Abstract
Glycogen synthase kinase 3 (GSK3) is a serine/threonine kinase that has been implicated in psychiatric diseases, neurodevelopment, and circadian regulation. Both GSK3 isoforms, α and β, exhibit a 24-hour variation of inhibitory phosphorylation within the suprachiasmatic nucleus (SCN), the primary circadian pacemaker. We examined the hypothesis that rhythmic GSK3 activity is critical for robust circadian rhythmicity using GSK3α21A/21A/β9A/9A knock-in mice with serine-alanine substitutions at the inhibitory phosphorylation sites, making both forms constitutively active. We monitored wheel-running locomotor activity of GSK3 knock-in mice and used loose-patch electrophysiology to examine the effect of chronic GSK3 activity on circadian behavior and SCN neuronal activity. Double transgenic GSK3α/β knock-in mice exhibit disrupted behavioral rhythmicity, including significantly decreased rhythmic amplitude, lengthened active period, and increased activity bouts per day. This behavioral disruption was dependent on chronic activation of both GSK3 isoforms and was not seen in single transgenic GSK3α or GSK3β knock-in mice. Underlying the behavioral changes, SCN neurons from double transgenic GSK3α/β knock-in mice exhibited significantly higher spike rates during the subjective night compared to those from WT controls, with no differences detected during the subjective day. These results suggest that constitutive activation of GSK3 results in loss of the typical day/night variation of SCN neuronal activity. Together, these results implicate GSK3 activity as a critical regulator of circadian behavior and neurophysiological rhythms. Because GSK3 has been implicated in numerous pathologies, understanding how GSK3 modulates circadian rhythms and neurophysiological activity may lead to novel therapeutics for pathological disorders and circadian rhythm dysfunction.
Keywords: Glycogen synthase kinase 3, circadian rhythms, suprachiasmatic nucleus, electrophysiology
INTRODUCTION
Circadian rhythms are endogenous 24-hour physiological and behavioral rhythms that are present in nearly all living organisms, ranging from bacteria to mammals (Bell-Pedersen et al., 2005). Circadian disturbance in humans has been implicated in a number of pathologies including psychiatric disorders, cardiometabolic disease, inflammatory disease and cancer (Takahashi et al., 2008). In all mammals, daily physiological and behavioral rhythms are orchestrated by a primary circadian pacemaker, the suprachiasmatic nucleus (SCN) of the hypothalamus (Welsh et al., 2010). SCN neurons generate characteristic, daily rhythms in electrical activity, exhibiting high activity during the day and low activity during the night (Inouye and Kawamura, 1979). The daily rhythm in the spontaneous firing rate (SFR) of SCN cells is important for synchronous output of the central pacemaker and is necessary for normal circadian behavior (Schwartz et al., 1987). At the molecular level, 24-hour timing is driven by transcriptional/translational feedback loops of primary “clock” genes which are present in almost all cell types throughout the body (Takahashi et al., 2008). Post-translational modifications, such as phosphorylation, of the core clock components contribute to the precise timing and robustness of the primary feedback loop (Gallego and Virshup, 2007), but the roles of some kinases, such as glycogen synthase kinase 3(GSK3), remain unclear.
GSK3 is a serine/threonine kinase that is able to phosphorylate nearly all of the circadian molecular clock components such as PER2, CLOCK, BMAL1, and REVERVBα (Iitaka et al., 2005, Wang et al., 2006, Spengler et al., 2009, Kurabayashi et al., 2010, Sahar et al., 2010). In addition, GSK3 is a therapeutic target of the mood-stabilizing agent, lithium (Klein and Melton, 1996), which can lengthen the period of behavioral and molecular rhythms of multiple organisms (LeSauter and Silver, 1993, Iwahana et al., 2004, Dokucu et al., 2005, Li et al., 2012). GSK3 has two isoforms, α and β, both of which are ubiquitously expressed throughout the brain (Woodgett, 1990). Unlike most kinases, GSK3 is by default active and can be inactivated by phosphorylation at serine-21 and serine-9 sites for α and β, respectively (Woodgett, 1990). Recent work has shown that GSK3 exhibits a daily rhythm in inhibitory phosphorylation within the SCN (Iwahana et al., 2004, Iitaka et al., 2005), yet little is known about what role this activity plays in overall circadian rhythmicity. In this study, we examine the function of rhythmic GSK3α/β phosphorylation using GSK3α21A/21A/β9A/9A (double knock-in, DKI) mice with serine-alanine substitutions at both inhibitory phosphorylation sites (McManus et al., 2005). With this model, we tested the hypothesis that rhythmic GSK3 activity is critical for generating robust circadian rhythms. Specifically, we measured the effect of chronic GSK3 activity on circadian wheel-running behavior in two different backgrounds of mice. We also examined the role of each GSK3 isoform individually using single transgenic animals (α-KI and β-KI). Finally, we examined whether chronic GSK3 activity disrupts day/night differences in SCN neuronal output (i.e., action potential frequency) using loose-patch recordings of SCN neurons from DKI and wild-type (WT) mice during the subjective day and subjective night.
MATERIALS/METHODS
Animals and Housing
Male, homozygous double transgenic GSK3 α21A/21A/β9A/9A (McManus et al., 2005) mice (5-7 months old) on a mixed (C57BL/6 X Balb/c) background (kindly provided by Dario R. Alessi, Dundee, Scotland) or back-crossed at least 10 generations to C57BL/6J were compared to WT mice that were strain- and age-matched (generated within the colony or purchased from Jackson Laboratories, Bar Harbor, ME). For single knock-in experiments, male, homozygous single transgenic, GSK3α21A/21A (α-KI, 8-11 months old) or GSK3β9A/9A (β-KI, 3-6 months old) on a C57BL/6J background were compared to WT mice that were strain- and age-matched. These serine-alanine substitutions resulted in loss of phosphorylation of GSK3α and/or GSK3β within the SCN of the transgenic mice (Fig. 1). In addition, these mice develop normally and do not display any obvious behavioral or physiological phenotype. Mice were genotyped for GSK3α using the forward primer TTGAAGTGGCTGGTACTGGCTCTG and the reverse primer GTGTGCTCCAGAGTAGTACCTAGC and for GSK3β using the forward primer TCACTGGTCTAGGGGTGGTGGAAG and the reverse primer GGAGTCAGTGACAACACTTAACTT according to the specifications in (McManus et al., 2005). Mice were housed in individual wheel cages (Coulbourn Instruments, Whitehall, PA) with standard rodent chow (#7917, Harlan Laboratories, Madison, WI) and water provided ad libitum. All mice were maintained in a 12:12 light-dark (LD) cycle for at least 9 days before being placed into constant dark (DD). All handling of animals was done in accordance with the University of Alabama at Birmingham (UAB) Institutional Animal Care and Use Committee (IACUC) and National Instit tes of Health (NIH) guidelines.
