Abstract
H2-M3-restricted T cells have a pre-activated surface phenotype, rapidly expand and produce cytokines upon stimulation and as such, are classified as innate T cells. Unlike most innate T cells, M3-restricted T cells also express CD8αβ co-receptors and a diverse TCR repertoire: hallmarks of conventional MHC Ia-restricted CD8+ T cells. Although iNKT cells are also innate lymphocytes, they are selected exclusively on hematopoietic cells (HC), while M3-restricted T cells can be selected on either hematopoietic or thymic epithelial cells (TEC). Moreover, their phenotypes differ depending on what cells mediate their selection. Though there is a clear correlation between selection on HC and development of innate phenotype, the underlying mechanism remains unclear. SAP is required for the development of iNKT cells and mediates signals from SLAM receptors that are exclusively expressed on HC. Based on their dual selection pathway, M3-restricted T cells present a unique model for studying the development of innate T cell phenotype. Using both polyclonal and transgenic mouse models we demonstrate that while M3-restricted T cells are capable of developing in the absence of SAP, SAP is required for HC-mediated selection, development of pre-activated phenotype and heightened effector functions of M3-restricted T cells. These findings are significant because they directly demonstrate the need for SAP in HC-mediated acquisition of innate T cell phenotype and suggest that due to their SAP-dependent HC-mediated selection, M3-restricted T cells develop a pre-activated phenotype and an intrinsic ability to proliferate faster upon stimulation, allowing for an important role in the early response to infection.
Keywords: T cell Development, SAP, MHC class Ib, CD8+ T cells, Innate T Lymphocytes
INTRODUCTION
Despite the classical distinctions between innate (immediate, non-specific) and adaptive (delayed, antigen specific) immune responses, the discovery of a relatively new group of adaptive immune cells (innate T cells) has revealed that the two branches of the immune system may not be as mutually exclusive as previously thought (1). Innate T cell subsets including CD1d-restricted invariant natural killer T (iNKT) cells and MHC Ib-restricted CD8+ T cells have unique phenotypes that set them apart from more “conventional” T cells. CD1d-restricted iNKT cells recognize lipid antigens presented by CD1d and are capable of rapidly producing large quantities of both Th1 and Th2 cytokines upon activation (2, 3). MHC Ib-restricted CD8+ T cells, recognize peptide antigens, and are also capable of responding rapidly to infection (4). H2–M3 (M3) is the best-characterized murine MHC Ib molecule, demonstrating specific affinity for N-formylated peptides (5) and is thus uniquely suited to present bacterially derived peptides. Like most MHC Ib-restricted CD8+ T cells, M3-restricted T cells can be distinguished from MHC Ia-restricted T cells based on an “innate-like” phenotype. This phenotype is characterized by up-regulation of cellular activation markers under naïve conditions as well as rapid cell proliferation and cytokine production following antigenic stimulation (6, 7). Upon intracellular bacterial infection, M3-restricted CD8+ T cells respond more quickly than MHC Ia-restricted CD8+ T cells (8). These unique features of innate T cells suggest a role in bridging innate and adaptive immune responses to infection and despite the relatively small number of innate T cells found within the total T cell population, these T cells have been shown to be important in shaping the overall immune response to infection. Illustrating the important role played by M3-restricted T cells in primary responses to bacterial infection, M3 deficiency results in diminished bacterial clearance in both the liver and spleen following infection, indicating that MHC Ia-restricted T cells are unable to compensate for a lack of M3-restricted T cells (9).
The majority of conventional T cells are positively selected on thymic epithelial cells (TEC) (10), however, certain innate T cell subsets can be selected on hematopoietic cells (HC) and this unique selection pathway has been linked to the development of innate phenotypes in these cells. While CD1d-restricted iNKT cells are exclusively selected on HC (11), we have recently demonstrated that M3-restricted T cells can be selected on both TEC and HC and that selecting cell type plays an important role in determining the phenotype of these cells (12). We have demonstrated that HC-selected M3-restricted T cells are characterized by a more profound pre-activated phenotype and more potent effector functions upon stimulation, suggesting that the mode of selection of innate T cells may be the key to understanding the unique phenotypes displayed by this important group of immune cells. Despite these advances in our understanding of innate T cell function, the underlying mechanisms responsible for the development of innate phenotypes remain largely undefined. It is still unclear how innate T cells are able to mount more rapid T cell responses, and the mechanism by which selecting cell type influences T cell phenotype is yet to be determined.
