Abstract
The major constituent of the eukaryotic ER protein-translocation channel (Sec61p in yeast, Sec61 in higher eukaryotes) shows a high degree of evolutionary conservation from yeast to humans. The vast majority of eukaryotic species have a conserved di-tyrosine in the 4th ER luminal loop. Previously, we discovered through a screen of ethylnitrosourea- (ENU-) mutagenized mice that substitution of the latter of these tyrosines with histidine (Y344H) of the murine Sec61 protein results in diabetes and hepatic steatosis in mice that is a result of ER stress. To further characterize the mechanism behind ER stress in these mice we made the homologous mutation in yeast Sec61p (Y345H). We found that this mutation increased sensitivity of yeast to ER stressing agents and to reduction of Inositol Requiring Enzyme 1 (IRE1) activity. Furthermore, we found that, while this mutation did not affect translocation, it did delay degradation of the model ER-associated degradation (ERAD) substrate CPY*. Replacing both ER luminal tyrosines with alanines resulted in a destabilization of the Sec61 protein that was rescued by over expression of Sss1p. This double mutant still lacked a noticeable translocation defect after stabilization by Sss1p, but exhibited a similar defect in CPY* degradation.
Keywords: Sec61, ER stress, ER associated degradation
Introduction
The eukaryotic translocon is a heterotrimeric channel in the ER membrane that allows for the entry of secretory and membrane bound proteins. It consists of a main 10-pass transmembrane protein, Sec61p (Sec61 in higher eukaryotes) that forms an aqueous pore in the membrane with two smaller proteins Sbh1p and Sss1p (Sec61 and Sec61 in higher eukaryotes, respectively). In yeast Sec61p and Sss1p are essential while Sbh1, and its homolog Sbh2, are dispensable for cell growth. The Sec61 translocon is responsible for both co- and post-translational translocation (reviewed in [1]).
In addition to its role in protein translocation, the Sec61 complex has also been shown to participate in the retro-translocation of misfolded proteins to the cytosol for degradation by the ubiquitin proteasome system, a process known as ERAD (reviewed in [2]). Briefly, in the case of glycoproteins with misfolded luminal domains, proteins are recognized by a surveillance complex that consists of the lectin Yos9p and the adaptor Hrd3p [3]. These proteins are then retro-translocated to the cytosol and ubiquitinated by a membrane-bound E3 ligase (Hrd1p in yeast) [4] where they are degraded by the proteasome.
Recently, we have isolated mice with an ENU-induced point mutation in the mouse Sec61a1 gene. This point mutation results in a tyrosine-to-histidine substitution at amino acid 344, which is located in the 4th ER luminal loop of the Sec61 l protein [5]. Mice homozygous for this mutation exhibit diabetes that is a result of -cell apoptosis brought on by ER stress. These mice also develop hepatic steatosis that is also the result of ER stress and not secondary to the development of diabetes. To better understand the mechanism of increased ER stress, we took advantage of the high degree of evolutionary conservation of the eukaryotic Sec61 protein and the existence of several genetic and biochemical tools by making the homologous mutation in SEC61 of Saccharomyces cerevisiae.
In this study we examine the influence this mutation has on Sec61p function including translocation and degradation of misfolded proteins. We show that this di-tyrosine motif in Sec61p is required for proper degradation of a model ER substrate and for protein stability, but is not required for other important Sec61p functions such as translocation.
Materials and Methods
Plasmids
Plasmids for split ubiquitin were provided by W. Lennarz (reference). Sec61Y345H-CUB-PLV was made from the SEC61 wildtype plasmid using a minigene construct that encoded the Y345H mutant allele inserted at novel PasI and PstI sites. SEC61 wildtype and mutant alleles were made on the pRS416 plasmid as follows. SEC61 and 500 bp of upstream promoter sequence were amplified from genomic DNA by PCR and cloned into pRS416 carrying a 3xHA tag and yADH1 terminator using KpnI and PacI. Mutant alleles were made using minigene constructs as above. CPY* and DPY* plasmids were provided by C. Stirling. These were cloned into pVP16, which contains a 2µ origin of replication, the LEU2 marker and the ADH1 promoter, using PCR primers that incorporated a c-terminal FLAG epitope tag using the restriction sites HindIII and EcoRI. All plasmids were verified via sequencing.
Strains
All experiments utilized yeast strains made on BY4742 (MAT his3Δ1 leu2Δ0 lys2Δ0 ura3Δ0, Open Biosystems) except for split-ubiquitin experiments which utilized L40 (MATa ade2 his3 leu2 trp1 LYS2::lexA-HIS3 URA3::lexA-LacZ, ATCC). Strains were made using a plasmid shuffling technique. BY4742 were simultaneously transformed with a G418 resistance cassette generated by PCR from the vector pFa6kanMX6 with homologous ends that removed the endogenous SEC61 gene and promoter, and a plasmid encoding one of the SEC61 wildtype or mutant alleles used in this study (see details above). Clones were selected on URA- plates supplemented with G418 (Invitrogen) at 200 g/mL and confirmed by sequencing of PCR products. The gal promoter was integrated into the promoter region of either the IRE1 or SSS1 gene by homologous recombination of PCR products generated using the pFa6MX6-PGAL1 plasmid. All primer sequences are listed in supplemental table 1.