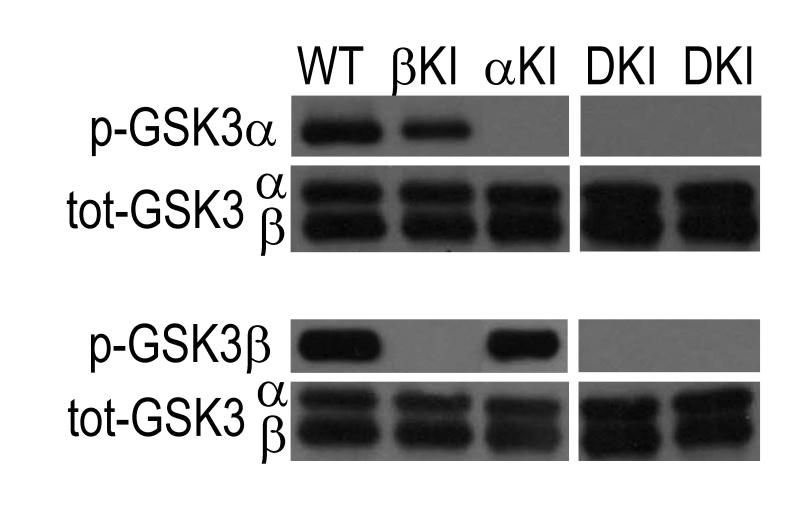
Fig. 1.
Representative immunoblots of p-GSK3α/β in SCN tissue of WT, α-KI, β-KI, and DKI mice. Immunostaining for p-GSK3α S21 (top) and p-GSK3β S9 (bottom) showing loss of inhibitory phosphorylation of GSK3α, GSK3β, or both in SCN of α-KI, β-KI, or DKI mice, respectively. Total GSK3α/β staining shows loading control for each blot.
Immunoblotting
In order to confirm the loss of phosphorylation of GSK3 in the SCN from the three transgenic models, the SCN was isolated from α-KI, β-KI, DKI and WT mice (2-3 months old) housed in a 12:12 LD cycle. Protein lysates were prepared and visualized using immunoblotting with an antibody to p-GSK3α S21 (1:750, Cell Signaling, Danvers, MA) or p-GSK3β S9 only (1:500, Cell Signaling, Danvers, MA). Total GSK3α/β (1:750, Cell Signal, Danvers, MA) staining was used on the same blot as a loading control.
Behavioral Analysis
Wheel-running activity was recorded and analyzed using ClockLab software (Actimetrics, Wilmette, IL). Actograms were generated using 6-min bins of activity and double plotted for ease of examination. Behavior was analyzed across 7-10 days for LD analysis, and 10 days of activity for DD analysis after the mice had been in constant conditions for 6-12 days. The activity levels were calculated using the batch analysis in ClockLab software. The free-running period (τ) and amplitude were determined by chi-squared (Χ2) periodogram analysis. Activity bout analyses were exported using the “bout” function in ClockLab, with a bout defined as a period where the activity level never fell below 3 count/min for longer than 30 minutes. Due to the low levels of activity seen in DKI mice on the mixed background, the threshold was reduced to 1 count/min for the bout analyses in those experiments. The length of the active period (α) was measured as the time between onset and offset of activity. Activity onset was fit by eye, and activity offset was defined as the last point at which the activity in three of the previous six bins exceeded the mean activity level (Gorman and Yellon, 2010). In 2 of 16 DKI mice, activity levels were too low to reliably detect activity onset/offset, and these animals were excluded from the statistical analysis of α. Because there were no significant differences in the behavior of the WT groups age-matched to the either the α-KI or β-KI mice, the two WT groups were combined into one control group for analysis of the single KI behavior.
Slice preparation and electrophysiological recording
Mice were individually housed in constant darkness for three weeks on running wheels and sacrificed at Circadian Time (CT) 4 and 16 (where CT 12 is conventionally defined as the onset of activity) by cervical dislocation and then enucleated with the aid of night-vision goggles. Brains were harvested, sectioned on a vibroslicer (Campden 7000SMZ, World Precision Instruments, Lafayette, IN) in cold, oxygenated sucrose saline (in mM: 250 sucrose, 26 NaHCO3, 1.25 Na2HPO4-7H2O, 1.2 MgSO4-7H2O, 10 glucose, 2.5 MgCl2, 3.5 KCl). Slices were transferred to a beaker containing 50% sucrose saline and 50% normal saline (in mM: 130 NaCl, 20 NaHCO3, 1 Na2HPO4-7H2O, 1.3 MgSO4-7H2O, 10 Glucose, 3.5 KCl, 2.5 CaCl2) at room temperature for 20 min and then transferred to an open recording chamber (Warner Instruments, Hambden, CT) that was continuously perfused at a rate of 2.0 ml/min with normal saline, bubbled with 5% CO2 / 95% O2 and heated to 34 ± 0.5 °C. Neurons were visualized with an Axio Examiner microscope (Carl Zeiss Inc., Thornwood, NY) equipped for near-IR-DIC. Loose patch recordings were made from CT 6-8 or from CT 18-20 in the medial (dorsal and ventral) SCN. Electrodes with a pipette resistance of ~3-5 MΩ were filled with filtered intracellular solution (in mM: 135 K-gluconate, 10 KCl, 10 HEPES, 0.5 EGTA; pH 7.4) (Kuhlman et al., 2003). Firing frequency was measured as the average of a 120-sec record. Electrophysiological signals were processed and controlled by a Multiclamp 700B amplifier, and pClamp 10.02 software (Axon Instruments, Union City, CA) in gap-free mode. Recordings were sampled at 20 kHz and filtered at 10 kHz (Besing et al., 2012).
Statistical Analysis
Data were analyzed using independent samples t-tests or one-way ANOVA with Tukey HSD post hoc analysis. For variables in which there were outliers and/or assumptions of normality and equal variances were violated and could not be corrected with logarithmic transformations of the data, we analyzed the data with a Mann-Whitney U test or an independent samples Kruskal-Wallis test with two-sided asymptotic significance post hoc analysis. Outliers were not excluded from the analysis. Data are presented as mean ± SEM. Significance was ascribed at p < 0.05.