An important component of HC-mediated positive selection is the signaling lymphocyte activation molecule (SLAM) associated protein (SAP) signaling pathway. SLAM receptors are found exclusively on thymocytes and other hematopoietic cells and are activated through homotypic interactions with one another (13–15). Upon receptor ligation, SAP is recruited to and associates with certain high affinity tyrosine-based motifs on the cytoplasmic ends of the receptor. SAP recruits and activates protein tyrosine kinases (including Fyn) enabling SLAM receptors to mediate downstream protein phosphorylation signaling events (16–18), culminating in cell activation and presumably development of innate phenotypes. The physiological importance of SAP is underscored by the discovery that the human immunodeficiency X-linked lymphoproliferative disease (XLP) is caused by a mutation in the gene SH2D1A that codes for SAP (19, 20). Positive selection of iNKT cells is severely impaired in the absence of SAP (21) and the development of innate CD4+ T cells (selected on HC) has also been shown to be dependent on this signaling pathway (22). Given the apparent link between SLAM/SAP signaling and the development of innate T cell phenotypes, we designed this study to assess the role of SAP in determining the phenotype and effector functions of M3-restricted T cells. Given their ability to be selected by both HC and TEC-mediated pathways, M3-restricted T cells provide a unique model to study the role played by SAP in these two selection pathways.
Using SAP deficient mice we demonstrate that while M3-restricted CD8+ T cells are capable of developing in the absence of SAP, the phenotype of these cells differs significantly from those that develop in the presence of SAP. We show that SAP is required for the development of the pre-activated phenotype characteristic of innate T cells and M3-restricted T cells that develop in a SAP-deficient background exhibit impaired expansion and less potent effector functions upon antigen stimulation. Most importantly, we demonstrate that selection of M3-restricted CD8+ T cells on HC, but not on TEC, is dependent on SAP expression. This finding is significant not only because it directly demonstrates the need for SAP in HC-mediated selection and acquisition of innate T cell phenotype, but also provides an explanation for the unique kinetics exhibited by these cells upon antigenic challenge.
MATERIALS AND METHODS
Mice
C57BL/6 (B6), C57BL/6 congenic (CD45.1), Rag-2-deficient (Rag−/−) and IL-4 deficient (IL-4−/−) mice were purchased from Jackson laboratories. D7 Tg (23) and M3−/− (9), and SAP−/− mice (24) have been described previously. D7 Tg mice were crossed onto the Rag−/− background and further crossed with SAP−/− mice for these studies. The Institutional Animal Care and Use Committee approved all animal work.
Antibodies and tetramers
FITC-conjugated anti-CD8β, CD44, Ly6c, CD24; PE-conjugated anti-CD8α, B220, β7 integrin; PerCP-conjugated anti-CD4, B220; allophycocyanin-conjugated INF-γ; PerCP Cy5.5-conjugated anti TCRβ Ly6c, CD62L; pacific̣ bluẽ conjugateḍ ant̃i B220, CD11b, and CD11c and biotin-conjugated anti-CD24; Kb-dimer were purchased from BD Biosciences or eBiosciences. H2-M3/LemA and M3/Fr38 tetramers were provided by the NIH MHC tetramer Core facility.
Flow cytometry
Single cell suspensions from the thymus, spleen and liver, were prepared by standard procedures. Cells were incubated with 2.4G2 Fcγ RII/RIII blocking mAb (hybridoma supernatant) for 15 min, then stained for 30 min on ice with the appropriate combinations of mAbs. Flow cytometry was performed with a FACSCanto II (BD Biosciences) and analyzed using FlowJo software (Tree Star Inc).
Tetramer enrichment
B6, SAP−/− and IL-4−/− splenocytes were stained with PE-M3/LemA tetramer, M3/Fr38 tetramer or PEKb/Ova dimer at 4°C for 1 hr, washed and incubated with anti-PE microbeads (Miltenyi) for 30min at 4°C. Cells were purified using a magnetized LS column and stained with allophycocyanin-M3/LemA tetramer or TCRβ (dimẹrsamples), anti-CD3 (FITC), anti-CD8 (V500), anti-CD44 (Alexa Fluor 700), and PerCP-conjugated anti-Ly6C and anti-CD62L mAb. Cells were also stained with pacific blue conjugated anti-B220, CD11b and CD11c, (dump gate). Cells were washed and analyzed with a FACS Canto II.
