Abstract
Purpose
To describe the incidence, microbiology, associated factors and clinical outcomes of patients with infectious keratitis progressing to endophthalmitis.
Design
Non-randomized, retrospective, consecutive case series.
Participants
All patients treated for culture-proven keratitis and endophthalmitis between January 1, 1995 and December 31, 2009 at the Bascom Palmer Eye Institute.
Methods
Ocular microbiology and medical records were reviewed on all patients with positive corneal and intraocular cultures over the period of the study. Univariate anaylsis was performed to obtain p values described in the study.
Main Outcome Measures
Microbial isolates, treatment strategies, visual acuity outcomes.
Results
A total of 9934 corneal cultures were performed for suspected infectious keratitis. Only 49 eyes (0.5%) progressed to culture-proven endophthalmitis. Fungi (n=26) were the most common responsible organism followed by gram positive bacteria (n=13) and gram negative bacteria (n=10). Topical steroid use (37/49[76%]) was the most common associated factor identified in the current study, followed by previous surgery (30/49[61%]), corneal perforation (17/49[35%]), dry eye (15/49[31%]), relative immune compromise (10/49[20%]), organic matter trauma (9/49[18%]) and contact lens wear (3/49[6%]). There were 27 patients in which a primary infectious keratitis developed into endophthalmitis, and 22 patients in which an infectious keratitis adjacent to a previous surgical wound progressed into endophthalmitis. Patients in the primary keratitis group were more likely to be male (22/27[81%] vs. 8/22[36%], p=0.001), have history of organic matter trauma (8/27[30%] vs. 1/22[5%]), p=0.030), and have fungal etiology (21/27[78%] vs. 5/22[23%], p<0.001). Patients in the surgical-wound-associated group were more likely to use topical steroids (20/22[91%] vs. 17/27[63%], p=0.024). Visual acuity of ≥20/50 was achieved in 7/49[14%] patients, but was <5/200 in 34/49[69%] patients at last follow up. Enucleation or evisceration was performed in 15/49[31%] patients.
Conclusions
Progression of infectious keratitis to endophthalmitis is relatively uncommon. The current study suggests that patients at higher risk for progression to endophthalmitis include patients using topical corticosteroids, patients with fungal keratitis, patients with corneal perforation, and patients with infectious keratitis developing adjacent to a previous surgical wound. Patients with sequential keratitis and endophthalmitis have generally poor visual outcomes.
Introduction
Infectious keratitis uncommonly progresses to endophthalmitis. While a number of small case series describing infectious keratitis associated with endophthalmitis exist, there are few consecutive case series on the subject.1–20 Additionally, because patients with infectious keratitis often present with marked visual loss, pain, hypopyon, and a poorly visualized posterior segment, distinguishing keratitis from endophthalmitis can sometimes be difficult. The purpose of the current study is to describe a consecutive series of patients with infectious keratitis progressing to endophthalmitis and to report the associated microbiology, associated factors, and clinical outcomes in these challenging patients.
Patients and methods
Institutional Review Board approval was obtained from the University of Miami Miller School of Medicine Sciences Subcommittee for the Protection of Human Subjects. The ocular microbiology department database was searched to identify all patients with positive corneal and intraocular cultures (anterior chamber and/or vitreous) between January 1, 1995 and December 31, 2009. To be considered in the current study, the same organism was required to be positive from both corneal and intraocular cultures. Therefore, only culture-proven cases of sequential keratitis and endophthalmitis were included. Microbiology department records were reviewed to identify the responsible microbial isolates and antibiotic sensitivities.
Corneal cultures were obtained at presentation, or within days of presentation, in all cases. Specimens were obtained via corneal scraping with a Beaver blade and plated directly onto several different culture media, which typically included chocolate agar, 5% sheep blood agar, and Sabouraud agar. Gram stains and giemsa stains were also performed. Blood and chocolate agars underwent incubation at 35 degrees Celsius for a period of up to 2 weeks. Sabouraud agars underwent incubation at 35 degrees Celsius for a period of 24 to 36 hours and then at 25 degrees Celsius for up to 2 more weeks. Additional culture media, including thioglycollate broth, Lowenstein-Jensen medium, and agar agar media were performed at the discretion of the ophthalmologist performing the culture.
Anterior chamber cultures were most often obtained at the time of penetrating keratoplasty. In a few instances, anterior chamber cultures were obtained from an anterior chamber paracentesis. In these instances, care was taken to pass the needle through clear cornea, to avoid contamination of the specimen by infected corneal tissue, and to avoid introduction of microbes into the anterior chamber. Vitreous cultures were obtained either at the time of vitreous tap and inject or during vitrectomy. Fluids from anterior chamber paracentesis or vitreous tap were plated directly on to culture media, and were handled in an identical fashion to corneal specimens. For vitrectomy specimens, 30–50 cubic centimeters (cc) of vitreous washings were filtered using a 0.45 micron filter. The resultant filter paper was divided into sections and was plated on to different culture media, which typically included chocolate agar, 5% sheep blood agar, and Sabouraud agar.
All cultures were read and classified by Ocular Microbiology Department staff. Antibiotic sensitivities were performed on all gram-positive and gram-negative bacteria. Antifungal sensitivities were not routinely assessed.
After analyzing microbiology records, the corresponding medical records of these patients were reviewed. Patient demographics, clinical characteristics, risk factors, treatment strategies and clinical outcomes were assessed. Exclusion criteria included patients with endophthalmitis occurring within 6 weeks of a previous surgery, inadequate clinical records (<30 days of follow up), bleb-associated infections, penetrating trauma, and cases of viral keratitis.