RESULTS
Chronic GSK3 activity disrupts circadian wheel-running behavior
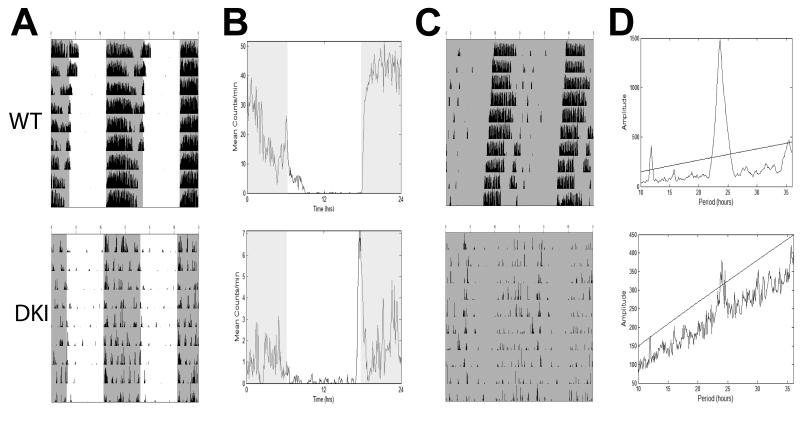
To determine the importance of rhythmic GSK3 phosphorylation on mammalian circadian rhythms we measured wheel running activity of DKI mice in which GSK3α and GSK3β have been mutated at the S21 and S9 inhibitory phosphorylation sites, respectively, rendering both forms constitutively active (McManus et al., 2005). First, we examined wheel-running behavior of DKI and WT mice on a mixed (C57BL/6 X Balb/c) background. In a 12:12h light-dark cycle (LD), both DKI and WT mice were capable of entraining to the light cycle, with the majority of activity occurring in the dark phase (Fig. 2A,B). This was reflected in the percentage of lights-on activity, which did not differ between the two genotypes (Table 1, U = 103, p > 0.05 ). The average activity in DKI mice (2.4 ± 0.7 rev/min) was significantly reduced from that of WT mice (12.0 ± 0.9 rev/min; U = 243; p < 0.001). This decrease in activity was seen in both the light and dark phases of the light cycle (Table 1).
Fig. 2.
Representative wheel-running behavior for WT (top) and DKI (bottom) mice on a mixed background. (A) Double-plotted actograms show behavior in a 12:12 light cycle (LD). (B) Activity profile plots showing averaged LD activity based on actograms in A. (C) Double-plotted actograms show behavior starting 6 days after release into constant dark (DD). (D) Representative Χ2-periodogram plots showing free-running period and amplitude for behavior in C. Line indicates 0.001% significance level. Shaded areas indicate lights off.
Table.
| WT | n | DKI | n | |
|---|---|---|---|---|
| LD | ||||
| Activity (rev/min) | 12.0 ± 0.9 | 16 | 2.4 ± 0.9** | 16 |
| Light Activity (rev/day) | 1235 ± 968 | 16 | 152 ± 158** | 16 |
| Dark Activity (rev/day) | 16062 ± 4443 | 16 | 3294 ± 5026** | 16 |
| Total Activity (rev/day) | 17297 ± 4912 | 16 | 6443 ± 5162** | 16 |
| % Lights on Activity (%) | 6.8 ± 4.8 | 16 | 8.7 ± 6.4 | 16 |
| ± | ± | |||
| DD | ± | ± | ||
| Tau (h) | 23.42 ± 0.16 | 17 | 23.81 ± 0.10** | 15 |
| Power | 1363 ± 343 | 17 | 524 ± 62** | 15 |
| Activity (rev/min) | 14.11 ± 1.04 | 17 | 0.83 ± 0.34** | 16 |
| Alpha Length (h) | 12.38 ± 0.73 | 17 | 14.44 ± 0.48** | 14 |
| Fragmentation (bouts/day) | 4.3 ± 0.5 | 17 | 6.1 ± 0.4* | 16 |
| Avg. Bout Length (min) | 133 ± 12.6 | 17 | 21.7 ± 2.3** | 16 |
p < 0.001
p < 0.01; Mann-Whitney U test
Under constant darkness (DD), several differences in the behavioral rhythms emerged (Fig. 2C). Periodogram analysis revealed that the free-running period (τ) of DKI mice was ~23 minutes longer than WT mice (Table 1; U = 30; p < 0.001). After only a short time in DD, 1 out of 20 DKI mice did not show a detectable rhythm and was classified as arrhythmic. The remaining DKI mice exhibited a significantly lower amplitude in circadian behavior than WT mice, as seen in the power of the Χ2-periodogram (Table 1; U = 240; p < 0.001; Fig. 2D). In addition, DKI mice showed significant fragmentation in their wheel-running rhythms, as indicated by an average of 6 activity bouts per day, compared to only 4 bouts per day in WT mice (Table 1; U = 55.5; p < 0.005). DKI mice also had significantly longer α or activity period (14.44 ± 0.48 h) than WT controls (12.38 ± 0.73 h; U = 52; p < 0.01), suggesting a lack of consolidation of activity.
