addition, we found that APX-1, the Notch ligand that induces the fate of half of the AB-derived cells (Mango et al. 1994; Mello et al. 1994), is not required to restrict developmental plasticity (Table 1). Furthermore, cell ablations (Fig. 2B,D; Supplemental Fig. S4A) indicated that a viable P1 cell is not required for commitment of AB descendants to a nonendodermal fate. However, this experiment does not eliminate the caveat that P1-derived signals might be produced by the ablated cell (e.g., Goldstein 1992). To eliminate all potential P1-derived Notch signal, we physically isolated and cultured AB blastomeres (Edgar and Goldstein 2012) and tested for GLP-1-dependent restriction of developmental plasticity. This manipulation is known to prevent all Notch signaling to the AB lineage (Gendreau et al. 1994; Moskowitz et al. 1994). The resultant partial embryos differentiated (Supplemental Fig. S5A,B) and produced endoderm when induced to express END-3 at early stages (Supplemental Fig. S5C,D). However, although they do not experience Notch signal from P1 descendants, the GLP-1-dependent block to reprogramming nonetheless persisted in such AB-derived embryos. Specifically, endoderm differentiation, as evident by elt-2∷GFP expression as well as the presence of birefringent gut granules, an endogenous marker of endoderm differentiation (Babu 1974), could be activated in late glp-1(−) but not glp-1(+) AB-derived partial embryos (Fig. 2C,E; data not shown). Thus, posterior-derived Notch signals are not necessary for developmental commitment; rather, the plasticity-regulating signals apparently arise from within the AB lineage (Model B, Fig. 2A). These intra-AB signals cannot be attributed to the few known (and very limited) intra-AB inductions received by LIN-12, as LIN-12 is not expressed in embryos lacking P1-derived Notch signals (Moskowitz and Rothman 1996).
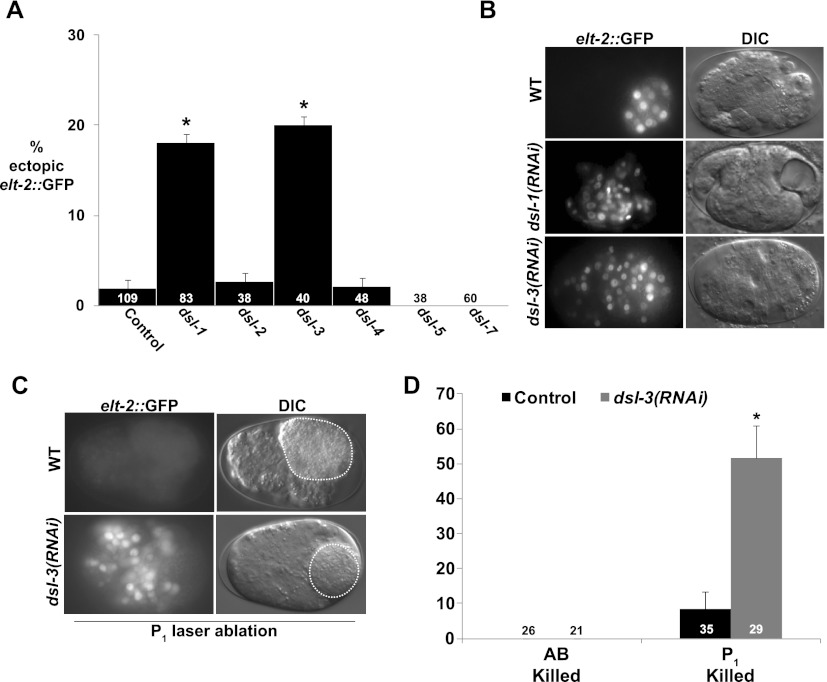
The only Notch ligand known to be expressed and function in the embryonic AB lineage is LAG-2, which activates zygotic expression of lin-12 and glp-1 (Moskowitz and Rothman 1996). However, we found that lag-2 knockdown did not affect the ability of late embryos to be reprogrammed (Table 1), suggesting the requirement for an unidentified AB-specific Notch ligand. The C. elegans genome encodes several other DSL-like Notch ligands (Chen and Greenwald 2004). We found that knocking down either of two DSL-encoding genes, dsl-1 or dsl-3, extended the period of developmental plasticity (Fig. 3A,B). Of these, only dsl-3 showed a requirement for commitment in the AB lineage specifically when partial embryos, obtained by laser ablation of AB or P1, were analyzed (Fig. 3C,D). Thus, like GLP-1/Notch, DSL-3, which is predicted to be a secreted protein (Chen and Greenwald 2004), apparently acts specifically on AB descendants to restrict developmental plasticity.