The remaining patients were divided into two categories: patients in which a primary keratitis developed into endophthalmitis (defined as “primary keratitis”), and patients in which an infectious keratitis associated with a previous surgical wound developed into endophthalmitis (defined as “surgical-wound-associated keratitis”). In the current study, endophthalmitis was defined by the presence of positive intraocular cultures. When anterior segment opacities prevented a view of the posterior segment, echography was consistently performed.
Pearson chi-squared test and Fisher exact test were used to compare basic characteristics of the two groups using SISA online statistical analysis (Uitenbroek DG. 1997. SISA. <http://www.quantitativeskills.com/sisa/index.htm>. Accessed September 4, 2011). The t test was used for 2 independent samples. All P-values are two-sided and nominal.
Results
Demographics and Clinical Features
Over the 15-year period of the study, 9934 corneal cultures were performed for cases of suspected infectious keratitis, of which 3724 corneal cultures (37.5%) were positive. During this time period, 68 eyes were identified to have both positive corneal and intraocular cultures, of which 19 were excluded from the study. Reasons for exclusion included: endophthalmitis occurring within 6 weeks of a previous surgery (9 patients), inadequate clinical records (5 patients), bleb-associated endophthalmitis (3 patients), and penetrating ocular trauma (2 patients). Thus of 9934 cases of clinically suspected keratitis, 49 eyes (0.5%) which had progressed to culture-proven endophthalmitis were included.
The average age of patients in the current series was 61.4 ± 17.6 years (median 57, range 10–96 years) and 30/49 [61.2%] were male. Patients in our series were divided into two classifications: patients in which a primary keratitis developed into endophthalmitis (defined as “primary keratitis”), and patients in which an infectious keratitis adjacent to a previous surgical wound developed into endophthalmitis (defined as “surgical-wound-associated keratitis”). Demographics and risk factors for these patients are presented in Table 1 and representative cases are shown in Figure 1. Patients in the primary keratitis groups were younger (average 54.8 years, range 10–96) compared to patients in the surgical-wound-associated keratitis group (average age 69.4 years, range 26–91). Additionally, patients in the primary keratitis group were more likely to be male (22/27 [81%]), to have a history of trauma (12/27 [44%]), to have a foreign body injury (11/27 [41%]), organic matter trauma (8/27 [30%]), and to have a fungal causative organism (21/27 [78%]). Patients in the surgical-wound-associated keratitis group were more likely to be female (14/22 [64%]), to have used topical steroids (20/22, [91%]), and to have a bacterial causative organism (17/22 [77%]).
Table 1.
Comparison of characteristics of patients with keratitis and subsequent endophthalmitis
| Primary Keratitis | Surgical-Wound- Associated Keratitis |
P valuea | |
|---|---|---|---|
| Average age | 54.8± 15.1 years | 69.4 ± 17.5 years | 0.003b |
| Male gender | 22/27 (81.5%) | 8/22 (36.4%) | 0.001 |
| Female gender | 5/27 (18.5%) | 14/22 (63.6%) | - |
| Contact lens wear | 3/27 (11.1%) | 0/22 (0.0%) | 0.242c |
| History of trauma | 12/27 (44.4%) | 3/22 (13.6%) | 0.029c |
| Foreign body injury | 11/27 (40.7%) | 2/22 (9.1%) | 0.021c |
| Organic matter trauma | 8/27 (29.6%) | 1/22 (4.5%) | 0.030c |
| Corneal perforation | 11/27 (40.7%) | 6/22 (27.3%) | 0.325 |
| Dry eye syndrome or blepharitis | 6/27 (22.2%) | 9/22 (40.9%) | 0.158 |