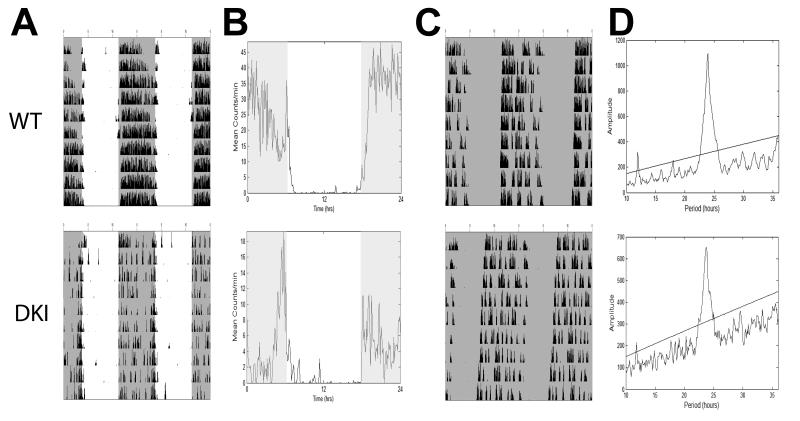
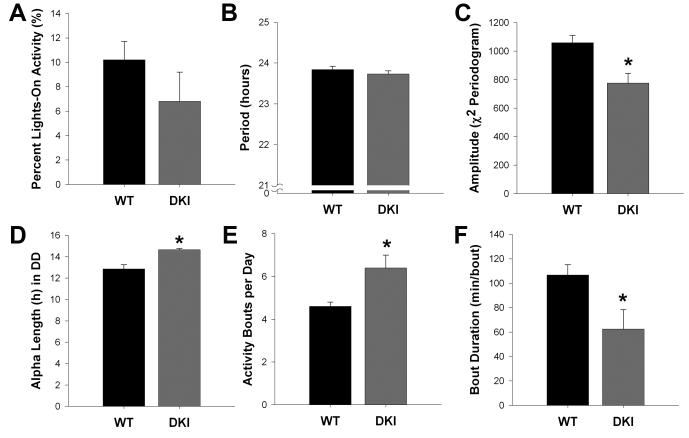
To ensure that the observed phenotype was not an effect of the mouse strain (Pendergast et al., 2010), we next examined the wheel-running behavior of DKI mice backcrossed to C57BL/6J (C57) for at least ten generations. When housed in LD, both groups successfully synchronized to the light cycle (Fig. 3A,B), and there was no difference in the percentage of lights-on activity observed between groups (Fig. 4A, t9 = −3.46, p > 0.05 ). As in the mixed background, overall wheel-running activity levels of back-crossed DKI mice (mean ± SEM: 8.1 ± 2.2 rev/min) were significantly reduced compared to WT mice (14.4 ± 1.0 rev/min; t6.7 = −2.6; p < 0.05; Fig. 3B); however, this difference was lost in DD (mean ± SEM; DKI, 7.2 ± 2.4 rev/min; WT, 10.5 ± 1.0 rev/min; t6.6 = −1.3; p > 0.05; Fig. 3C). Additionally, back-crossed DKI mice no longer exhibited a lengthened τ in DD (Fig. 4B; t9 = −0.96; p > 0.05). However, the DKI mice showed noticeably dampened activity rhythms, as seen in the significantly reduced amplitude of the Χ2-periodogram (Figs. 3D and 4C; t9 = −3.26; p < 0.05). Even though C57 DKI mice exhibited normal levels of activity in DD, the backcrossed mutants continued to show the same fragmented phenotype seen on the mixed background (Fig. 3C). Specifically, the mean α length of C57 DKI mice was nearly 2 hours longer than that of WT mice (Fig. 4D; t4.6 = 4.28; p < 0.01). Also, activity bouts of DKI mice were significantly greater in number per day (Fig 4E; t9 = 2.59; p < 0.05) and shorter in mean duration (Fig 4F; t9 = −2.29; p < 0.05).
Fig. 3.
Representative wheel-running behavior for C57BL/6J WT (top) and DKI (bottom) mice. (A) Double-plotted actograms show entrained behavior in LD. (B) Activity profile plots show averaged LD activity based on behavior in A. (C) Double-plotted actograms show behavior starting 12 days after release into DD. (D) Representative Χ2-periodogram plots showing free-running period and amplitude for behavior in C. Line indicates 0.001% significance level. Shaded areas indicate lights off.
Fig. 4.
Summary of circadian behavioral parameters for C57BL/6J DKI mice. Bar graphs indicating the mean ± SEM of percent activity during lights on in LD (A), free-running period (B), Χ2-periodogram amplitude (C), alpha length (D), activity fragmentation (E), and bout duration (F) for C57BL/6J WT (n = 5) and DKI (n = 6) mice. *p < 0.05
Circadian behavior is unaltered in single transgenic, α-KI and β-KI, mice
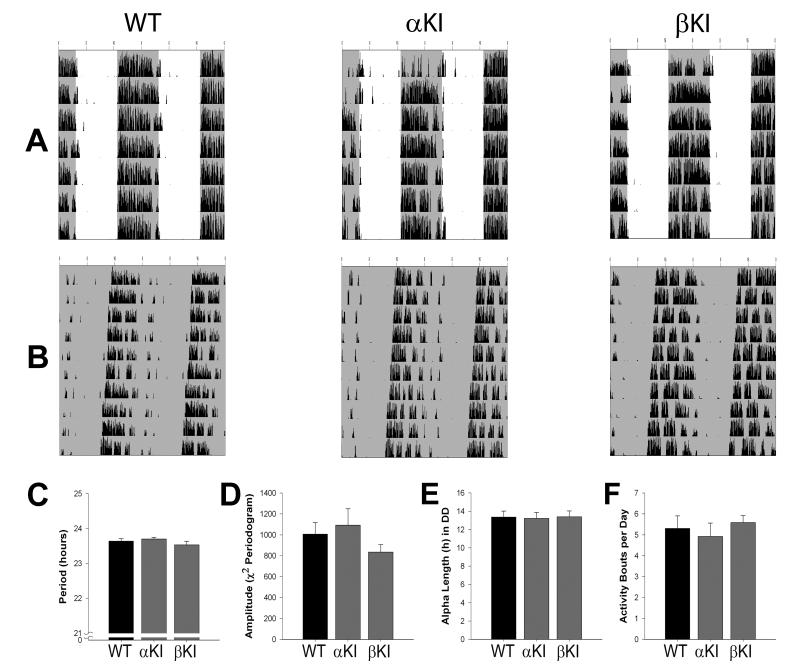
To determine the role of rhythmic activity of each GSK3 isoform, we measured wheel-running behavior of homozygous single transgenic, GSK3α21A/21A (α-KI) or GSK3β9A/9A (β-KI) mice and WT controls in the same manner as above. Both α-KI and β-KI mice exhibited typical entrainment to the light cycle (Fig. 5A) and similar levels of activity (rev/min) to WT mice in LD (mean ± SEM; α-KI, 12.0 ± 1.4; β-KI, 9.9 ± 1.7; WT, 12.4 ± 2.2; Kruskal-Wallis, H2 = 0.72; p > 0.05). In DD, Χ2-periodogram analysis revealed no significant differences in τ (F2,18 = 0.94; p > 0.05) or amplitude (F2,18 = 1.03; p > 0.05) among all three groups (Fig. 5B-D). The mean α lengths for α-KI and β-KI mice were essentially identical to the WT controls (F2,18 = 0.02; p > 0.05; Fig. 5E). Finally, single KI and WT mice had similarly consolidated behavior, with no significant differences in the number of activity bouts per day (F2,18 = 0.25; p > 0.05; Fig. 5F).
Fig. 5.
Summary of circadian locomotor activity for α-KI and β-KI mice. (A,B) Representative wheel-running behavior for WT (left), α-KI (middle), and β-KI (right) mice. Double-plotted actograms show locomotor activity in LD (A) and DD (B). Shaded areas indicate lights off. (C-F) Bar graphs indicating the mean ± SEM of free-running period (C), Χ2-periodogram amplitude (D), alpha length (E), and activity bouts per day (F) for WT (n = 10), α-KI (n = 5), and β-KI (n = 6) mice. One-way ANOVA; p > 0.05 for all graphs.