RNA extraction and Quantitative-RT-PCR
RNA was extracted from 2 OD of yeast growing in log phase by 1mL of Trizol reagent (Invitrogen), as per manufacturer’s directions. cDNA was synthesized from 100 ng of total RNA using the qScript cDNA supermix kit from Quanta Biosciences. cDNA was amplified using the Perfecta SYBR green Fast Mix Kit from Quantas Biosciences. Primers for amplification of actin and spliced Hac1 are listed in supplemental table 1.
Translocation and pulse-chase assay
Yeast in log phase growth (5 OD per timepoint) were labeled with 10 Ci of EasyTag™ EXPRESS35S Protein Labeling Mix (Perkin Elmer) per OD for 15 minutes. For translocation assays yeast were lysed immediately, or for pulse-chase assays labeling was quenched with 2 mM cold cysteine and methionine and incubated for the indicated time points before lysis by beating with glass beads. Supernatants were immunoprecipitated with anti-FLAG antibody (clone M2, Stratagene) followed by addition of Protein A/G agarose (Cal Biochem). Precipitated protein was fractionated by SDS-PAGE using 4–12% NuPage gels (Invitrogen), dried under vacuum and exposed to film. Band density was quantified using ImageJ software (NIH).
Western blotting
2 OD of yeast in log phase growth were collected and lysed by beating with glass beads. Lysates were fractionated by SDS-PAGE using 4–12% NuPage gels (Invitrogen) and transferred to PVDF membranes. Membranes were probed with anti-HA (Roche, clone 12CA5, 2 g) in TBS + 0.1% Tween, followed by anti-mouse HRP (Pierce) used at 1:10,000 and detected using chemilluminescence (Pierce). Blots were then stripped and reprobed with anti-PGK1 (Invitrogen, clone 22C5D8, 1ug).
Results
Y345H mutation of Sec61p is sensitive to ER stress
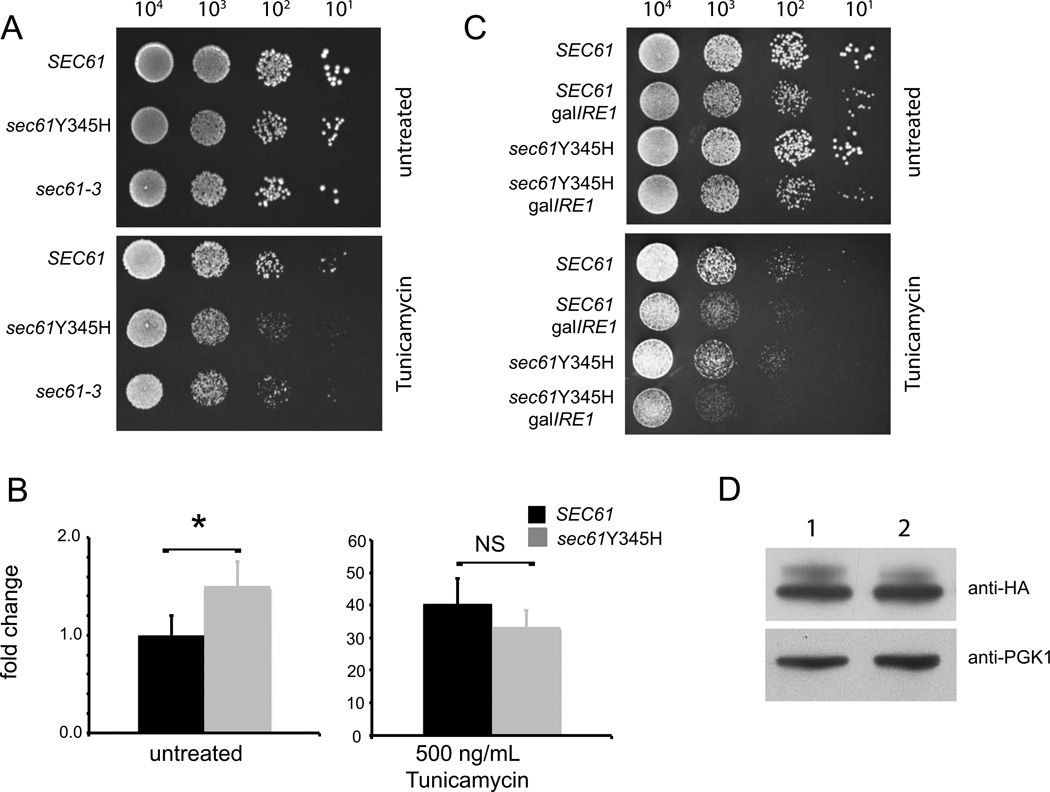
The Sec61 protein contains two consecutive tyrosines in its 4th ER luminal loop that exhibit a high degree of conservation in many different eukaryotic species (Supplemental Fig. 1, marked with plus sign). Interestingly, these residues lie four amino acids downstream of another highly conserved glycine residue (Supplemental Fig. 1, marked with asterisk). Mutation of this glycine to glutamic acid (sec61-3) results in cold sensitivity, ER stress sensitivity, and defects in translocation [6]. With this in mind we investigated the effect of mutation of the second tyrosine of Sec61p to histidine - Y345H, which is analogous to the Y344H mutation that causes -cell failure and diabetes in mice - on sensitivity to the ER stress-inducing agent tunicamycin. Yeast that expressed sec61Y345H showed enhanced sensitivity to 500 ng/mL of Tunicamycin when compared to yeast expressing wild-type SEC61 (Fig. 1A), these yeast were also more sensitive to tunicamycin than sec61-3. Sec61Y345H yeast showed little cold sensitivity when grown at 17° C in contrast to greatly reduced growth at this temperature by sec61-3 yeast (Supplemental Fig. 2A).