| Diabetes mellitus | 3/27 (11.1%) | 3/22 (13.6%) | 1.000c |
| Immune suppression | 3/27 (11.1%) | 1/22 (4.5%) | 0.617c |
| Oral steroid use | 1/27 (3.7%) | 0/22 (0.0%) | 1.000c |
| Topical steroid use | 17/27 (63.0%) | 20/22 (90.9%) | 0.024 |
| Previous surgery | 8/27 (29.6%) | 22/22 (100%) | <0.001 |
| Average number previous surgeries | 0.37 | 2.36 | - |
| Fungal etiology | 21/27 (77.8%) | 5/22 (22.7%) | <0.001 |
| Bacterial etiology | 6/27 (22.2%) | 17/22 (77.3%) | - |
Notes:
= χ2 test,
=Student t test,
= Fisher exact test
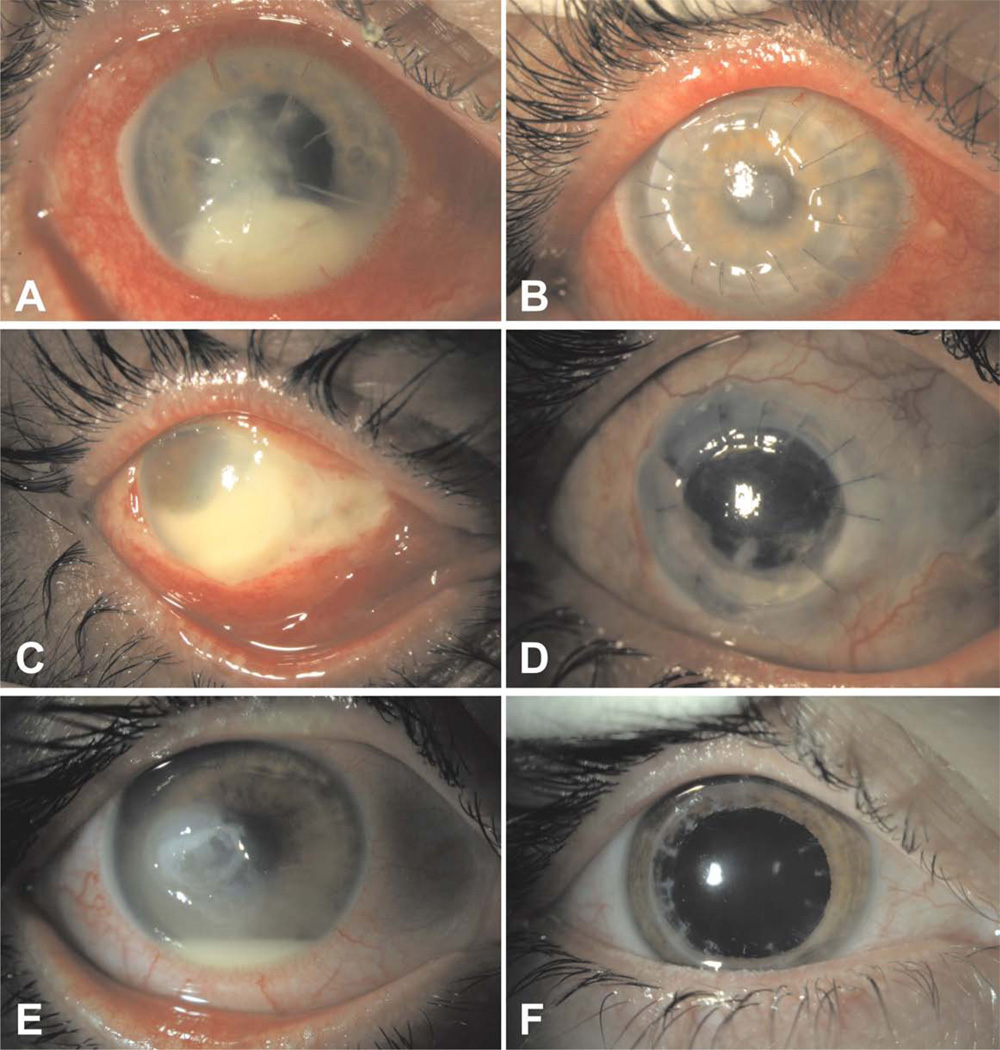
Figure 1.
A, A 56-year male with a history of radial keratotomy developed Fusarium keratitis and consequent endophthalmitis after a weekend of camping. Presenting visual acuity was 6/200.
B, Appearance of the patient from figure A one month after undergoing treatment with topical natamycin, intracameral voriconazole, and penetrating keratoplasty. The patient eventually regained 20/20 visual acuity with use of a hard contact lens.
C, A 47-year-old male developed Pseudomonas aeruginosa keratitis and subsequent endophthalmitis after a non-perforating corneal abrasion from a tree branch.
D, Appearance of the patient from figure C two years following management with topical tobramycin, intravitreal ceftazidime, penetrating keratoplasty, and subsequent extracapsular cataract extraction with secondary intraocular lens. He regained hand motions vision.
E, A 26-year-old male developed Mycobacterium chelonae endophthalmitis after a 4 month history of chronic keratitis following LASIK surgery. Presenting vision was hand motions.
F, Appearance of the patient from figure E eight months after management with topical amikacin, topical clarithromycin, subconjunctival amikacin, and penetrating keratoplasty with anterior chamber washout. The patient recovered 20/30 visual acuity.
At the time of presentation, most patients were using topical steroids (37/49 [76%]), representing the most common associated factor identified in the current study. Only 3 of 49 (6%) patients were contact-lens wearers, all of which had a fungal etiology. A history of prior intraocular surgery was noted in thirty of 49 (61%) patients. The average number of previous intraocular surgeries for all patients was 1.27 (range 0–7). In patients with a previous surgical history, the average length of time from most recent surgery to the date of presentation was 1087 days (median 255.5, range 48–8349 days). All patients with keratitis and endophthalmitis occurring within 6 weeks of a previous surgery were excluded from the current study.
Clinical features at presentation included conjunctival injection (49/49 [100%]), hypopyon (45/49 [92%]), documented vitritis on clinical exam and/or echography (25/49 [51%]), and moderate to severe pain (38/49 [78%]). Corneal perforation was common, occurring in 17/49 eyes (35%). Of patients in the primary keratitis group, 11/27 eyes (41%) had a corneal perforation compared to 6/22 eyes (27%) in the surgical-wound-associated keratitis group, which was not a statistically significant difference (p=0.325).
The clinical diagnosis of endophthalmitis was suspected by physicians based on characteristic features in 44 of 49 patients (90%). In 5 of 49 patients (10%), physicians were uncertain of the diagnosis at the time of intraocular cultures due to equivocal clinical findings or patients presenting early in the disease course. Due to the retrospective nature of this study, a full description of which clinical features led to the diagnosis of endophthalmitis were not always available in the medical record.