Bone Marrow Chimeras
Donor BM cells were depleted of mature T cells using anti-Thy1.2 mAb (AT83.A-6) and rabbit complement (Cedarlane). 1×107 T cell-depleted BM cells were injected i.v. per irradiated recipient. 7–8 weeks later, chimeras were immunized, sacrificed and analyzed by flow cytometry.
BMDC generation and DC immunization
BMDC were prepared from BM cells eluted from the tibia and femur of B6 mice. Cells were cultured with RPMI-10 (10% FBS, 25mM HEPES, 2mM L-glutamin 100U/ml penicillin, 100mg/ml streptomycin, and 50mM 2-ME) supplemented with GM-CSF and IL-4 (Pepro Tech) for 7 days. DC were matured with 100ng/mL LPS, pulsed with 1 μM of LemA peptide (fMIGWII, GenScript) or Ova peptides (SIINFEKL, GenScript) for 6 hr and injected i.v. into BM chimeric mice (1× 106 peptide-coated DC per mouse).
Bacteria and LM infection
The recombinant L. monocytogenes (LM) strain rLM-OVA was grown in brain-heart infusion broth supplemented with 5μg/ml erythromycin. Mice were infected i.v. with 5 ×103 CFU rLM-OVA (1/100 LD50).
Intracellular cytokine staining assay
Splenocytes were stimulated with LemA peptide (5 μM) for 3–5 hours in the presence of 10 μM monensin. Cells were washed and stained for cell surface markers CD8α and TCRβ. After fixation with 4% paraformaldehyde and permeabilization with 0.15 % saponin, cells were then stained with anti-IFN-γ antibody for 30 min in PBS containing 1% bovine serum albumin and 0.1% saponin. Flow cytometry was performed as described above.
RNA extraction and quantitative real-time PCR
D7+SAP−/− and D7+SAP+ thymocytes were isolated from the respective mice. Immature thymocytes were depleted using anti-CD24 mAb and complement. Single positive CD8 (CD8SP) thymocytes were sorted by flow cytometry (Northwestern University Flow Core). Total RNA was isolated from purified D7+SAP−/− and D7+SAP+ SP CD8SP thymocytes using an RNeasy kit and reverse-transcribed using Superscript II reverse transcriptase and random hexamer primers. Real-time PCR was performed on an i-cycler using SYBR Green Master Mix. Transcripts for murine Eomes and T-bet were quantified with primers (forward primer: 5'-TGAATGAACCTTCCAAGACTCAGA-3'; reverse: 5'-TGAATGAACCTTCCAAGACTCAGA-3') and (forward primer: 5'-CCAGCACCAGACAGAGATGA-3'; reverse: 5'- GCTTCCCAAATGAAACTTCC-3') respectively and normalized to GAPDH (forward primer: 5′-TTCACCACCATGGAGAAGGC-3'; reverse primer, 5′-GGCATGGACTGTGGTCATGA-3′).
Statistical analysis
Statistical analyses were performed using PRISM software (GraphPad). Statistical significance of differences was calculated using two-tailed Student's t tests. P-values <0.05 were considered significant (*, P < 0.05)
RESULTS
Differences between conventional and M3-restricted T cell kinetics reflect differences in naïve T cell precursor frequency and intrinsic T cell phenotype
A number of recent studies have demonstrated a link between naïve antigen specific precursor frequency and T cell immune responses. Using magnetic bead-assisted tetramer enrichment, these studies quantified the number of T cells specific for particular antigens in individual naïve animals and demonstrated that this precursor frequency is predictive of the magnitude and kinetics of the T cell responses to the various antigens (25, 26). Antigens with higher precursor frequencies were found to elicit more rapid and more robust immune responses than those with lower precursor frequency. Based on these results we designed experiments to characterize the M3-restricted CD8+ T cell population in naïve mice and compare the precursor frequencies of these cells with those of conventional CD8+ T cells.