Figure 3.
dsl-3 is required for commitment to differentiation in the AB lineage. (A) Proportion of embryos subjected to RNAi of the indicated gene that showed ectopic elt-2∷GFP expression. (B) Endogenous and ectopic elt-2∷GFP in terminal dsl-1(RNAi) and dsl-3(RNAi) embryos. (C) elt-2∷GFP in terminal P1-ablated wild-type and dsl-3(RNAi) embryos. Ablated blastomeres are denoted by dashed lines. (D) Proportion of isolated P1 or AB descendants of wild-type and dsl-3(RNAi) embryos expressing ectopic elt-2∷GFP. In all experiments, END-3 was induced by heat shock when the number of cells corresponded approximately to the 20E stage in intact wild-type embryos. Numbers in bars are the total number of embryos carrying the END-3 transgene. (*) Fisher's exact test, P < 0.01. Error bars represent standard error.
Consistent with its potential action on Notch signaling to the AB lineage, microarrays (Baugh et al. 2003) and expression patterns reported in the NEXTDB database (The Nematode Expression Pattern Database, http://nematode.lab.nig.ac.jp) indicate that dsl-3 expression overlaps temporally with that of the GLP-1 and LIN-12 Notch receptors. Moreover, we detected dsl-3 transcripts by in situ hybridization throughout the embryo as early as the two-cell stage, with stronger, more restricted staining primarily in AB-derived embryonic cells later in development (Supplemental Fig. S6). While knockdown of dsl-3 under conditions that eliminate a detectable message (Supplemental Fig. S6) impairs developmental commitment, we observed no overt phenotype of larvae arising from such embryos, consistent with other reports (Kamath et al. 2003; Sonnichsen et al. 2005). Thus, while dsl-3 transcripts are present throughout the early embryo, DSL-3 may block reprogramming by acting specifically through Notch receptors expressed only in AB descendants. These findings suggest two fundamentally different modes of action for Notch signaling in development, with one set of ligands that controls specification of cell identity and another that mediates an auxiliary role in regulating developmental plasticity (Supplemental Fig. S7).
Previous work identified a role for a member of the C. elegans polycomb repressor complex, MES-2, in restricting developmental plasticity (Yuzyuk et al. 2009). In that study, it was shown that removal of mes-2 function enhances developmental plasticity at the 8E stage. It is therefore possible that MES-2 acts downstream from Notch signaling to promote commitment to differentiation. We found that RNAi knockdown of mes-2 in glp-1(−) embryos did not significantly (P = 1) increase the fraction of embryos (53.9% ± 9.8%; n = 26) that were reprogrammed, consistent with the possibility that Notch signaling and MES-2 function in the same pathway to restrict developmental plasticity. However, the findings that MES-2 function is not lineage-specific (Yuzyuk et al. 2009) and that inactivation of GLP-1 causes cells to remain plastic longer than those in embryos lacking MES-2 function suggest that MES-2 may act in a global pathway with multiple inputs, while Notch apparently acts in an AB-specific pathway that may impinge on MES-2 and other regulators of chromatin function.
In other systems, Notch signaling acts to coordinate proliferation and differentiation, as observed, for example, with mammalian Notch1, which is necessary for both the proliferation of neuronal stem cell populations and their differentiation into specific neuronal types (Hitoshi et al. 2002; Wang et al. 2009; Ables et al. 2010; Zhou et al. 2010; Matsumoto et al. 2011). We showed that in C. elegans, Notch can act not only as a factor in specifying cell types, but also, through an apparently distinct process, in the general restriction of developmental plasticity. The diversification of Notch function through the action of different ligands may point to a new role for Notch signaling in regulating the multipotential, stem cell-like properties of developing cells.