Figure 1. Yeast expressing Y345H mutant Sec61 are sensitive to ER stress.
Yeast strains were constructed in which the endogenous Sec61 gene was knocked out and rescued by episomal expression of wild-type Sec61p (SEC61), sec61Y345H, or sec61G341E (sec61-3). (A) Yeast of the indicated genotype were grown at 30° to an OD of 0.8 and plated at the indicated concentrations on URA- plates containing either no stressor (top) or 500 ng/mL tunicamycin (bottom) and incubated at 30°. (B) Yeast of the indicated genotype were grown to an OD of 0.8, and then treated with 0 or 500 ng/mL of tunicamycin for 1 hour followed by SYBR green qPCR specific for the spliced form of HAC1. Expression is normalized to β-actin and relative to SEC61, untreated yeast (mean ± SD), *= p<0.05 by Student’s t-test. (C) Yeast of the indicated genotypes were grown at 30° to an OD of 0.8 and then plated at the indicated density on plates containing 2% glucose to suppress IRE1 expression. Plates either had no stressor (top) or 500 ng/mL of tunicamycin (bottom). (D) Lysates from SEC61 (lane 1) or sec61Y345H (lane 2) yeast were fractionated by SDS-PAGE and transferred to PVDF membrane and probed with an anti-HA antibody; the same membrane was stripped and then re-probed with anti PGK1 antibody, which served as a loading control.
The transcription factor Hac1p mediates yeast’s transcriptional response to ER stress. The ER transmembrane kinase/endoribonuclease IRE1 senses unfolded proteins in the ER, and initiates splicing of HAC1 mRNA to form the transcript for the active transcription factor [7]. As measured by qRT-PCR for HAC1 splicing, sec61Y345H yeast also showed a small but significant increase in the level of spliced HAC1 under unstressed conditions but no detectable defect in activation in response to 500 ng/mL tunicamycin (Fig. 1B). Sec61-3 however showed a marked reduction of Hac1p splicing under stressed conditions (Supplemental Fig. 2B). To further test the sensitivity of the Y345H mutation we created a strain of yeast that contained the IRE1 gene under the control of the galactose (gal) promoter. It has previously been shown that combing IRE1 knockout with mutations that disrupt protein folding or ERAD enhance sensitivity to tunicamycin [8] When grown on plates that contain glucose, a repressor of the gal promoter, gal-IRE1 yeast that expressed sec61Y345H showed much greater sensitivity to tunicamycin than SEC61-expressing yeast (Fig. 1C), suggesting some defect in protein processing or ERAD in sec61Y345H yeast.
One possible explanation for such a defect would be instability of Sec61p, as is the case with the sec61-3 protein product [9]. In light of this we asked if there was a defect in the stability of the sec61Y345H mutant that could account for its defect in growth on tunicamycin plates. We could detect no lack of stability of the Y345H protein by western blot analysis (Fig. 1D).
Recently, another group has shown that the Y344H mutation may disrupt cellular calcium homeostasis in a mammalian cell culture system [10]. To test whether the sec61Y345H mutation leads to improper calcium homeostasis in yeast we performed growth assays on plates containing the calcium-chelating agent EGTA. 10 mM EGTA had no effect on sec61Y345H growth when compared to wild-type SEC61 (Supplemental Fig. 2C), however, there was a marked reduction of growth of yeast with deletion of PMR1, a golgi localized calcium ion pump [11].
These results suggest that the sec61Y345H mutation led to defective growth on tunicamycin not through decreased protein stability or a decreased stress response, as is the case with the sec61-3 mutation, but through some effect on protein processing, folding or degradation. We therefore investigated the role of Sec61p in protein translocation and processing.