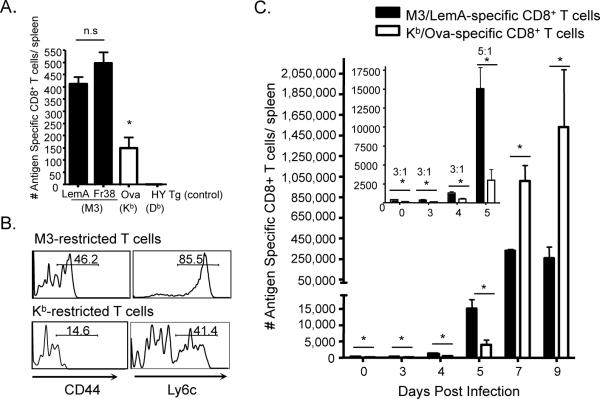
Using peptide-MHC tetramers coupled to two previously identified M3-restricted peptides isolated from Listeria monocytogenes (LM) (LemA and Fraction 38) (4, 27) as well as an MHC dimer bound to a conventional T cell epitope (Kb/Ova), we compared the M3-restricted and conventional Ova-specific T cell precursor populations in naïve wildtype (B6) mice. While we found no significant difference between the number of LemA and Fr38 specific precursors, the M3-restricted CD8+ T cell precursor frequency was approximately 3 times that of the conventional Ova-specific CD8+ T cell population (Figure 1A). In addition to the differences in precursor frequency, M3-restricted CD8+ T cells also exhibited a more activated surface phenotype (CD44hi, Ly6chi) than conventional Ova-specific CD8+ T cells (Figure 1B). This is an important finding since all previous reports on the phenotype of M3-restricted CD8+ T cells have relied on T cell receptor (TCR) Tg mice and as such have been unable to rule out the possibility that the activated phenotype observed was a result of homeostatic proliferation known to occur within TCR Tg mice (28, 29).
Figure 1. Differences between conventional and M3-restricted T cell kinetics reflect differences in naïve T cell precursor frequency and intrinsic T cell phenotype.
Antigen specific precursors from B6 mice were isolated and analyzed via magnetic bead assisted tetramer enrichment. (A) Bars represent the number of LemA (n=10), Fr38 (n=3) (M3-restricted) and Ova (n=3) (Kb-restricted)-specific CD8+ T cell precursors per spleen of naïve B6 mice. (The number of M3/LemA specific T cell precursors/spleen in an HY TCR Tg mouse serves as a negative control). (B) Histograms represent surface activation marker expression of M3/LemA and Kb/Ova specific CD8+ T cells isolated from spleens of B6 mice. (C) Bars represent numbers of M3/LemA and Kb/Ova specific CD8+ T cells isolated from B6 mice at different days post infection with LM-Ova. Inset: Early response (Days 0–5). Numbers indicate the ratio of LemA-specific: Ova-specific CD8+ T cells. Data are representative of at least two independent experiments and bars represent at least 3 mice per group.
Given the apparent differences in precursor frequency and surface phenotype between M3-restricted CD8+ T cells and conventional CD8+ T cells, we infected B6 mice with rLM-Ova and used magnetic bead-assisted tetramer enrichment to follow the expansion of antigen specific CD8+ T cells from as early as 1 day post infection (Figure 1C). No appreciable expansion was detected until 4 days post-infection and initial differences between M3/LemA- and Kb/Ova- specific CD8+ T cell populations reflected the naïve precursor frequency. Interestingly, between day 4 and day 5 post-infection, we observed a sharp expansion in M3-restricted CD8+T cells, but only a very moderate expansion in Kb -restricted CD8+ T cells. Based on this result, it is clear that while differences in precursor frequency certainly contribute to the differences in the magnitudes of the M3- and Kb-restricted CD8+ T cell responses, M3-restricted CD8+T cells also appear to possess an intrinsic ability (most likely reflected by their pre-activated phenotype) to expand faster than conventional CD8+ T cells. It is important to note that although the conventional CD8+ T cell response takes a longer time to expand, it does eventually establish itself as the dominant T cell response (possibly explaining the lack of a significant memory response in the M3-restricted T cell population).