Materials and methods
Worm culture and strains
Unless otherwise noted, strains were reared at 20°C on NGM agar plates fed on OP50 Escherichia coli. glp-1(e2144) worms were reared at 15°C, and all experiments with these mutants were performed at 25°C, except where indicated. The following strains were constructed for this study: JR1753 unc-119(ed4) III; wEx690 [unc-119(+) hsp16-2/41∷end-3(+)]; wIs84 [rol-6(su1006) elt-2∷GFP] and JR3279 unc-119(ed4) glp-1(e2144ts) III; wEx690 [unc-119(+) hsp16-2/41∷end-3(+)]; wIs84 [rol-6(su1006) elt-2∷GFP]. Strain JG7 cals6 [hsp16-2∷elt-1(+)]; ijIs12 [dpy7∷GFP rol-6(su1006)] was obtained from John Gilleard.
RNAi
E. coli RNAi feeding strains for glp-1, lin-12, lag-2, dsl-1, dsl-4, lag-1, and ref-1 were obtained from the Ahringer RNAi library (Fraser et al. 2000; Kamath et al. 2003). The dsl-2, dsl-3, dsl-5, dsl-7, and apx-1 genes were cloned into plasmid L4440 using appropriate primers carrying added restriction sites to facilitate cloning (sequences furnished on request) and were transformed into HT115 for RNAi feeding. In the case of glp-1, apx-1, and lag-1, which show lethal RNAi phenotypes, the efficacy of RNAi was determined by scoring for embryonic lethality. L3 worms were fed on RNAi feeding bacteria for 3 d at 15°C. Embryos were dissected from gravid adults on the third day and used in heat-shock experiments.
Analysis of responsiveness to cell fate reprogramming
Embryos from strain JR1753 or the indicated mutant or knockdown strains were isolated at the two-cell stage, and END-3 expression was induced via heat shock after they had been allowed to develop to various stages of E development. Embryos were returned to 20°C, allowed to develop overnight, and scored for expression of elt-2∷GFP. We found that induction of END-3 at any stage leads to ectopic expression of elt-2∷GFP in a low number of small nuclei; however, this expression did not appear to reflect bona fide reprogramming, as ectopic expression of other markers of gut differentiation was not observed in such cells. We scored only embryos with widespread elt-2∷GFP expression as positive in all experiments (e.g., Supplemental Fig. S2). For analysis of differentiation markers, terminal embryos were prepared for immunofluorescence. In all heat-shock experiments, embryos were shifted for 30 min to 33°C, with the exception that embryos from the laser ablation and in vitro culture experiments were heat-shocked for 15 min at 33°C.
Temperature shift experiments
To block GLP-1 function continuously, glp-1(e2144) embryos were isolated at the two- to four-cell stage and incubated immediately at the nonpermissive temperature (25°C) through to the 20E stage, at which point they were heat-shocked. To block GLP-1 function later, glp-1(e2144) embryos were isolated at the permissive temperature (15°C) in a 15°C temperature-controlled room. Following incubation at 15°C past the 28-cell stage or 8E stage, embryos were shifted to the nonpermissive temperature and incubated until the 20E stage, at which point they were heat-shocked. Shift-down assays were performed by isolating and incubating glp-1(e2144) embryos at the nonpermissive temperature from the two- to four-cell stages and shifting to the permissive temperature after the 28-cell stage. After allowing them to develop to the 20E cell stage, embryos were heat-shocked.
Laser ablation
AB or P1 blastomeres were ablated at the two-cell stage with a pulsed laser microbeam (Photonic Instruments, Inc.) as described in Bowerman et al. (1992). After ablation, embryos from either wild-type or glp-1(e2144) worms were incubated to the equivalent of the 20E stage at 25°C, based on observation of total cell number. Embryos were then heat-shocked for 15 min at 33°C and incubated overnight at 20°C before scoring.