Chronic GSK3 activity alters SFR rhythms in SCN neurons
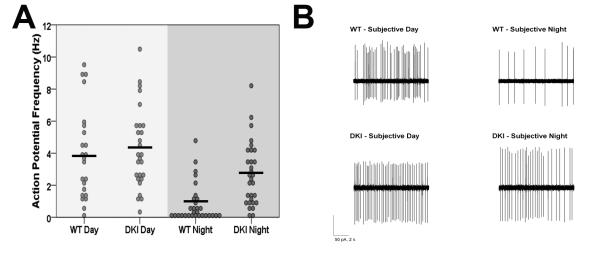
In order to determine whether DKI mice have a disruption in normal pacemaker function at the level of the SCN, we next examined neurophysiological activity of SCN neurons from these mice. SCN neurons exhibit a circadian variation in the frequency of spontaneous action potential generation, with high activity during the day and low activity during the night (Inouye and Kawamura, 1979). These rhythms are a major output signal of the SCN and are important in the regulation of circadian behavior (Schwartz et al., 1987). To test the effect of chronic GSK3 activation on SFR rhythms in the SCN, we used loose-patch electrophysiological recordings of SCN neurons from WT and DKI mice during the subjective day and night. After behavioral analysis in DD, the same C57BL/6J WT and DKI animals were sacrificed, and extracellular recordings were made from SCN neurons at CT 6-8 or CT 18-20 for day or night recordings, respectively. During the subjective day, SCN neurons exhibited similarly elevated firing in both WT and DKI mice (Fig. 6; WT, 3.9 ± 0.6 Hz, n = 23; DKI, 4.3 ± 0.5 Hz, n = 27). As expected, WT neurons had a significantly lower SFR at night (0.8 ± 0.3 Hz, n = 30; H3 = 40.7; p < 0.001; Kruskal-Wallis test). Unlike WT neurons, DKI SCN cells did not exhibit a significant day/night difference in neuronal activity (DKI-night, 2.7 ± 0.4 Hz; n = 30; p > 0.05). The loss of rhythmic activity in the SCN of DKI mice was driven by the hyperactivity of DKI neurons at night, which were firing at a rate over three times faster than WT cells (p < 0.05; two-sided asymptotic significance post hoc analysis). This same pattern of high excitability at night and normal activity during the day was also replicated in SCN neurons from DKI mice on the mixed background (mean ± SEM; DKI-night, 4.1 ± 0.8 Hz, n = 20; WT-night, 1.0 ± 0.4 Hz, n = 11; DKI-day, 5.8 ± 1.0 Hz, n = 11; WT-day, 4.8 ± 1.0 Hz, n = 17; 1-2 slices per genotype per timepoint).
Fig. 6.
Neurophysiological activity of SCN neurons from C57 DKI mice. (A) Spontaneous action potential frequencies of SCN neurons during the subjective day (CT 6-8) and subjective night (CT 18-20) from WT and DKI mice housed in DD. Black bars indicate the means for each group. (B) Representative cell-attached loose-patch traces (10 s) from results quantified in A. *Significantly different from all other groups, p < 0.005, 2-3 slices per genotype per time point.
DISCUSSION
The role of GSK3 as a critical regulator of the molecular clock is supported by reports showing that GSK3 phosphorylates nearly all of the core clock components in vitro (Iitaka et al., 2005, Wang et al., 2006, Spengler et al., 2009, Kurabayashi et al., 2010, Sahar et al., 2010). In addition to regulating the molecular clock, GSK3 exhibits a 24-hour variation of inhibitory phosphorylation within the SCN from mice held in an LD cycle (Iitaka et al., 2005). However, the role that this inactivation rhythm plays in the mammalian circadian system is not yet known. The present study shows, for the first time, that rhythmic GSK3 activity is critical for robust circadian rhythmicity. Specifically, our results indicate that: (1) chronic activation of GSK3 disrupts circadian behavior by decreasing circadian amplitude and increasing fragmentation; (2) chronic activation of either GSK3 isoform alone does not alter behavioral rhythms; and (3) chronic activation of GSK3α/β eliminates rhythms in SCN neuronal activity.
Our first finding that chronic GSK3 disrupts behavioral rhythms is supported by our results that DKI mice exhibit dampened and fragmented wheel-running activity. Previous work on GSK3 and circadian behavior has predominantly focused on changes in the free-running period. For example, lithium, a known inhibitor of GSK3, lengthens the free-running period of a variety of organisms (LeSauter and Silver, 1993, Iwahana et al., 2004, Dokucu et al., 2005). Conversely, Drosophila that over-express GSK3β have a shortened τ (Martinek et al., 2001). Surprisingly, our results show that GSK3 that is chronically active (but expressed at physiological levels) causes only a slight increase in τ in mutant mice on a mixed background. In rodents, the act of running on a wheel is known to influence τ, with wheel-access being associated with a shorter period (Yamada et al., 1988). Thus, it is possible that the lengthened τ in mixed DKI mice was a result of the severely reduced activity levels seen in these animals. This explanation is supported by the lack of period change in DKI mice on the C57 background, which also did not differ from WT mice in DD activity levels. Instead, our results revealed the importance of GSK3 phosphorylation rhythms in the generation of robust circadian behavior. On both backgrounds of mice, chronic GSK3 activity reduced the amplitude of behavioral rhythms and expanded the active phase (α). This reduced amplitude was coupled with significant behavioral fragmentation, as seen in the quantification of activity bouts, which were shorter in length and greater in number. These results are consistent with recent in vitro data that GSK3 inhibition increases the molecular clock amplitude (Li et al., 2012). Taken together, these findings suggest a critical role for GSK3 phosphorylation state balance in driving circadian clock amplitude.
Although the two GSK3 isoforms express 98% homology in amino acid sequence (Kaidanovich and Eldar-Finkelman, 2002), differences in α and β isoforms function has been noted in muscle metabolism (McManus et al., 2005), ischemic injury (Lal et al., 2012), and anti-depressant response (Polter and Li, 2011). Different functions of the isoforms have also been implicated within the circadian system. For example, GSK3α, but not β, interacts in vitro with Receptor for Activated C-Kinase 1 (RACK1) (Zeidner et al., 2011), a known regulator of the circadian clock (Robles et al., 2010). In spite of this, the differential roles of GSK3α and β in regulating circadian rhythms in vivo remain unclear; furthermore, the distinct function of each isoforms’ rhythmic activity has been largely unexplored. In the present study, we examined the role of inhibitory phosphorylation of GSK3α and GSK3β separately by using transgenic mouse models. Interestingly, the single transgenic α-KI and β-KI mice did not exhibit any of the same behavioral disruptions that were characteristic of the DKI mice. These results suggest that, in terms of circadian rhythm function, there may be compensation for the loss of phosphorylation of one isoform. However, peak levels of p-GSK3 in these mice do not suggest that this compensation occurs at the level of GSK3 S21/S9 phosphorylation, consistent with findings of McManus et al. (2005).