SAP is required for the development of a pre-activated phenotype in M3-restricted CD8+ T cells and functions in an IL-4 independent manner
We have previously demonstrated that unlike iNKT cells, M3-restricted CD8+ T cells can be selected on both TEC and HC and acquire different phenotypes depending on the type of cell responsible for their selection (12). Given that SAP is such an important component of innate T cell development and has been shown to be necessary, and sufficient to mediate selection of iNKT cells and thymocyte-selected innate CD4+ T (T-CD4+) cells on HC (21, 22), we hypothesized that SAP might also play a role in the selection and development of M3-restricted CD8+ T cells. The “dual” selection pathways of M3-restricted T cells provide a unique model to not only study the role of SAP in mediating T cell selection on HC, but the development of innate phenotype as well.
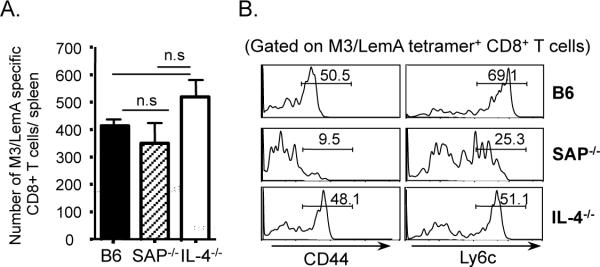
To determine the role of SAP in the development of innate phenotype in M3-restricted CD8+ T cells, we first compared the frequency and phenotype of M3/LemA -specific CD8+ T cells from naive SAP-deficient mice with those isolated from naïve B6 mice. Interestingly, unlike iNKT cells that are absent in SAP-deficient mice (21), the absence of SAP did not affect the overall numbers of M3-restricted CD8+ T cells (Figure 2A). We did however observe a significant difference in the phenotype of these cells. The pre-activated phenotype characteristic of M3-restricted CD8+ T cells was severely decreased in SAP-deficient mice as illustrated by the fact that most of these cells were CD44lo and Ly6clo (Figure 2B). Given that a number of recent studies have demonstrated that the innate phenotype exhibited by MHC Ia-restricted CD8+ T cells found in Kruppel-Like Factor 2 (KLF2)- and Id3-deficient animals is dependent on IL-4 produced by a subset of SAP-dependent PLZF-expressing T cells (30, 31), we also assessed the phenotype of M3-restricted CD8+ T cells in IL-4 deficient mice. Importantly, IL-4 deficiency did not have any effect on the frequency or phenotype of M3-restricted CD8+ T cells, suggesting that SAP is involved directly in the development of M3-restricted CD8+ T cells most likely through mediating selection on HC. Unlike iNKT cells, M3-restricted CD8+ T cells can therefore develop in the absence of SAP; however, the phenotype of the population is significantly altered due to the loss of SAP-dependent pre-activation.
Figure 2. SAP is required for the development of pre-activated phenotype in M3-restricted T cells in an IL-4 independent manner.
M3/LemA specific CD8+ T cells were isolated from the spleens of B6, SAP−/− and IL-4−/− mice via tetramer enrichment and analyzed by FACS. (A) Bars represent the number of M3/LemA specific CD8+ T cells per spleen of naïve B6 (n=11), SAP−/− (n=5) and IL-4−/− (n=3) mice, respectively. (B) Histograms represent surface activation marker expression of M3/LemA specific CD8+ T cells isolated from spleens of indicated mice. Data are representative of at least three independent experiments.
SAP-deficient animals have impaired M3-restricted CD8+ T cell responses to bacterial antigens
Given that M3-restricted CD8+ T cells that develop in a SAP-deficient background exhibit a significantly less activated phenotype than those that develop in B6 mice, we were interested in the effect that the loss of SAP-dependent pre-activation would have on M3-restricted CD8+ T cell responses. We therefore compared the expansion of these cells in B6 and SAP-deficient mice after exposure to bacterial antigens.