In vitro culture
The methods for culturing isolated blastomeres were adapted from Edgar and Goldstein (2012). After enzymatic digestion of the eggshell, blastomeres were separated by forcing embryos through a pulled glass needle. AB or P1 blastomeres were identified by size and mounted in 14 μL of Edgar's growth medium on a glass slide under a coverslip mounted with clay feet. Slides were sealed with Vaseline to prevent desiccation. To assess the ability of cells grown in isolation to differentiate, AB blastomeres from strain JG7 were incubated overnight at 20°C and scored for expression of the epidermal marker dpy-7∷GFP (Gilleard and McGhee 2001). P1 blastomeres from strain JR3339 were grown overnight at 20°C and scored for expression of muscle marker unc-54∷GFP. To test whether cultured AB blastomeres exhibit the same transition from developmental plasticity to commitment as intact embryos, AB isolates from wild-type embryos were allowed to develop at 20°C to the equivalent of the 2E and 20E stage, as determined by cell number; heat-shocked for 15 min at 33°C; returned to 20°C; and scored for elt-2∷GFP expression after overnight incubation. To test for GLP-1 dependence in commitment to differentiation, AB blastomeres were isolated from wild-type and glp-1(e2144) embryos, grown in vitro at 25°C to the equivalent of the 20E stage, and heat-shocked for 15 min at 33°C. After overnight incubation at 20°C, descendants were scored for elt-2∷gfp expression.
In situ hybridization
Mixed-stage embryos from N2, dsl-1(ok810), dsl-3(RNAi), or glp-1(e2144) worms were stained with DIG-labeled RNA probes for dsl-1 and dsl-3. Probes were made as described (http://www.faculty.ucr.edu/mmaduro) with appropriate primers (sequences provided on request). BLASTN analysis of the genomic sequences amplified by these primers indicated that only the targeted gene would be predicted to hybridize significantly.
Embryos obtained from bleached gravid adults were forced through 27.5-gauge needles to break up adult corpses and then washed three times in M9. Embryos in M9 suspension were immediately dispensed in 10-μL aliquots onto 14 × 14-mm square wells of polylysine-coated slides, covered with coverslips, and frozen. After freeze-crack, the Kohara Laboratory protocol for “large-scale fixation of embryos” was followed (http://nematode.lab.nig.ac.jp/method/protocol.php?docbase=insitu_embryo), replacing the dehydration (C.II.11–C.II.1112) and proteinase K treatment steps (D.I.1–D.I.19) with two 5-min washes in PBT. Hybridizations and subsequent warm temperature washes were performed at 58°C. For probe detection, slides were immersed in 400 μL of premixed NBT+BCIP solution (Roche Diagnostics GmbH, reference 11-681-451-001) mixed with 40 mL of staining buffer and developed overnight at room temperature.
Imaging and immunofluorescence analysis
The embryonic stage in any given experiment was determined by observing the number of elt-2∷gfp-expressing cells with an Olympus SZX12 fluorescence dissecting microscope. Terminal embryos were scored for ectopic elt-2∷gfp expression on either a Zeiss Axioskop 2 or Nikon Microphot SA fluorescence compound microscope. Embryos were mounted on 3% agar pads in egg salts (Edgar and Goldstein 2012). Images were processed with NIH ImageJ. For immunofluorescence analyses, embryos were mounted and stained with antibodies MH33 and 1CB4, as described in Zhu et al. (1998).
Acknowledgments
We are grateful to M. Kourakis, B. Birsoy, L. Chen, P.M. Joshi, and J. Casanova for comments on the manuscript. Some nematode strains were provided by the Caenorhabditis Genetics Center, which is funded by the NIH National Center for Research Resources (NCRR). N.J.-V.D. was supported in part by a training grant from the California Institute of Regenerative Medicine. This work was supported by grants from the NIH (HD062922) and March of Dimes (FY2007-804) to J.H.R.
Footnotes
Supplemental material is available for this article.
Article is online at http://www.genesdev.org/cgi/doi/10.1101/gad.199588.112.