Microbial Isolates and Antibiotic Sensitivities
Of the 49 eyes included in the current study, all had documented keratitis before the development of endophthalmitis. A single organism was identified in 39 eyes and multiple organisms were identified in 10 eyes. Fungi (n=26) were the most common responsible organism followed by gram positive bacteria (n=13) and gram negative bacteria (n=10). A summary of all responsible microbial isolates is shown in Table 2 (available online at http://aaojournal.org). Corneal cultures were performed in 49 patients, anterior chamber cultures were performed in 34 patients, and vitreous cultures were performed in 22 patients. All 49 patients had positive corneal cultures, 31 patients had positive anterior chamber cultures and 22 patients had positive vitreous cultures. Four patients had positive corneal, anterior chamber, and vitreous cultures. Fungi responsible for endophthalmitis were isolated from the anterior chamber alone in 20 cases, from the vitreous alone in 5 cases, and from both in 1 case. Bacteria responsible for endophthalmitis were isolated from the anterior chamber alone in 7 cases, from the vitreous alone in 13 cases, and from both in 3 cases.
In the primary keratitis group, fungi accounted for the majority of cases (21/27 [78%])) progressing to endophthalmitis. There were 6 of 27 cases in the primary keratitis group where bacterial keratitis progressed to endophthalmitis, each of which had special circumstances which predisposed the patients to serious infection including: a recent history of organic matter trauma (n=2), Mooren’s ulcer (n=1), neurotrophic keratopathy (n=1), and a previous history of herpes zoster ophthalmicus in the setting of human immunodeficiency virus (HIV) infection (n=1). A final patient with sequential Pseudomonas keratitis and endophthalmitis had a history of urine exposure to the eye as an attempted treatment for conjunctivitis.
In the surgical-wound-associated group, bacteria accounted for the majority of cases (17/22 [77%])). Scenarios associated with infection included suture abscess (n=6), wound dehiscence (n=3), or wound leak (n=3) occurring months to years after the original surgery. These cases were distinct from cases of postoperative endophthalmitis, which were excluded from the current study.
Antibiotic sensitivities were assessed for gram positive and gram negative bacteria. Among gram positive isolates, 100% (13/13) were sensitive to vancomycin, 55% (6/11) were sensitive to fluoroquinolones, and 30% (3/10) were sensitive to trimethoprim/sulfa. The single case of Mycobacterium chelonae was sensitive to amikacin, but was resistant to trimethoprim/sulfa and ciprofloxacin. Gram negative isolates were found to be sensitive to a wide range on antibiotics with 100% of gram negative isolates (10/10) sensitive to amikacin, ceftazidime, tobramycin, gentamicin, and ciprofloxacin. All gram negative bacteria were resistant to cefazolin and ampicillin.
Treatment Strategies
Nearly all patients, 47 of 49 (96%), were treated with topical antibiotics or antifungals before and/or after the diagnosis of keratitis and endophthalmitis. (Table 3) The two patients who were not treated with topical antimicrobial agents underwent primary enucleation. Of the fungal cases, 26 of 26 patients (100%) received topical antifungal drops, most commonly natamycin (18/26 [69%]), amphotericin B (5/26 [19%]) or multiple antifungal agents (2/26 [8%]). Topical amphotericin B was used in all five cases caused by Candida. Of the bacterial cases, 20 of 22 (91%) received topical antibiotics which were tailored based on antibiotic sensitivities.
Table 3.
Treatment strategies in patients with keratitis and subsequent endophthalmitis
| Primary Keratitis | Surgical-Wound- ssociated Keratitis |
P valuea | |
|---|---|---|---|
| Topical antimicrobials | 27/27 (100%) | 20/22 (90.9%) | 0.056 |
| Intraocular antimicrobials | 23/27 (85.2%) | 16/22 (72.7%) | 0.282 |
| Intraocular steroids | 1/27 (3.7%) | 10/22 (45.5%) | <0.001b |
| Pars Plana Vitrectomy | 7/27 (25.9%) | 10/22 (45.5%) | 0.153 |
| Penetrating Keratoplasty | 22/27 (81.5%) | 11/22 (50.0%) | 0.019 |
| Enucleation or Evisceration | 7/27 (25.9%) | 8/22 (36.4%) | 0.430 |
Notes:
= χ2 test
=Fisher exact test
Intraocular antimicrobials were administered in 39 of 49 patients (80%). Among patients with fungal infections, intraocular antifungals were used in 23 of 26 cases (88%): most commonly amphotericin B (16/23 [70%]), voriconazole (2/23 [9%]), or multiple antifungal agents 5/23 [22%]). In three patients with fungal keratitis and endophthalmitis, penetrating keratoplasty (PKP) and anterior chamber irrigation alone were performed. In these patients, the diagnosis endophthalmitis at the time of PKP was uncertain, and intraocular antifungals were not administered. Among patients with bacterial infections, intraocular antibiotics were used in 16 of 23 cases (70%), with ceftazidime and vancomycin being the antibiotics of choice. Of the seven patients with bacterial keratitis and endophthalmitis who were not treated with intraocular antimicrobial agents, five underwent primary enucleation, and two underwent PKP with anterior chamber irrigation and culture. In these two patients, the anterior chamber culture was obtained at the time of surgery, and the diagnosis of endophthalmitis was not made until culture results returned.
Intraocular steroids were used in 11 of 49 (22%) patients, and were used more commonly in patients with surgical-wound associated keratitis and endophthalmitis. (Table 3) Intraocular steroids were avoided in cases of suspected or confirmed fungal endophthalmitis, accounting for the relatively low rates of use.
Pars plana vitrectomy (PPV) was performed after the diagnosis of endophthalmitis in 17 of 49 patients (35%). The median length of time from presentation to vitrectomy was 2 days (average 25, range 0–195 days). Clinical data pertaining to the subset of patients undergoing PPV is highlighted in Table 4 (available online at http://aaojournal.org). Globe salvage, defined as avoidance of enucleation or evisceration, was achieved in 13 of 17 (76%) patients undergoing PPV.