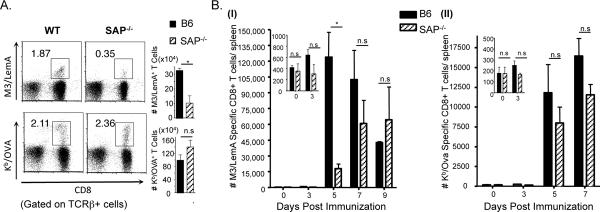
B6 and SAP-deficient mice were infected with rLM-Ova and the number of M3/LemA- and Kb/Ova- specific CD8+ T cells was assessed 7 days post-infection. Interestingly, while there was not a significant difference in the number of Ova-specific CD8+ T cells isolated from the two groups of mice, the M3-restricted CD8+ T cell response was significantly lower in SAP-deficient mice (Figure 3A). In order to control for any differences in antigen presentation between the two groups, we also assessed the M3/LemA and Kb/Ova- specific CD8+ T cell responses in mice immunized with LemA/Ova-pulsed bone marrow-derived dendritic cells (BMDC) (Figure 3B). Once again, there was no difference in the conventional Ova-specific response in B6 and SAP-deficient mice. However, the M3-restricted CD8+ T cell response showed significant impairment in the absence of SAP; at 5 days post-immunization, there were 5 times as many M3/LemA-specific CD8+ T cells in the B6 mice as there were in the SAP-deficient mice. The M3/LemA- specific response in B6 mice peaked around 5 days post-immunization, whereas the response in SAP-deficient animals peaked between 7and 9 days post-immunization and the overall magnitude of the response was lower than in the B6 mice. SAP-deficient M3-restricted CD8+ T cells therefore exhibited both slower kinetics and less robust expansion following bacterial stimulation compared to M3-restricted CD8+ T cells that developed in the presence of SAP. These data suggest therefore that SAP is not only involved in the development of pre-activated phenotype, but may also affect the ability of M3-restricted CD8+ T cells to expand in response to infection.
Figure 3. SAP−/− mice have impaired M3-restricted T cell responses upon exposure to bacterial antigens.
(A) B6 and SAP−/− mice were infected with rLM-Ova. 7 days post-infection splenocytes were isolated from infected mice and the LemA and Ova-specific responses analyzed by flow cytometry. Percentages of M3/LemA- (upper panel) and Kb/Ova- (lower panel) specific T cells within the total T cell population are shown. Bars represent total numbers of antigen specific CD8+ T cells per spleen. (B) B6 and SAP−/− mice were immunized with LPS-matured BMDC pulsed with LemA and Ova peptides. Antigen specific CD8+ T cells were isolated at subsequent days post immunization via tetramer enrichment and analyzed by FACS. Bar graphs represent the mean ± SEM number of (I) M3/LemA and (II) Kb/Ova-specific CD8+ T cells per spleen of mice at different days post immunization. Data are representative of at least two independent experiments and bars represent 3–4 mice per group.
SAP is required for selection of M3 restricted CD8+ T cells on hematopoietic cells
Given the similar phenotypes observed between M3-restricted CD8+ T cells selected exclusively on TEC (D7TEC) (12) and SAP−/− M3-restricted CD8+ T cells as well as the well-documented role of SAP in mediating the selection of iNKT and T-CD4 T cells on HC (21, 22), we hypothesized that SAP might be necessary for the selection of M3-restricted CD8+ T cells on HC. According to this hypothesis, in the absence of SAP, M3-restricted CD8+ T cells would only be selected on TEC, explaining the more naïve phenotypic characteristics exhibited by M3-restricted CD8+ T cells that develop in the absence of SAP.