References
- Ables JL, Decarolis NA, Johnson MA, Rivera PD, Gao Z, Cooper DC, Radtke F, Hsieh J, Eisch AJ 2010. Notch1 is required for maintenance of the reservoir of adult hippocampal stem cells. J Neurosci 30: 10484–10492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artavanis-Tsakonas S, Muskavitch MA 2010. Notch: The past, the present, and the future. Curr Top Dev Biol 92: 1–29 [DOI] [PubMed] [Google Scholar]
- Babu P 1974. Biochemical genetics of Caenorhabditis elegans. Mol Genet Genomics 135: 39–44 [Google Scholar]
- Baugh LR, Hill AA, Slonim DK, Brown EL, Hunter CP 2003. Composition and dynamics of the Caenorhabditis elegans early embryonic transcriptome. Development 130: 889–900 [DOI] [PubMed] [Google Scholar]
- Berry LW, Westlund B, Schedl T 1997. Germ-line tumor formation caused by activation of glp-1, a Caenorhabditis elegans member of the Notch family of receptors. Development 124: 925–936 [DOI] [PubMed] [Google Scholar]
- Bowerman B, Tax FE, Thomas JH, Priess JR 1992. Cell interactions involved in development of the bilaterally symmetrical intestinal valve cells during embryogenesis in Caenorhabditis elegans. Development 116: 1113–1122 [DOI] [PubMed] [Google Scholar]
- Chen N, Greenwald I 2004. The lateral signal for LIN-12/Notch in C. elegans vulval development comprises redundant secreted and transmembrane DSL proteins. Dev Cell 6: 183–192 [DOI] [PubMed] [Google Scholar]
- Christensen S, Kodoyianni V, Bosenberg M, Friedman L, Kimble J 1996. lag-1, a gene required for lin-12 and glp-1 signaling in Caenorhabditis elegans, is homologous to human CBF1 and Drosophila Su(H). Development 122: 1373–1383 [DOI] [PubMed] [Google Scholar]
- Edgar LG, Goldstein B 2012. Culture and manipulation of embryonic cells. Methods Cell Biol 107: 151–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser AG, Kamath RS, Zipperlen P, Martinez-Campos M, Sohrmann M, Ahringer J 2000. Functional genomic analysis of C. elegans chromosome I by systematic RNA interference. Nature 408: 325–330 [DOI] [PubMed] [Google Scholar]
- Fukushige T, Krause M 2005. The myogenic potency of HLH-1 reveals wide-spread developmental plasticity in early C. elegans embryos. Development 132: 1795–1805 [DOI] [PubMed] [Google Scholar]
- Gendreau SB, Moskowitz IP, Terns RM, Rothman JH 1994. The potential to differentiate epidermis is unequally distributed in the AB lineage during early embryonic development in C. elegans. Dev Biol 166: 770–781 [DOI] [PubMed] [Google Scholar]
- Gilleard JS, McGhee JD 2001. Activation of hypodermal differentiation in the Caenorhabditis elegans embryo by GATA transcription factors ELT-1 and ELT-3. Mol Cell Biol 21: 2533–2544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein B 1992. Induction of gut in Caenorhabditis elegans embryos. Nature 357: 255–257 [DOI] [PubMed] [Google Scholar]
- Henderson ST, Gao D, Christensen S, Kimble J 1997. Functional domains of LAG-2, a putative signaling ligand for LIN-12 and GLP-1 receptors in Caenorhabditis elegans. Mol Biol Cell 8: 1751–1762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitoshi S, Alexson T, Tropepe V, Donoviel D, Elia AJ, Nye JS, Conlon RA, Mak TW, Bernstein A, van der Kooy D 2002. Notch pathway molecules are essential for the maintenance, but not the generation, of mammalian neural stem cells. Genes Dev 16: 846–858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horner MA, Quintin S, Domeier ME, Kimble J, Labouesse M, Mango SE 1998. pha-4, an HNF-3 homolog, specifies pharyngeal organ identity in Caenorhabditis elegans. Genes Dev 12: 1947–1952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutter H, Schnabel R 1994. glp-1 and inductions establishing embryonic axes in C. elegans. Development 120: 2051–2064 [DOI] [PubMed] [Google Scholar]