In addition to disrupting circadian behavior, we found that chronic GSK3 activity altered SFR rhythms in the SCN. Our results show that loss of GSK3 inactivation, as in the DKI mice, eliminated the day/night difference in SCN neuronal activity. This loss was due to the elevated “day”-like activity of DKI neurons during the subjective night. Importantly, the activity of SCN DKI neurons did not differ from that of WT neurons during the day, suggesting that the effects of constitutive GSK3 activation are phase-specific. These results suggest that the phosphorylation rhythm may be necessary for normal rhythmicity of spike rate in SCN neurons. Our results are consistent with a model in which active GSK3 enhances excitability while inactivation or inhibition decreases excitability. This idea is supported by previous work examining the effect of lithium on SCN activity. In hamsters, application of lithium to acute brain slices suppressed day-phase SCN neuronal firing (Mason and Biello, 1992), but this suppression may have occurred through a non-GSK3 related action of the drug. In the future, it will be important to replicate this effect with specific, small-molecule inhibitors of GSK3.
The mechanism by which GSK3 activity controls neurophysiological rhythms in the SCN remains unclear. One possible explanation for the disrupted SCN activity seen in DKI mice is an indirect consequence of disruption in the core molecular clock. However, further study in vivo is needed to determine how the molecular clock is affected by chronic GSK3 activity. Another possibility is that, downstream of the core clock, GSK3 activity directly influences membrane properties through an unknown mechanism. Interestingly, the effects of lithium on SCN activity are seen within 5-10 min of treatment (Mason and Biello, 1992). The rapid onset of this effect supports the notion that GSK3 is able to exert control over SCN neurophysiology without acting through the core clock. The ionic mechanisms underlying the daily rhythms in SCN activity have been extensively studied, and many of these currents have been shown to be rhythmic themselves (Pennartz et al., 2002, Itri et al., 2005, Pitts et al., 2006, Itri et al., 2010). One of the biggest questions left unanswered is how ion channels are being regulated to produce these rhythms (Colwell, 2011). In cortical and hippocampal cell culture, GSK3 has been shown to regulate expression and function of multiple receptors including, NMDAR, AMPAR, and GABAAR, through mechanisms like trafficking, clustering, and phosphorylation (Chen et al., 2007, Wei et al., 2010, Tyagarajan et al., 2011). Future research should examine the possibility that rhythmic GSK3 regulates ion channels in the SCN in a similar manner.
One notable candidate target of GSK3 is large-conductance Ca2+ activated K+ (BK) channels which contribute to the nightly silencing of SCN neurons (Meredith et al., 2006). In other cell types, GSK3 can directly associate with the BKα sub-unit and regulate membrane expression of BK channels in vitro (Bian et al., 2011, Sokolowski et al., 2011). Furthermore, kcnma1−/− mice, which lack the pore-forming α subunit of BK channels, show strikingly similar circadian phenotypes to DKI mice, in both behavior and SCN activity (Meredith et al., 2006). Determining the role of rhythmic GSK3 activity in regulating membrane properties of the SCN could provide a missing link between the molecular clock and the circadian output signal (i.e., spike rate). With the growing number of reports showing disrupted circadian behavior together with altered neuronal rhythms (Meredith et al., 2006, Kudo et al., 2011a, Kudo et al., 2011b, Nakamura et al., 2011, Farajnia et al., 2012), understanding the link between the core clock and membrane excitability is an important direction for future circadian research.
Dysregulation of GSK3 has been implicated in many of the same pathological conditions that are linked with circadian rhythm disturbance, such as psychiatric and aging-related disorders (Gomez-Sintes et al., 2011, Jope, 2011). Previously, DKI mice have been used as a model for bipolar disorder, with the mice displaying increased susceptibility to both manic- and depressive-like behaviors (Polter et al., 2010). In human bipolar patients, phosphorylation levels of GSK3 are decreased in peripheral blood mononuclear cells and fibroblasts (Yang et al., 2009, Polter et al., 2010). Circadian disruption is commonly seen in aging (Kondratova and Kondratov, 2012), and loss of p-GSK3 rhythms has been demonstrated in the SCN of aged hamsters (Iwahana et al., 2007). Because of its involvement in these and other pathologies, understanding how GSK3 modulates circadian rhythms and neurophysiological activity may lead to novel therapeutics for pathological disorders and circadian rhythm dysfunction.
Highlights.
Mice with constitutively active GSK3α/β (KI) exhibit disrupted circadian behavior.
GSK3α/β KI mice show dampened and fragmented wheel-running activity rhythms.
Chronic activation of GSK3α or β alone did not produce the same altered phenotype.
SCN neurons from GSK3α/β KI have elevated neuronal activity during the night.
Chronic GSK3α/β activity results in the loss of typical SCN firing rate rhythms.
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health, Grants R00 GM086683 (KLG) and R01 MH038752 (RSJ). We thank Xiaohua Li for backcrossing the mice, Lauren Hablitz for technical assistance, and Rachel Besing for advice on the manuscript.