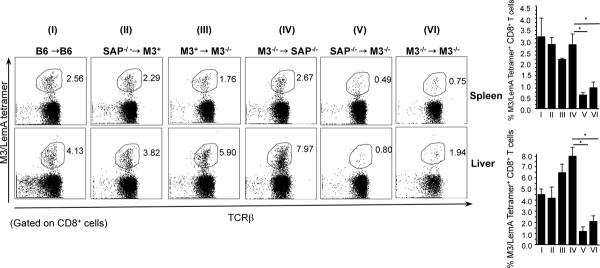
To directly test this hypothesis, we generated chimeric mice by adoptive transfer of bone marrow (BM) from either B6, SAP−/− or M3−/− donors into irradiated B6, M3−/− or SAP−/− recipients. We generated 6 different groups of chimeric mice: (I) B6 →B6 (positive control), (II) SAP−/−→M3+ (SAP−/− BM control), (III) M3+→M3−/− (M3 expression limited to SAP-sufficient HC), (IV) M3−/−→SAP−/− (M3 expression limited to SAP-deficient TEC), (V) SAP−/−→M3−/− (M3 expression limited to SAP-deficient HC) and (VI) M3−/−→M3−/− (negative control). Since significant numbers of LemA-specific M3-restricted CD8+ T cells are only detectable after antigen-induced cellular expansion, 6–7 weeks following adoptive transfer, mice were immunized with LemA-pulsed BMDC to determine the relative abilities of chimeric mice to mount LemA specific immune responses. Six days post-immunization, a significant number of M3/LemA- specific CD8+ T cells were detected in both the spleen and liver of mice in groups I–IV and very few cells detected in the organs of mice in group VI (negative control) (Figure 4). Interestingly, there were also significantly fewer cells found in the SAP−/−→M3−/− chimeric mice (group V). In these mice, M3 expression is limited to SAP−/− HC, and unlike in group III where SAP is expressed in the donor cells, M3-expression on the SAP−/− donor (hematopoietic) cells is unable to rescue the selection of M3-restricted CD8+ T cells. This demonstrates very clearly that SAP is required for the selection of M3-restricted CD8+ T cells on HC. It is also important to note that based on the results from group IV, selection on TEC appears to be SAP-independent, suggesting another aspect of similarity between TEC-selected M3-restricted T cells and conventional CD8+ T cells.
Figure 4. SAP is required for hematopoietic cell-mediated selection of M3-restricted CD8+ T cells.
BM chimeric mice were immunized with LemA-pulsed BMDC. Six days post-immunization, the M3/LemA-specific CD8+ T cell response was analyzed. Percentages of CD8+ M3/LemA- tetramer+ T cells isolated from spleen and liver of immunized chimeric mice are shown. Data represents 3–4 mice per group.
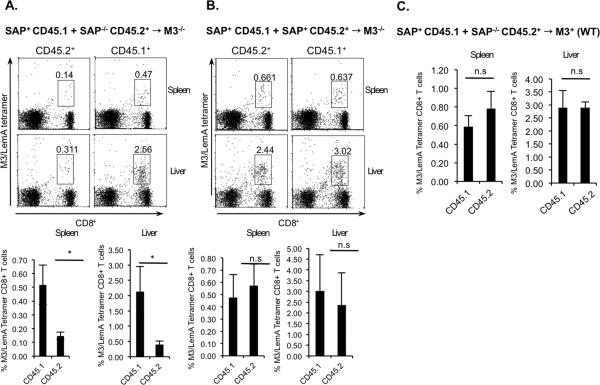
While these results demonstrate the importance of SAP for the selection of M3-restricted CD8+ T cells they do not eliminate the possibility that SAP expression during HC-mediated selection may not be intrinsic to M3-restricted T cells. To determine whether SAP expression on M3-restricted thymocytes is required for HC-mediated selection or if SAP expression on other cell types is sufficient to mediate selection of M3-restricted CD8+ T cells on HC, we generated mixed bone marrow chimeric mice. These mice were generated by the adoptive transfer of a mixture of B6 BM (expressing the CD45.1 congenic marker) with either SAP−/− or SAP+ BM (expressing the CD45.2 marker) into irradiated M3−/− CD45.2 expressing mice. As was done previously, 6–7 weeks following adoptive transfer, mice were immunized with LemA-pulsed BMDC to compare the abilities of the chimeric mice to mount LemA-specific responses. 5 days post-immunization, significant numbers of both CD45.1 and CD45.2-expressing CD8+ T cells were detected in the spleen and liver of mice that received a mixture of SAP+ CD45.1+ and SAP+ CD45.2+ BM, consistent with our earlier results demonstrating that in the presence of SAP, M3-restricted CD8+ T cells can be selected on M3-expressing HC (Figure 5B). Interestingly, while a good number of CD45.1+ M3-restricted CD8+ T cells were detected, very few CD45.2+ M3-restricted CD8+ T cells were detected in chimeric mice that received a mixture of SAP+ CD45.1+ BM and SAP−/− CD45.2+ BM (Figure 5A). This result demonstrates that SAP expression during HC-mediated selection of M3-restricted CD8+ T cells is intrinsic to M3-restricted thymocytes and cannot be compensated for by SAP expressed on other hematopoietic cells. To confirm that the our results did not represent a migration defect, we compared numbers of SAP-sufficient and deficient M3-restricted T cells in the peripheral organs of immunized mixed bone marrow chimeric mice generated by transferring a mixture of CD45.1 SAP+ and CD45.2 SAP−/− BM into irradiated wildtype mice (Figure 5C).