- Joshi PM, Riddle MR, Djabrayan NJ, Rothman JH 2010. Caenorhabditis elegans as a model for stem cell biology. Dev Dyn 239: 1539–1554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamath RS, Fraser AG, Dong Y, Poulin G, Durbin R, Gotta M, Kanapin A, Le Bot N, Moreno S, Sohrmann M, et al. 2003. Systematic functional analysis of the Caenorhabditis elegans genome using RNAi. Nature 421: 231–237 [DOI] [PubMed] [Google Scholar]
- Kodoyianni V, Maine EM, Kimble J 1992. Molecular basis of loss-of-function mutations in the glp-1 gene of Caenorhabditis elegans. Mol Biol Cell 3: 1199–1213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mango SE, Thorpe CJ, Martin PR, Chamberlain SH, Bowerman B 1994. Two maternal genes, apx-1 and pie-1, are required to distinguish the fates of equivalent blastomeres in the early Caenorhabditis elegans embryo. Development 120: 2305–2315 [DOI] [PubMed] [Google Scholar]
- Matsumoto A, Onoyama I, Sunabori T, Kageyama R, Okano H, Nakayama KI 2011. Fbxw7-dependent degradation of Notch is required for control of ‘stemness’ and neuronal–glial differentiation in neural stem cells. J Biol Chem 286: 13754–13764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGovern M, Voutev R, Maciejowski J, Corsi AK, Hubbard EJ 2009. A ‘latent niche’ mechanism for tumor initiation. Proc Natl Acad Sci 106: 11617–11622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello CC, Draper BW, Priess JR 1994. The maternal genes apx-1 and glp-1 and establishment of dorsal–ventral polarity in the early C. elegans embryo. Cell 77: 95–106 [DOI] [PubMed] [Google Scholar]
- Moskowitz IP, Rothman JH 1996. lin-12 and glp-1 are required zygotically for early embryonic cellular interactions and are regulated by maternal GLP-1 signaling in Caenorhabditis elegans. Development 122: 4105–4117 [DOI] [PubMed] [Google Scholar]
- Moskowitz IP, Gendreau SB, Rothman JH 1994. Combinatorial specification of blastomere identity by glp-1-dependent cellular interactions in the nematode Caenorhabditis elegans. Development 120: 3325–3338 [DOI] [PubMed] [Google Scholar]
- Priess JR. 2005 Notch signaling in the C. elegans embryo. In WormBook (ed. The C. Elegans Community), WormBook . doi: 10.1895/wormbook.1.4.1. [Google Scholar]
- Priess JR, Schnabel H, Schnabel R 1987. The glp-1 locus and cellular interactions in early C. elegans embryos. Cell 51: 601–611 [DOI] [PubMed] [Google Scholar]
- Schnabel R, Priess JR 1997. Specification of cell fates in the early embryo. In C. elegans II, 2nd ed. (ed. DL Riddle et al.), pp. 361–382. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- Shaye DD, Greenwald I 2005. LIN-12/Notch trafficking and regulation of DSL ligand activity during vulval induction in Caenorhabditis elegans. Development 132: 5081–5092 [DOI] [PubMed] [Google Scholar]
- Sonnichsen B, Koski LB, Walsh A, Marschall P, Neumann B, Brehm M, Alleaume AM, Artelt J, Bettencourt P, Cassin E, et al. 2005. Full-genome RNAi profiling of early embryogenesis in Caenorhabditis elegans. Nature 434: 462–469 [DOI] [PubMed] [Google Scholar]
- Wang Y, Tu W, Lou Y, Xie A, Lai X, Guo F, Deng Z 2009. Mesenchymal stem cells regulate the proliferation and differentiation of neural stem cells through Notch signaling. Cell Biol Int 33: 1173–1179 [DOI] [PubMed] [Google Scholar]
- Yuzyuk T, Fakhouri TH, Kiefer J, Mango SE 2009. The Polycomb complex protein mes-2/E(z) promotes the transition from developmental plasticity to differentiation in C. elegans embryos. Dev Cell 16: 699–710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou ZD, Kumari U, Xiao ZC, Tan EK 2010. Notch as a molecular switch in neural stem cells. IUBMB Life 62: 618–623 [DOI] [PubMed] [Google Scholar]
- Zhu J, Fukushige T, McGhee JD, Rothman JH 1998. Reprogramming of early embryonic blastomeres into endodermal progenitors by a Caenorhabditis elegans GATA factor. Genes Dev 12: 3809–3814 [DOI] [PMC free article] [PubMed] [Google Scholar]