ABBREVIATIONS
- α-KI
GSK3α21A/21A knock-in
- β-KI
GSK3β9A/9A knock-in
- CT
circadian time
- C57
C57BL/6J
- DD
constant dark
- DKI
GSK3α21A/21A/β9A9A knock-in
- GSK3
glycogen synthase kinase 3
- LD
12:12 light-dark cycle
- SCN
suprachiasmatic nucleus
- SFR
spontaneous firing rate
- WT
wild-type
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Bell-Pedersen D, Cassone VM, Earnest DJ, Golden SS, Hardin PE, Thomas TL, Zoran MJ. Circadian rhythms from multiple oscillators: lessons from diverse organisms. Nat Rev Genet. 2005;6:544–556. doi: 10.1038/nrg1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besing RC, Hablitz LM, Paul JR, Johnson RL, Prosser RA, Gamble KL. Neuropeptide Y-induced phase shifts of PER2::LUC rhythms are mediated by long-term suppression of neuronal excitability in a phase-specific manner. Chronobiol Int. 2012;29:91–102. doi: 10.3109/07420528.2011.649382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bian S, Bai JP, Chapin H, Le Moellic C, Dong H, Caplan M, Sigworth FJ, Navaratnam DS. Interactions between beta-catenin and the HSlo potassium channel regulates HSlo surface expression. PLoS One. 2011;6:e28264. doi: 10.1371/journal.pone.0028264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P, Gu Z, Liu W, Yan Z. Glycogen synthase kinase 3 regulates N-methyl-D-aspartate receptor channel trafficking and function in cortical neurons. Mol Pharmacol. 2007;72:40–51. doi: 10.1124/mol.107.034942. [DOI] [PubMed] [Google Scholar]
- Colwell CS. Linking neural activity and molecular oscillations in the SCN. Nat Rev Neurosci. 2011;12:553–569. doi: 10.1038/nrn3086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dokucu ME, Yu L, Taghert PH. Lithium- and valproate-induced alterations in circadian locomotor behavior in Drosophila. Neuropsychopharmacology. 2005;30:2216–2224. doi: 10.1038/sj.npp.1300764. [DOI] [PubMed] [Google Scholar]
- Farajnia S, Michel S, Deboer T, vanderLeest HT, Houben T, Rohling JH, Ramkisoensing A, Yasenkov R, Meijer JH. Evidence for neuronal desynchrony in the aged suprachiasmatic nucleus clock. J Neurosci. 2012;32:5891–5899. doi: 10.1523/JNEUROSCI.0469-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallego M, Virshup DM. Post-translational modifications regulate the ticking of the circadian clock. Nat Rev Mol Cell Biol. 2007;8:139–148. doi: 10.1038/nrm2106. [DOI] [PubMed] [Google Scholar]
- Gomez-Sintes R, Hernandez F, Lucas JJ, Avila J. GSK-3 Mouse Models to Study Neuronal Apoptosis and Neurodegeneration. Front Mol Neurosci. 2011;4:45. doi: 10.3389/fnmol.2011.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman MR, Yellon S. Lifespan daily locomotor activity rhythms in a mouse model of amyloid-induced neuropathology. Chronobiol Int. 2010;27:1159–1177. doi: 10.3109/07420528.2010.485711. [DOI] [PubMed] [Google Scholar]
- Iitaka C, Miyazaki K, Akaike T, Ishida N. A role for glycogen synthase kinase-3beta in the mammalian circadian clock. J Biol Chem. 2005;280:29397–29402. doi: 10.1074/jbc.M503526200. [DOI] [PubMed] [Google Scholar]
- Inouye ST, Kawamura H. Persistence of circadian rhythmicity in a mammalian hypothalamic “island” containing the suprachiasmatic nucleus. Proc Natl Acad Sci U S A. 1979;76:5962–5966. doi: 10.1073/pnas.76.11.5962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itri JN, Michel S, Vansteensel MJ, Meijer JH, Colwell CS. Fast delayed rectifier potassium current is required for circadian neural activity. Nat Neurosci. 2005;8:650–656. doi: 10.1038/nn1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itri JN, Vosko AM, Schroeder A, Dragich JM, Michel S, Colwell CS. Circadian regulation of a-type potassium currents in the suprachiasmatic nucleus. J Neurophysiol. 2010;103:632–640. doi: 10.1152/jn.00670.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwahana E, Akiyama M, Miyakawa K, Uchida A, Kasahara J, Fukunaga K, Hamada T, Shibata S. Effect of lithium on the circadian rhythms of locomotor activity and glycogen synthase kinase-3 protein expression in the mouse suprachiasmatic nuclei. Eur J Neurosci. 2004;19:2281–2287. doi: 10.1111/j.0953-816X.2004.03322.x. [DOI] [PubMed] [Google Scholar]
- Iwahana E, Hamada T, Uchida A, Shibata S. Differential effect of lithium on the circadian oscillator in young and old hamsters. Biochem Biophys Res Commun. 2007;354:752–756. doi: 10.1016/j.bbrc.2007.01.042. [DOI] [PubMed] [Google Scholar]
- Jope RS. Glycogen synthase kinase-3 in the etiology and treatment of mood disorders. Front Mol Neurosci. 2011;4:16. doi: 10.3389/fnmol.2011.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaidanovich O, Eldar-Finkelman H. The role of glycogen synthase kinase-3 in insulin resistance and type 2 diabetes. Expert Opin Ther Targets. 2002;6:555–561. doi: 10.1517/14728222.6.5.555. [DOI] [PubMed] [Google Scholar]
- Klein PS, Melton DA. A molecular mechanism for the effect of lithium on development. Proc Natl Acad Sci U S A. 1996;93:8455–8459. doi: 10.1073/pnas.93.16.8455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondratova AA, Kondratov RV. The circadian clock and pathology of the ageing brain. Nat Rev Neurosci. 2012;13:325–335. doi: 10.1038/nrn3208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudo T, Loh DH, Truong D, Wu Y, Colwell CS. Circadian dysfunction in a mouse model of Parkinson’s disease. Exp Neurol. 2011a;232:66–75. doi: 10.1016/j.expneurol.2011.08.003. [DOI] [PubMed] [Google Scholar]
- Kudo T, Schroeder A, Loh DH, Kuljis D, Jordan MC, Roos KP, Colwell CS. Dysfunctions in circadian behavior and physiology in mouse models of Huntington’s disease. Exp Neurol. 2011b;228:80–90. doi: 10.1016/j.expneurol.2010.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhlman SJ, Silver R, Le Sauter J, Bult-Ito A, McMahon DG. Phase resetting light pulses induce Per1 and persistent spike activity in a subpopulation of biological clock neurons. J Neurosci. 2003;23:1441–1450. doi: 10.1523/JNEUROSCI.23-04-01441.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurabayashi N, Hirota T, Sakai M, Sanada K, Fukada Y. DYRK1A and glycogen synthase kinase 3beta, a dual-kinase mechanism directing proteasomal degradation of CRY2 for circadian timekeeping. Mol Cell Biol. 2010;30:1757–1768. doi: 10.1128/MCB.01047-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lal H, Zhou J, Ahmad F, Zaka R, Vagnozzi RJ, Decaul M, Woodgett J, Gao E, Force T. Glycogen synthase kinase-3alpha limits ischemic injury, cardiac rupture, post-myocardial infarction remodeling and death. Circulation. 2012;125:65–75. doi: 10.1161/CIRCULATIONAHA.111.050666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeSauter J, Silver R. Lithium lengthens the period of circadian rhythms in lesioned hamsters bearing SCN grafts. Biol Psychiatry. 1993;34:75–83. doi: 10.1016/0006-3223(93)90259-g. [DOI] [PubMed] [Google Scholar]