Y345H mutation of Sec61p does not alter interaction with subunits of the oligosaccharyltransferase (OST) complex
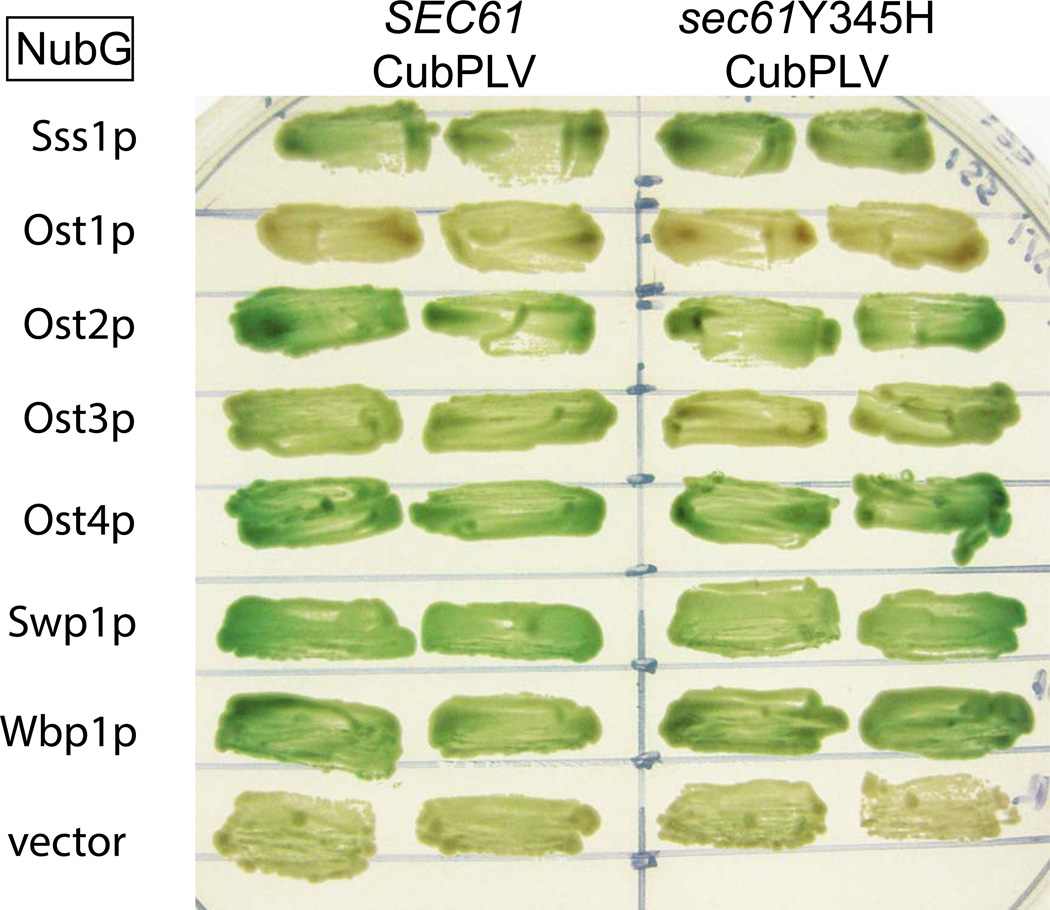
Since Sec61p has been shown to interact with members of the OST complex [12], and since alterations in glycosylation could account for enhanced sensitivity to ER stressors, we undertook experiments to determine the integrity of the OST-Sec61p complex in yeast. Using the split-ubiquitin system we examined pair-wise interactions between WT or Y345H Sec61p and subunits of the OST complex. The split-ubiquitin system allows for the analysis of interactions between membrane bound proteins by activation of a β-gal reporter [13, 14]. As shown in Figure 2, we successfully reproduced interactions between Sec61p and subunits of the OST complex [12], and observed no differences between the interactions of WT or Y345H Sec61p with Ost2p, Ost3p, Ost4p, Wbp1p and Swp1p. Additionally, we saw no difference in the interactions of either protein with Sss1p.
Figure 2. Y345H mutant sec61 preserves normal OST interactions.
Yeast strain L40 was transformed with C-terminally Cub-PLV tagged SEC61 or sec61Y345H and the indicated OST subunit tagged with NubG. Two independent clones from each transformation were patched onto Ura-Trp-Lue-Lys- plates containing 100 µg/mL X-gal and grown at 30°.