PKP was performed in 33 of 49 patients (67%). The median length of time from presentation to PKP was 12 days (average 56.5, range 0–641 days). In the primary keratitis group, 22 of 27 patients (81%) underwent PKP; 19 for therapeutic purposes and 3 for optical purposes. Of these, 12 of 22 (55%) had eventual graft failure and 9 of 22 (41%) underwent subsequent PKP. In the surgical-wound-associated keratitis group, 11 of 22 patients (50%) underwent PKP; 9 for therapeutic purposes and 2 for optical purposes. Of these, 3 of 11 (27%) had eventual graft failure, and 3 of 11 (27%) underwent subsequent PKP.
Patients with fungal keratitis and endophthalmitis underwent PKP more commonly (24/26 [92%]) than patients with bacterial keratitis and endophthalmitis (9/23 [39%]), which was a statistically significant difference (p < 0.001). Among patients with fungal keratitis and endophthalmitis undergoing PKP, 12 of 24 (50%) had eventual graft failure and 10 of 24 patients (42%) underwent subsequent PKP.
Clinical Outcomes
Best corrected visual acuity (VA) outcomes at last examination ranged from 20/20 to no light perception (NLP). (Table 5) Among all patients, only 7 of 49 (14%) achieved a VA of 20/50 or greater at last follow up. A VA of 20/400 or better was achieved in 12 of 49 patients (24%). NLP vision was present in 17 of 49 patients (35%), including 15 of 49 patients (31%) who ultimately underwent enucleation or evisceration.
Table 5.
Visual Acuity at last follow up visit
| ≥ 20/50 | ≥ 20/400 | < 5/200 | No Light Perception |
|
|---|---|---|---|---|
|
All Patients Primary Keratitis Wound-Associated Keratitis |
14% (7/49) 19% (5/27) 9% (2/22) |
24% (12/49) 26% (7/27) 23% (5/22) |
69% (34/49) 70% (19/27) 68% (15/22) |
35% (17/49) 30% (8/27) 41% (9/22) |
|
Fungi Fusarium sp Candida sp Other fungi |
19% (5/26) 14% (2/14) 25% (1/5) 29% (2/7) |
31% (8/26) 21% (3/14) 40% (2/5) 43% (3/7) |
62% (16/26) 71% (10/14) 40% (2/5) 57% (4/7) |
27% (7/26) 36% (5/14) 0% (0/5) 29% (2/7) |
|
Gram Positive Bacteria Streptococcus species Staphylococcus species |
8% (1/13) 0% (0/10) 0% (0/2) |
23% (3/13) 0% (0/10) 100% (2/2) |
69% (9/13) 90% (9/10) 0% (0/2) |
31% (4/13) 40% (4/10) 0% (0/2) |
|
Gram Negative Bacteria Pseudomonas aeruginosa Serratia Marcescens |
10% (1/10) 13% (1/8) 0% (0/2) |
10% (1/10) 13% (1/8) 0% (0/2) |
90% (9/10) 88% (7/8) 100% (2/2) |
60% (6/10) 50% (4/8) 100% (2/2) |
VA outcomes were similar among patients in the primary keratitis and surgical-wound-associated keratitis groups. Though a higher percentage of patients in the primary keratitis group achieved VA ≥ 20/50 (5/27[19%] vs. 2/22[9%]), this was not a statistically significant difference (p=0.436, Fisher exact test). For patients with gram negative infections, VA outcomes were particularly poor; all patients with Serratia marcescens infections were NLP at last follow up visit and 7 of 8 (88%) patients with Pseudomonas aeruginosa infections were hand motion VA or worse. For patients with streptococcal infections, 9 of 10 (90%) patients had hand motions or worse VA at last follow up. More patients with fungal keratitis and endophthalmitis achieved a VA ≥ 20/50 than those with bacterial keratitis and endophthalmitis (5/26 [19%]) vs. 2/23 [9%]), but this was not a statistically significant difference (p=0.430, Fisher exact test). In patients who had a corneal perforation, only 2/17 patients (12%) achieved VA ≥ 20/50, while 13/17 patients (76%) had worse than 5/200 VA at last visit, including 6/17 (35%) who ultimately underwent enucleation.
Secondary complications of patients included retinal detachment (5/52 [10%]), secondary glaucoma (20/52 [38%]) and irregular astigmatism (18/52 [35%]).
Among the 15 patients presenting with LP vision at presentation, 2 of 4 (50%) undergoing PPV achieved a VA ≥ 20/400 at last visit compared to 0 of 11 (0%) not undergoing PPV, which approached statistical significance (p=0.057). Patients undergoing PKP did relatively well with 12 of 33 (36%) patients achieving a VA ≥20/400 and 7 of 33 (21%) with ≥20/50 VA. Two patients undergoing PKP achieved 20/20 visual acuity.
Discussion
Microbial keratitis is common, with an estimated incidence of 30,000 cases per year in the United States.21–23 Progression of keratitis to endophthalmitis, on the other hand, is considered to be uncommon. This was confirmed in the current study, in which only 0.5% of eyes with clinically suspected keratitis progressed to culture-proven endophthalmitis (49 cases of endophthalmitis/9934 corneal cultures performed). Some patients presenting to with small or peripheral corneal ulcers did not undergo corneal cultures, meaning the true incidence of endophthalmitis developing from keratitis may be even lower. This was balanced somewhat by the fact that a few patients underwent multiple corneal cultures over the period of the study.