- Li J, Lu WQ, Beesley S, Loudon AS, Meng QJ. Lithium impacts on the amplitude and period of the molecular circadian clockwork. PLoS One. 2012;7:e33292. doi: 10.1371/journal.pone.0033292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinek S, Inonog S, Manoukian AS, Young MW. A role for the segment polarity gene shaggy/GSK-3 in the Drosophila circadian clock. Cell. 2001;105:769–779. doi: 10.1016/s0092-8674(01)00383-x. [DOI] [PubMed] [Google Scholar]
- Mason R, Biello SM. A neurophysiological study of a lithium-sensitive phosphoinositide system in the hamster suprachiasmatic (SCN) biological clock in vitro. Neurosci Lett. 1992;144:135–138. doi: 10.1016/0304-3940(92)90734-o. [DOI] [PubMed] [Google Scholar]
- McManus EJ, Sakamoto K, Armit LJ, Ronaldson L, Shpiro N, Marquez R, Alessi DR. Role that phosphorylation of GSK3 plays in insulin and Wnt signalling defined by knockin analysis. EMBO J. 2005;24:1571–1583. doi: 10.1038/sj.emboj.7600633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith AL, Wiler SW, Miller BH, Takahashi JS, Fodor AA, Ruby NF, Aldrich RW. BK calcium-activated potassium channels regulate circadian behavioral rhythms and pacemaker output. Nat Neurosci. 2006;9:1041–1049. doi: 10.1038/nn1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura TJ, Nakamura W, Yamazaki S, Kudo T, Cutler T, Colwell CS, Block GD. Age-related decline in circadian output. J Neurosci. 2011;31:10201–10205. doi: 10.1523/JNEUROSCI.0451-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pendergast JS, Friday RC, Yamazaki S. Distinct functions of Period2 and Period3 in the mouse circadian system revealed by in vitro analysis. PLoS One. 2010;5:e8552. doi: 10.1371/journal.pone.0008552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennartz CM, de Jeu MT, Bos NP, Schaap J, Geurtsen AM. Diurnal modulation of pacemaker potentials and calcium current in the mammalian circadian clock. Nature. 2002;416:286–290. doi: 10.1038/nature728. [DOI] [PubMed] [Google Scholar]
- Pitts GR, Ohta H, McMahon DG. Daily rhythmicity of large-conductance Ca2+ -activated K+ currents in suprachiasmatic nucleus neurons. Brain Res. 2006;1071:54–62. doi: 10.1016/j.brainres.2005.11.078. [DOI] [PubMed] [Google Scholar]
- Polter A, Beurel E, Yang S, Garner R, Song L, Miller CA, Sweatt JD, McMahon L, Bartolucci AA, Li X, Jope RS. Deficiency in the inhibitory serine-phosphorylation of glycogen synthase kinase-3 increases sensitivity to mood disturbances. Neuropsychopharmacology. 2010;35:1761–1774. doi: 10.1038/npp.2010.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polter AM, Li X. Glycogen Synthase Kinase-3 is an Intermediate Modulator of Serotonin Neurotransmission. Front Mol Neurosci. 2011;4:31. doi: 10.3389/fnmol.2011.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robles MS, Boyault C, Knutti D, Padmanabhan K, Weitz CJ. Identification of RACK1 and protein kinase Calpha as integral components of the mammalian circadian clock. Science. 2010;327:463–466. doi: 10.1126/science.1180067. [DOI] [PubMed] [Google Scholar]
- Sahar S, Zocchi L, Kinoshita C, Borrelli E, Sassone-Corsi P. Regulation of BMAL1 protein stability and circadian function by GSK3beta-mediated phosphorylation. PLoS One. 2010;5:e8561. doi: 10.1371/journal.pone.0008561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz WJ, Gross RA, Morton MT. The suprachiasmatic nuclei contain a tetrodotoxin-resistant circadian pacemaker. Proc Natl Acad Sci U S A. 1987;84:1694–1698. doi: 10.1073/pnas.84.6.1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokolowski B, Orchard S, Harvey M, Sridhar S, Sakai Y. Conserved BK channel-protein interactions reveal signals relevant to cell death and survival. PLoS One. 2011;6:e28532. doi: 10.1371/journal.pone.0028532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spengler ML, Kuropatwinski KK, Schumer M, Antoch MP. A serine cluster mediates BMAL1-dependent CLOCK phosphorylation and degradation. Cell Cycle. 2009;8:4138–4146. doi: 10.4161/cc.8.24.10273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi JS, Hong HK, Ko CH, McDearmon EL. The genetics of mammalian circadian order and disorder: implications for physiology and disease. Nat Rev Genet. 2008;9:764–775. doi: 10.1038/nrg2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyagarajan SK, Ghosh H, Yevenes GE, Nikonenko I, Ebeling C, Schwerdel C, Sidler C, Zeilhofer HU, Gerrits B, Muller D, Fritschy JM. Regulation of GABAergic synapse formation and plasticity by GSK3beta-dependent phosphorylation of gephyrin. Proc Natl Acad Sci U S A. 2011;108:379–384. doi: 10.1073/pnas.1011824108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Yin L, Lazar MA. The orphan nuclear receptor Rev-erb alpha regulates circadian expression of plasminogen activator inhibitor type 1. J Biol Chem. 2006;281:33842–33848. doi: 10.1074/jbc.M607873200. [DOI] [PubMed] [Google Scholar]
- Wei J, Liu W, Yan Z. Regulation of AMPA receptor trafficking and function by glycogen synthase kinase 3. J Biol Chem. 2010;285:26369–26376. doi: 10.1074/jbc.M110.121376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh DK, Takahashi JS, Kay SA. Suprachiasmatic nucleus: cell autonomy and network properties. Annu Rev Physiol. 2010;72:551–577. doi: 10.1146/annurev-physiol-021909-135919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodgett JR. Molecular cloning and expression of glycogen synthase kinase-3/factor A. EMBO J. 1990;9:2431–2438. doi: 10.1002/j.1460-2075.1990.tb07419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada N, Shimoda K, Ohi K, Takahashi S, Takahashi K. Free-access to a running wheel shortens the period of free-running rhythm in blinded rats. Physiol Behav. 1988;42:87–91. doi: 10.1016/0031-9384(88)90265-x. [DOI] [PubMed] [Google Scholar]
- Yang S, Van Dongen HP, Wang K, Berrettini W, Bucan M. Assessment of circadian function in fibroblasts of patients with bipolar disorder. Mol Psychiatry. 2009;14:143–155. doi: 10.1038/mp.2008.10. [DOI] [PubMed] [Google Scholar]
- Zeidner LC, Buescher JL, Phiel CJ. A novel interaction between Glycogen Synthase Kinase-3alpha (GSK-3alpha) and the scaffold protein Receptor for Activated C-Kinase 1 (RACK1) regulates the circadian clock. Int J Biochem Mol Biol. 2011;2:318–327. [PMC free article] [PubMed] [Google Scholar]