Y345H mutation of Sec61p preserves translocation of both co-and posttranslational translocation substrates
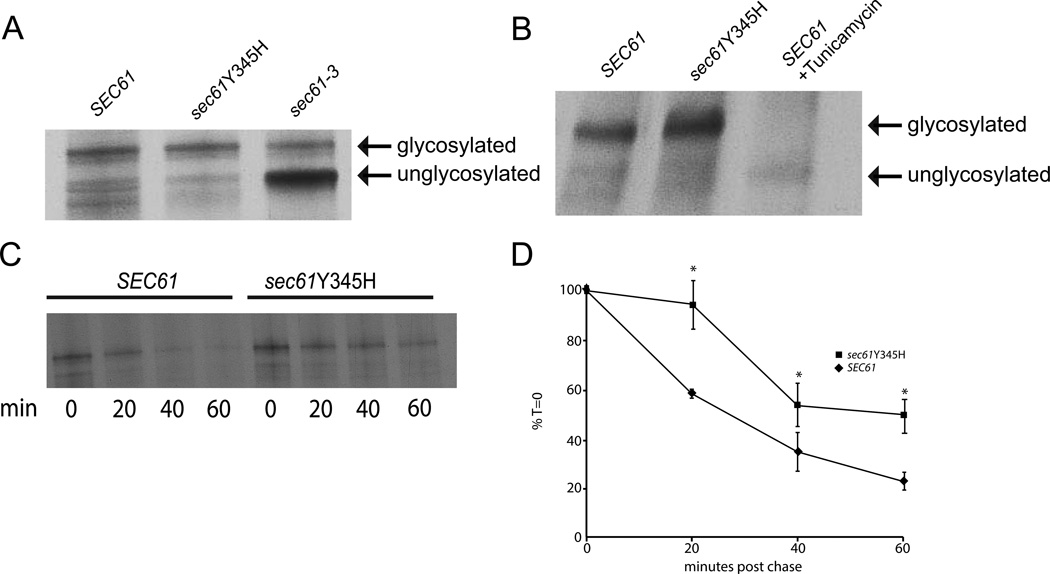
Since the Y345H mutation lies in close proximity to the mutation in sec61-3 that leads to defects in post-translational translocation, we tested weather sec61Y345H yeast possessed a similar defect using the model substrate CPY* appended with a FLAG epitope tag in conjunction with 35S labeling. The CPY* protein possesses a glycine-to-asparagine mutation at amino acid 255 which leads to incomplete glycosylation and rapid degradation. Recently, Willer et al. [15] developed a system whereby CPY* could be directed to either the co- or post-translational translocation pathway based upon different signal sequences. In yeast transformed with CPY*-FLAG, which is directed to the post translational translocation pathway, only yeast expressing sec61-3 showed a defect in total glycosylation (Fig 3A) representing a defect in translocation, as has been previously reported [15]. Sec61Y345H yeast exhibited full glycosylation of CPY*-FLAG (Fig. 3A) when compared to SEC61 yeast, indicating proper translocation and glycosylation of a post-translational substrate.
Figure 3. Y345H mutant Sec61 preserves translocation.
(A) 5 ODs of yeast transformed with CPY*-FLAG in log phase growth were labeled with 50 Ci of 35S-cysteine/methionine, lysed, immunoprecipitated with anti-FLAG antibody and fractionated by SDS-PAGE. The upper band represents fully glycosylated and translocated CPY*; the lower band represents an unglycosylated and untranslocated form. (B) Yeast transformed with DPY*-FLAG were treated as in A. SEC61 yeast treated with tunicamycin, which blocks N-linked glycosylation serves as a control for unglycosylated DPY*. For analysis of CPY* degradation (C) yeast expressing the indicated Sec61 allele and transformed with CPY*-FLAG growing in log phase were harvested, labeled with 35S-cysteine/methionine and chased with cold media for the indicated time points. Yeast were then lysed, immunoprecipitated with anti-FLAG antibody and fractionated by SDS-PAGE. (D) Quantitation of three independent experiments. Shown are mean ± SD *= p<0.05 by Student’s t-test.
We also performed this experiment, with DPY*-FLAG-expressing yeast. DPY*-FLAG possesses a different N-terminal signal sequence than CPY*-FLAG directing it to the co-translational translocation pathway. Again both SEC61 and sec61Y345H yeast exhibited full glycosylation of this substrate (Fig. 3B), when compared with SEC61 yeast pre-treated with tunicamycin, which blocks N-linked glycosylation. Taken together we concluded that Y345H mutant Sec61p is fully competent for both protein glycosylation and import through both the co- and post-translational translocation pathways, and that defects in these pathways likely do not explain increased sensitivity to ER stress agents
CPY* exhibits delayed degradation in yeast expressing Y345H mutation
We examined sec61Y345H yeast for their ability to properly degrade the CPY*-FLAG protein using pulse-chase labeling. Due to the G225N mutation, CPY* does not progress through the ER and sort to vacuoles as it normally would, but is instead fated for degradation by ERAD. When CPY*-FLAG was expressed in SEC61 yeast it was degraded over the course of 60 min (Fig. 3C and D). In contrast, sec61Y345H yeast expressing CPY*-FLAG exhibited a significant reduction in degradation at 20 minutes post chase, and CPY*-FLAG was still present in higher amounts at 60 minutes post chase (Fig. 3C and D). These data show that Y345H mutation of Sec61p leads to a defect in ERAD of a model misfolded luminal substrate.