There were a number of associated factors that may have predisposed patients to infection in the current study. The use of topical corticosteroids was seen in 37 of 49 patients (76%), and was the most common associated factor identified. Patients tended to be older, with an average age of 61.4. Relative immune compromise from HIV, leukemia/lymphoma, or diabetes mellitus was seen in over a fifth of patients (10/49 [20%]) which is higher than the prevalence in the general population of 8.3% for diabetes mellitus (25.8 million Americans) and 0.4% for HIV (1.2 million Americans).31,32 Dry eye syndrome (15/49 [31%]), foreign body injury (13/49 [27%]), and organic matter exposure (9/49 [18%]) were also relatively common risk factors in our series. The use of contact lenses was not a common risk factor in our series as only 3 patients had contact-lens related ulcers progressing to endophthalmitis. Corneal perforation occurred in 17 of 49 (35%) cases and was particularly common in cases of primary keratitis progressing to endophthalmitis (11/27 [41%]).
Among the cases of primary keratitis progressing to endophthalmitis, fungi accounted for 21 of 27 cases (78%), suggesting that a fungal etiology may increases the risk of progression to endophthalmitis. This makes sense mechanistically, because fungi such as Fusarium have demonstrated the ability to penetrate an intact cornea.14 In the current study, fungi causing keratitis and endophthlamitis were preferentially isolated from the anterior chamber, and in many cases infections appeared to be contained within the anterior chamber without spreading to the vitreous.
Topical corticosteroids have been previously demonstrated to potentiate the growth and invasiveness of bacteria and fungi by suppressing immune defense mechanisms, particularly when used without concomitant antimicrobial agents.14, 23–29 In addition, the use of topical corticosteroids has been shown to increase the risk ulcerative keratitis in patients with pre-existing corneal disesases.23,29 The use of corticosteroids prior to the development of keratitis can lead to worse treatment outcomes and an increased likelihood of antibiotic failure.23 Very recently, the steroids for corneal ulcers trial (SCUT), a randomized, placebo-controlled, double-masked trial of 500 patients, compared clinical outcomes with and without the use of topical corticosteroids as adjunctive therapy in the treatment of bacterial corneal ulcers. In this study, no safety concerns were reported with the use of topical corticosteroids, including no cases of endophthalmitis and no increased risk of corneal perforation. Primary outcomes, however, were assessed only through 3 months in this trial, and safety outcomes after long-term use of corticosteroids in patients with a history of microbial keratitis remain unknown.33
Topical cyclosporine A, in contrast, has been shown to inhibit the growth of fungi in vitro.25 More recently, topical tacrolimus (FK506), also a calcineurin inhibitor, has also been shown to inhibit fungal growth in a murine model of Aspergillus keratitis when used in combination with topical amphotericin B, voriconazole, or polyhexamethylene biguanide.34 Calcineurin inhibitors should be considered as an alternative agent to corticosteroids, especially in cases of fungal keratitis.
Topical and intravitreal antimicrobials were the mainstays of treatment in the current series. In cases that present with a bacterial etiology, antibiotic sensitivity results from the current study reinforce the standard use of intravitreal vancomycin and ceftazidime. However, antimicrobial agents did not prove sufficient in many cases as PPV and/or PKP were required in 35% and 67% of cases respectively. In the Endophthalmitis Vitrectomy Study, patients with light perception vision who underwent vitrectomy had superior visual outcomes compared to intravitreal antibiotics alone.30 In our study, 15 patients presented with light perception vision of which 50% undergoing PPV achieved a vision ≥ 20/400 at final outcome compared to 0% not undergoing PPV, which approached statistical significance (p=0.057). The retrospective nature of this study and small sample size, however, prevented definitive conclusions regarding benefits of PPV in this population. In patients undergoing PKP in the current study, initial graft failure was common (15 of 33 cases, 45%), however, many of these patents ultimately did well with 36% (12/33) achieving a visual acuity ≥20/400 and 21% (7/33) with ≥20/50 vision.
There are a few smaller case series in the literature that have evaluated sequential keratitis and endophthalmitis. A previous study by Scott et al. looked at patients presenting with positive corneal and intraocular cultures between 1990 and 1995.1 1699 corneal cultures were performed over the period of their study, of which, 14 eyes (0.8%) progressed to culture proven endophthalmitis. Similar to our study, topical corticosteroid use (93%) and a history of ocular surgery (57%) were common. Additionally, visual acuity outcomes in this series were poor with only 43% of patients achieving vision ≥20/200. In contrast to our study, gram negative bacteria were the most commonly isolated organisms, though the study size was smaller. A second study by Dursun et al. looked at 159 cases of Fusarium keratitis, of which 10 (6.3%) progressed to endophthalmitis, which supports the theory that fungal keratitis is more likely than bacterial keratitis to progress to endophthalmitis.14 Risk factors in this series included trauma (60%), topical steroid use (20%), contact lens use (10%) and LASIK surgery followed by minor trauma (10%). All patients in the Dursun series were 1immunocompetent, and developed endophthalmitis via contiguous intraocular spread. Other case reports in the literature suggest relative immune compromise, topical steroid use, previous surgical history, and pre-existing corneal disease as possible risk factors for progression of keratitis to endophthlamitis.4–20
Progression of infectious keratitis to endophthalmitis is relatively uncommon. The current study suggests that patients at higher risk for progression to endophthalmitis include patients using topical corticosteroids, patients with fungal keratitis, patients with corneal perforation, and patients with infectious keratitis developing adjacent to a previous surgical wound. Patients with sequential keratitis and endophthalmitis have generally poor visual outcomes.