Mutation of tyrosines at positions 344 and 345 of Sec61p results in protein instability and a defect in CPY* degradation
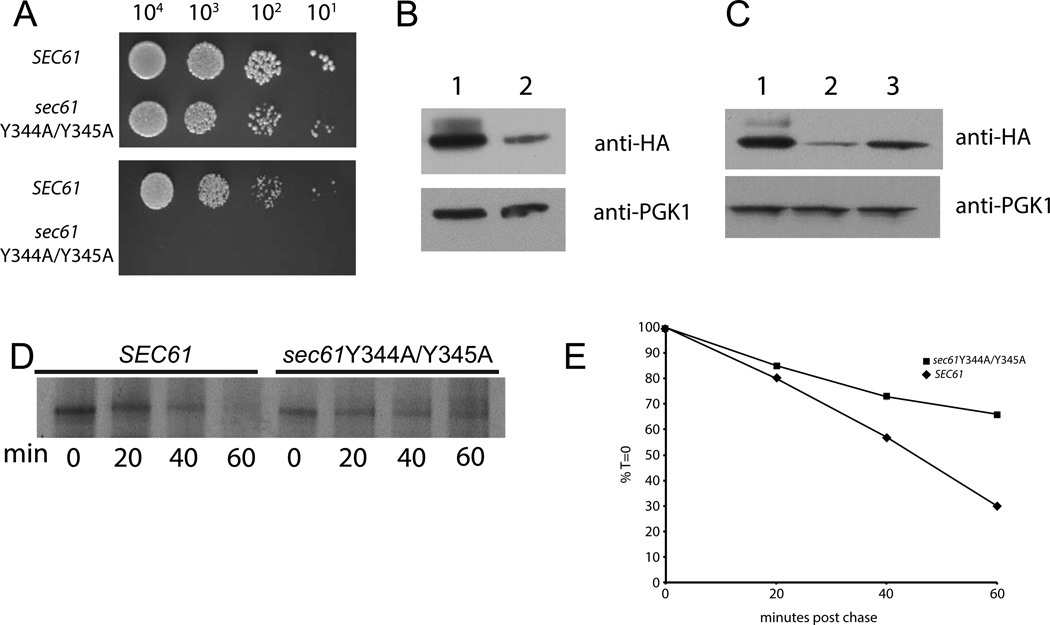
To further investigate the role of the di-tyrosine motif at positions 344 and 345 in Sec61p function, we made a mutant strain in which both of these residues were converted to alanine (Y344A/Y345A). We observed a complete lack of growth of sec61Y344A/Y345A yeast on plates that contained 500 ng/mL of tunicamycin (Fig. 4A). Subsequently, when we performed western blot analysis we noticed a significant reduction in steady-state levels of the mutant protein (Fig.4B). Since the stability of the sec61-3 protein can be enhanced by overexpression of Sss1p [9], we asked if overexpression of Sss1p would affect stability of the Y344A/Y345A mutant. To do this, we replaced the endogenous promoter of SSS1 with the gal promoter element. When gal-SSS1yeast were grown in the presence of galactose, which induces the gal promoter, we observed a significant degree of stabilization of the Y344A/Y345A mutant, as assessed by western blot analysis (Fig. 4C). As with the sec61Y345H mutant we saw no defect in translocation of CPY*-FLAG in sec61Y344A/Y345A yeast indicating that these two residues are dispensable for proper protein translocation (data not shown). Also, as with the sec61Y345H mutant, we saw a lag in degradation of CPY*-FLAG by the sec61Y344A/Y345A (Fig. 4D and E). Taken together these observations indicate that the tyrosines at positions 344 and 345 are critical both for the stability of Sec61p, and proper ERAD of a luminal substrate.
Figure 4. Tyrosines 344 and 345 are necessary for stability of the Sec61 protein.
Yeast strains were made as in Figure 1, except that sec61Y344A/Y345A was used instead of sec61Y345H. (A) Yeast of the indicated genotype were grown at 30° to an OD of 0.8 and plated at the indicated concentrations on plates containing either no stressor (top) or 500 ng/mL tunicamycin (bottom) and incubated at 30°. (B) Lysates from either SEC61 (lane 1) or sec61Y344A/Y345A (lane 2) yeast were fractionated by SDS-PAGE and transferred to PVDF membrane and probed with an anti-HA antibody; the same membrane was stripped and then re-probed with anti PGK1 antibody which serves as a loading control. (C) SEC61 (lane 1), sec61Y344A/Y345A (lane 2), sec61Y344A/Y345A gal-SSS1 (lane3) yeast were grown at 30° in media with 2% galactose as a carbon source to induce expression of Sss1p. Lysates were prepared and treated as in B. For CPY* degradation (D) yeast expressing CPY*-FLAG, gal-SSS1 and the indicated Sec61 allele growing in log phase in galactose-containing medium were harvested and labeled with 35S-cysteine/methionine and chased with cold media for the indicated time points. Yeast were then lysed, immunoprecipitated with anti-FLAG antibody and fractionated by SDS-PAGE. A representative experiment is shown. (E) Quantitation of the experiment shown in D.
Discussion
In this study we have examined the role that a conserved double tyrosine motif in the 4th ER luminal loop of Sec61 plays in the overall function of this protein. The impetus for these studies was to gain insight into the mechanism of Sec61 dysfunction seen in an ENU mouse mutant that develops diabetes and hepatic steatosis due to a Y344H mutation in Sec61 1. By taking advantage of the strong evolutionary conservation of this protein across eukaryotes we were able discern that this double tyrosine motif is necessary for proper degradation of a model ERAD-L substrate and the overall stability of the Sec61 protein.
Sec61Y345H yeast were more sensitive to the effects of tunicamycin than SEC61 yeast. These yeast also have elevated baseline UPR signaling as evidenced by increased HAC1 splicing, along with greater sensitivity to tunicamycin in the absence of proper Ire1p signaling. Western blot experiments showed that the stability of this protein did not appear to be affected by this mutation, and when we probed interactions between sec61Y345H and known interactors, such as OST subunits and Sss1p, we saw no difference when compared to SEC61. Taken together these observations argue that the effect of this mutation on ER stress is not mediated by stability of the translocon. When we examined translocation efficiency in sec61Y345H mutant yeast we saw no reduction in ER import by either the co- or post-translational translocation pathway. However, when we examined the rate of degradation of the model substrate CPY* by pulse-chase analysis we noticed a significant reduction in degradation by sec61Y345H yeast when compared with SEC61.
Yeast expressing the sec61Y344A/Y345A mutant showed a very high sensitivity to tunicamycin. When we performed western blot on these yeast we saw much lower levels of Sec61 protein when compared to WT. This enhanced sensitivity to tunicamycin, may result from accelerated degradation of the already unstable sec61Y344A/Y345A allele by ERAD machinery, which are induced by tunicamycin. This is in agreement with previous data showing that sec61-2, another unstable SEC61 allele exhibits greatly reduced growth at permissive temperatures when combined with overexpression of ERAD components [16]. Overexpression of the Sec61p accessory protein Sss1p via the gal promoter had the effect of partially restoring Y344A/Y345A levels to those of the WT protein, arguing that these two tyrosine residues are critical for stability of this protein. Furthermore, when we rescued expression levels of the double mutant by overexpressing SSS1 we saw a similar defect in ERAD as with the single mutant.
A recent report indicates that the mammalian Y344H mutation results in increased calcium leakage from the ER in a mammalian cell culture system [10]. However, we could find no sensitivity of sec61Y345H yeast to growth on plates containing the calcium chelator EGTA. This divergent result may be accounted for by the difference between yeast and vertebrate systems with regard to calcium homeostasis. S. cerevisiae lacks ER based calcium channels, exhibits a significantly lower ER calcium concentration and the main response to calcium shock is largely transcriptionally based [17]. More specifically Sec61p might not act as a calcium channel in yeast, as in vitro assays show that Sec61 does not bind calmodulin unlike its mammalian homolog [18, 19].
The Y345H mutant occurs just 4 amino acids downstream of a cold sensitive Sec61 allele sec61-3, yet there is significant divergence in the phenotypes of the two alleles. While sec61-3 is cold sensitive, unstable and defective for post-translational translocation, the Y345H mutant exhibits little cold sensitivity, is expressed at wildtype levels and is fully competent with regards to both co- and post-translational translocation. Clearly, this region of the 4th ER luminal loop is critical for Sec61p stability as the Y344A/Y345A mutant exhibits cold sensitivity and reduced protein levels. Since the Y345H mutant represents a stable Sec61 mutant that is defective for ERAD it lends weight to the hypothesis that Sec61p plays an active role in the degradation process either by directly binding and “handing off” misfolded substrates with the help of other luminal proteins, or by recruiting ERAD machinery, such as the Hrd3p complex.
Our results demonstrate a novel Sec61 point mutation that clearly implicates the 4th ER luminal loop of this protein in the ERAD process. It will be interesting to determine whether this specific portion of Sec61p interacts with ERAD machinery or temporarily holds misfolded substrates for a luminal surveillance complex.
Supplementary Material
Highlights.
Y345H mutation of Sec61p preserves translocation of a model substrate.
Y345H mutation of Sec61p results in reduced degradation of a model ERAD-L substrate.
Mutation of tyrosines at positions 344 and 345 results in instability of Sec61p.
Rescue of stability of the Y344A/Y345A results in reduced degradation of a model ERAD-L substrate.
These data propose a mechanism of action for Sec61 mutation in a diabetic mouse model.
Acknowledgments
We would like to acknowledge William J. Lennarz, Curt Wittenberg and Colin J. Stirling for plasmids used in this study and Mario Bengtson for experimental advice and discussion. This work was supported by NIH grant R01DK079925 to NG and K01DK090185 to MCW.