Supplementary Material
Acknowledgments
Sources of Financial Support: This research was funded in part by an unrestricted grant from Research to Prevent Blindness Inc., New York, NY and the National Institutes of Health NEI Center Grant P30 EY014801.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: CRH: None, HWF: Consultant for Alimera, Pfizer, Santen. DM: None, RKF: None, ECA: Advisor for Bio-Tissue and receives grant/research support from Alcon, Allergan, and Bausch & Lomb.
Meeting Presentation: This paper was presented in part at the annual meeting of the Association of Research in Vision and Ophthalmology (ARVO), 2011, Fort Lauderdale, FL.
This article contains online-only material. The following should appear online-only: Table 2 and Table 4.
REFERENCES
- 1.Scott IU, Flynn HW, Jr, Feuer W, et al. Endophthalmitis associated with microbial keratitis. Ophthalmology. 1996;103:1864–1870. doi: 10.1016/s0161-6420(96)30415-6. [DOI] [PubMed] [Google Scholar]
- 2.Wykoff CC, Flynn HW, Jr, Miller D, et al. Exogenous fungal endophthalmitis: microbiology and clinical outcomes. Ophthalmology. 2008;115:1501–1507. doi: 10.1016/j.ophtha.2008.02.027. [DOI] [PubMed] [Google Scholar]
- 3.Pflugfelder SC, Flynn HW, Jr, Zwickey TA, et al. Exogenous fungal endophthalmitis. Ophthalmology. 1988;95:19–30. doi: 10.1016/s0161-6420(88)33229-x. [DOI] [PubMed] [Google Scholar]
- 4.Shah CV, Jones DB, Holz ER. Microsphaeropsis olivacea keratitis and consecutive endophthalmitis. Am J Ophthalmol. 2001;131:142–143. doi: 10.1016/s0002-9394(00)00715-7. [DOI] [PubMed] [Google Scholar]
- 5.Marcus DM, Hull DS, Rubin RM, Newman CL. Lecythophora mutabilis endophthalmitis after long-term corneal cyanoacrylate. Retina. 1999;19:351–353. doi: 10.1097/00006982-199919040-00018. [DOI] [PubMed] [Google Scholar]
- 6.Borderie VM, Bourcier TM, Poirot JL, et al. Endophthalmitis after asiodiplodia theobromae corneal abscess. Graefes Arch Clin Exp Ophthalmol. 1997;235:259–261. doi: 10.1007/BF00941769. [DOI] [PubMed] [Google Scholar]
- 7.Elliott ID, Halde C, Shapiro J. Keratitis and endophthalmitis caused by Petriellidium boydii. Am J Ophthalmol. 1977;83:16–18. doi: 10.1016/0002-9394(77)90184-2. [DOI] [PubMed] [Google Scholar]
- 8.Shen YC, Wang CY, Tsai HY, Lee HN. Intracameral voriconazole injection in the treatment of fungal endophthalmitis resulting from keratitis. Am J Ophthalmol. 2010;149:916–221. doi: 10.1016/j.ajo.2010.01.024. [DOI] [PubMed] [Google Scholar]
- 9.Proenca-Pina J, Ssi Yan Kai I, Bourcier T et al. Fusarium keratitis and endophthalmitis associated with lens contact wear. Int Ophthalmol. 2010;30:103–107. doi: 10.1007/s10792-008-9290-7. [DOI] [PubMed] [Google Scholar]
- 10.Peponis V, Rosenberg P, Chalkiadakis SE, et al. Fungal scleral keratitis and endophthalmitis following pterygium excision. Eur J Ophthalmol. 2009;19:478–480. doi: 10.1177/112067210901900326. [DOI] [PubMed] [Google Scholar]
- 11.Rosenberg KD, Flynn HW, Jr, Alfonso EC, Miller D. Fusarium endophthalmitis following keratitis associated with contact lenses. Ophthalmic Surg Lasers Imaging. 2006;37:310–313. doi: 10.3928/15428877-20060701-08. [DOI] [PubMed] [Google Scholar]
- 12.Garcia-Valenzuela E, Song CD. Intracorneal injection of amphotericin B for recurrent fungal keratitis and endophthalmitis. Arch Ophthalmol. 2005;123:1721–1723. doi: 10.1001/archopht.123.12.1721. [DOI] [PubMed] [Google Scholar]
- 13.Marangon FB, Miller D, Giaconi JA, Alfonso EC. In vitro investigation of voriconazole susceptibility for keratitis and endophthalmitis fungal pathogens. Am J Ophthalmol. 2004;137:820–825. doi: 10.1016/j.ajo.2003.11.078. [DOI] [PubMed] [Google Scholar]
- 14.Dursun D, Fernandez V, Miller D, Alfonso EC. Advanced Fusarium keratitis progressing to endophthalmitis. Cornea. 2003;22:300–303. doi: 10.1097/00003226-200305000-00004. [DOI] [PubMed] [Google Scholar]
- 15.Ansari EA, McVerry BA. Consecutive keratitis and Candida endophthalmitis in an immunocompromised patient with chronic lymphocytic leukaemia [letter] Eye (Lond) 1997;11:943–945. doi: 10.1038/eye.1997.238. [DOI] [PubMed] [Google Scholar]
- 16.Davis MJ, Packo KH, Epstein RJ, et al. Acanthamoeba endophthalmitis following penetrating keratoplasty for Acanthamoeba keratitis [letter] Arch Ophthalmol. 2010;128:505–506. doi: 10.1001/archophthalmol.2010.33. [DOI] [PubMed] [Google Scholar]
- 17.Ornek K, Ozdemir M, Ergin A. Burkholderia cepacia keratitis with endophthalmitis. J Med Microbiol. 2009;58:1517–1518. doi: 10.1099/jmm.0.011197-0. [DOI] [PubMed] [Google Scholar]
- 18.Ritterband D, Shah M, Cohen K, et al. Burkholderia gladioli keratitis associated with consecutive recurrent endophthalmitis. Cornea. 2002;21:602–603. doi: 10.1097/00003226-200208000-00014. [DOI] [PubMed] [Google Scholar]
- 19.Ritterband D, Shah M, Kresloff M, et al. Gemella haemolysans keratitis and consecutive endophthalmitis. Am J Ophthalmol. 2002;133:268–269. doi: 10.1016/s0002-9394(01)01271-5. [DOI] [PubMed] [Google Scholar]
- 20.Wynants S, Koppen C, Tassignon MJ. Spontaneous corneal perforation and endophthalmitis in Pseudomonas aeruginosa infection in a ventilated patient: a case report. Bull Soc Belge Ophtalmol. 2000;276:53–56. [PubMed] [Google Scholar]
- 21.Poggio EC, Glynn RJ, Schein OD, et al. The incidence of ulcerative keratitis among users of daily-wear and extended-wear soft contact lenses. N Engl J Med. 1989;321:779–783. doi: 10.1056/NEJM198909213211202. [DOI] [PubMed] [Google Scholar]
- 22.Erie JC, Nevitt MP, Hodge DO, Ballard DJ. Incidence of ulcerative keratitis in a defined population from 1950 through 1988. Arch Ophthalmol. 1993;111:1665–1671. doi: 10.1001/archopht.1993.01090120087027. [DOI] [PubMed] [Google Scholar]
- 23.Wilhelmus KR. Indecision about corticosteroids for bacterial keratitis: an evidence-based update. Ophthalmology. 2002;109:835–842. doi: 10.1016/s0161-6420(02)00963-6. [DOI] [PubMed] [Google Scholar]
- 24.Wang MX, Shen DJ, Liu JC, et al. Recurrent fungal keratitis and endophthalmitis. Cornea. 2000;19:558–560. doi: 10.1097/00003226-200007000-00031. [DOI] [PubMed] [Google Scholar]
- 25.Bell NP, Karp CL, Alfonso EC, et al. Effects of methyprednisolone and cyclosporine A on fungal growth in vitro. Cornea. 1999;18:306–313. doi: 10.1097/00003226-199905000-00012. [DOI] [PubMed] [Google Scholar]
- 26.O’Day DM. Fungal keratitis. In: Leibowitz HM, Waring GO III, editors. Corneal Disorders: Clinical Diagnosis and Management. 2nd ed. Philadelphia, PA: Saunders; 1998. pp. 711–718. [Google Scholar]
- 27.Nelson PE, Digani MC, Anaissie EJ. Taxonomy, biology, and clinical aspects of Fusarium species. Clin Microbiol Rev. 1994;7:479–504. doi: 10.1128/cmr.7.4.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Suie T, Taylor FW. The effect of cortisone on experimental Pseudomonas corneal ulcers. Arch Ophthalmol. 1956;56:53–56. doi: 10.1001/archopht.1956.00930040059008. [DOI] [PubMed] [Google Scholar]
- 29.Luchs JI, Cohen EJ, Rapuano CJ, Laibson PR. Ulcerative keratitis in bullous keratopathy. Ophthalmology. 1997;104:816–822. doi: 10.1016/s0161-6420(97)30228-0. [DOI] [PubMed] [Google Scholar]
- 30.Endophthalmitis Vitrectomy Study Group. Results of the Endophthalmitis Vitrectomy Study: a randomized trial of immediate vitrectomy and of intravenous antibiotics for the treatment of postoperative bacterial endophthalmitis. Arch Ophthalmol. 1995;113:1479–1496. [PubMed] [Google Scholar]
- 31.Centers for Disease Control and Prevention. National Diabetes Fact Sheet, 2011. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; 2011. [Accessed June 2, 2012]. Available at: http://www.cdc.gov/diabetes/pubs/pdf/ndfs_2011.pdf. [Google Scholar]
- 32.Centers for Disease Control and Prevention. HIV in the United States: An overview. Atlanta, GA: U. S. Department of Health and Human Services, Centers for Disease Control and Prevention; 2011. Aug, [Accessed June 2, 2012]. Available at: http://www.cdc.gov/hiv/topics/surveillance/resources/factsheets/pdf/HIV-US-overview.pdf. [Google Scholar]
- 33.Srinivasan M, Mascarenhas J, Rajaraman R, et al. Steroids for Corneal Ulcers Trial Group. Corticosteroids for bacterial keratitis: the Steroids for Corneal Ulcers Trial (SCUT) Arch Ophthalmol. 2012;130:143–150. doi: 10.1001/archophthalmol.2011.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rebong RA, Santaella RM, Goldhagen BE, et al. Polyhexamethylene biguanide and calcineurin inhibitors as novel antifungal treatments for Aspergillus keratitis. Invest Ophthalmol Vis Sci. 2011;52:7309–7315. doi: 10.1167/iovs.11-7739. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.