Abbreviations used are
- ERAD
ER associated degradation
- ENU
ethylnitrosourea
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Park E, Rapoport TA. Mechanisms of Sec61/SecY-Mediated Protein Translocation Across Membranes. Annual review of biophysics. 2011;41:21–40. doi: 10.1146/annurev-biophys-050511-102312. [DOI] [PubMed] [Google Scholar]
- 2.Smith MH, Ploegh HL, Weissman JS. Road to ruin: targeting proteins for degradation in the endoplasmic reticulum. Science. 2011;334:1086–1090. doi: 10.1126/science.1209235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gauss R, Jarosch E, Sommer T, Hirsch C. A complex of Yos9p and the HRD ligase integrates endoplasmic reticulum quality control into the degradation machinery. Nat Cell Biol. 2006;8:849–854. doi: 10.1038/ncb1445. [DOI] [PubMed] [Google Scholar]
- 4.Gardner RG, Swarbrick GM, Bays NW, Cronin SR, Wilhovsky S, et al. Endoplasmic reticulum degradation requires lumen to cytosol signaling. Transmembrane control of Hrd1p by Hrd3p. J Cell Biol. 2000;151:69–82. doi: 10.1083/jcb.151.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lloyd DJ, Wheeler MC, Gekakis N. A point mutation in Sec61alpha1 leads to diabetes and hepatosteatosis in mice. Diabetes. 2010;59:460–470. doi: 10.2337/db08-1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stirling CJ, Rothblatt J, Hosobuchi M, Deshaies R, Schekman R. Protein translocation mutants defective in the insertion of integral membrane proteins into the endoplasmic reticulum. Mol Biol Cell. 1992;3:129–142. doi: 10.1091/mbc.3.2.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cox JS, Walter P. A novel mechanism for regulating activity of a transcription factor that controls the unfolded protein response. Cell. 1996;87:391–404. doi: 10.1016/s0092-8674(00)81360-4. [DOI] [PubMed] [Google Scholar]
- 8.Travers KJ, Patil CK, Wodicka L, Lockhart DJ, Weissman JS, et al. Functional and genomic analyses reveal an essential coordination between the unfolded protein response and ER-associated degradation. Cell. 2000;101:249–258. doi: 10.1016/s0092-8674(00)80835-1. [DOI] [PubMed] [Google Scholar]
- 9.Esnault Y, Feldheim D, Blondel MO, Schekman R, Kepes F. SSS1 encodes a stabilizing component of the Sec61 subcomplex of the yeast protein translocation apparatus. J Biol Chem. 1994;269:27478–27485. [PubMed] [Google Scholar]
- 10.Schauble N, Lang S, Jung M, Cappel S, Schorr S, et al. BiP-mediated closing of the Sec61 channel limits Ca(2+) leakage from the ER. Embo J. 2012;31:3282–3296. doi: 10.1038/emboj.2012.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Durr G, Strayle J, Plemper R, Elbs S, Klee SK, et al. The medial-Golgi ion pump Pmr1 supplies the yeast secretory pathway with Ca2+ and Mn2+ required for glycosylation, sorting, and endoplasmic reticulum-associated protein degradation. Mol Biol Cell. 1998;9:1149–1162. doi: 10.1091/mbc.9.5.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chavan M, Yan A, Lennarz WJ. Subunits of the translocon interact with components of the oligosaccharyl transferase complex. J Biol Chem. 2005;280:22917–22924. doi: 10.1074/jbc.M502858200. [DOI] [PubMed] [Google Scholar]
- 13.Johnsson N, Varshavsky A. Split ubiquitin as a sensor of protein interactions in vivo. Proceedings of the National Academy of Sciences of the United States of America. 1994;91:10340–10344. doi: 10.1073/pnas.91.22.10340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stagljar I, Korostensky C, Johnsson N, te Heesen S. A genetic system based on split-ubiquitin for the analysis of interactions between membrane proteins in vivo. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:5187–5192. doi: 10.1073/pnas.95.9.5187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Willer M, Forte GM, Stirling CJ. Sec61p is required for ERAD-L: genetic dissection of the translocation and ERAD-L functions of Sec61P using novel derivatives of CPY. J Biol Chem. 2008;283:33883–33888. doi: 10.1074/jbc.M803054200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bordallo J, Plemper RK, Finger A, Wolf DH. Der3p/Hrd1p is required for endoplasmic reticulum-associated degradation of misfolded lumenal and integral membrane proteins. Mol Biol Cell. 1998;9:209–222. doi: 10.1091/mbc.9.1.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cunningham KW. Acidic calcium stores of Saccharomyces cerevisiae. Cell calcium. 50:129–138. doi: 10.1016/j.ceca.2011.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harsman A, Kopp A, Wagner R, Zimmermann R, Jung M. Calmodulin regulation of the calcium-leak channel Sec61 is unique to vertebrates. Channels (Austin, Tex. 2011;5:293–298. doi: 10.4161/chan.5.4.16160. [DOI] [PubMed] [Google Scholar]
- 19.Erdmann F, Schauble N, Lang S, Jung M, Honigmann A, et al. Interaction of calmodulin with Sec61alpha limits Ca2+ leakage from the endoplasmic reticulum. Embo J. 2011;30:17–31. doi: 10.1038/emboj.2010.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.