Figure 5. SAP dependence exhibited during HC-mediated selection of M3-restricted CD8+ T cells is intrinsic to M3-restricted thymocytes.
Mixture of BM cells from CD45.1 congenic B6 mice (SAP+CD45.1) with either SAP−/− (SAP−/−CD45.2) or B6 mice (SAP+CD45.2) were transferred into irradiated M3−/− (Figure 5A, B) or wildtype (Figure 5C) recipients. After 6 wk, BM chimeric mice were immunized with LemA-pulsed BMDC. Five days post-immunization, the M3/LemA-specific CD8+ T cells response was analyzed. Percentages of CD8+ M3/LemA-tetramer+ T cells isolated from the spleen and liver of immunized mixed BM chimeric mice are shown. Data represents 5–6 mice per group.
SAP-deficient M3-restricted CD8+ T cells exhibit diminished effector function
In order to further explore the phenotype of SAP-deficient M3-restricted CD8+ T cells and better explain the diminished M3-restricted immune response we observed in SAP-deficient mice, we took advantage of previously generated TCR transgenic (Tg) mice that express a TCR specific for LemA presented by M3 (D7 Tg) (23). By crossing D7 TCR Tg mice onto a SAP-deficient background we were able to compare the phenotypes and effector functions of M3-restricted CD8+ T cells, expressing identical TCR that only differed in whether they developed in the presence or absence of SAP. (To avoid complications with unwanted TCR recombination events, all Tg mice were further crossed onto a Rag-2-deficient background).
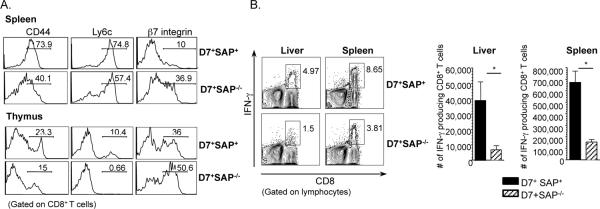
Similar to what we observed with polyclonal populations of M3-restricted CD8+ T cells (Figure 2), D7 CD8+ T cells isolated from both the spleen and thymus of naïve D7 SAP−/− Tg mice were less activated than those that developed in SAP-sufficient D7 Tg mice (Figure 6A). In agreement with our earlier studies (12), overall levels of pre-activation were lower in thymocytes, suggesting that M3-restricted CD8+ T cells acquire a certain baseline level of pre-activation through SAP-dependent interactions in the thymus and undergo further activation in the periphery.
Figure 6. SAP-deficient D7Tg T cells exhibit a less pre-activated surface phenotype and diminished effector function upon antigen stimulation as compared to SAP-sufficient D7 Tg T cells.
(A) Splenocytes from D7+ SAP+/+ and D7+ SAP−/− mice were isolated and stained with antibodies to various cell surface activation markers. Histograms represent surface activation marker expression in each group of mice. (B) Splenocytes and liver leukocytes were also isolated from D7+ SAP+/+ and D7+ SAP−/− mice and stimulated in vitro with LemA. Intracellular IFN-γ production was analyzed. Bars represent the percentage and total number of IFN-γ producing CD8+ T cells isolated each group. Data are representative of at least two independent experiments with 3 mice per group.
In trying to explain the impaired immune responses observed in SAP-deficient M3-restricted CD8+ T cells, we hypothesized that these cells might exhibit weaker effector functions than those selected in the presence of SAP. To test this hypothesis, we took advantage of the large numbers of monoclonal M3/LemA specific CD8+ T cells that could be isolated from D7 Tg mice and compared the relative abilities of D7+SAP+/+ and D7+SAP−/− CD8+ T cells to secrete the pro-inflammatory cytokine IFN-γ following antigenic stimulation. (IFN-γ is the dominant cytokine produced by D7 Tg CD8+ T cells, in addition to lesser amounts of TNF-α and barely detectable levels of IL-17). Splenocytes and liver leukocytes were isolated from each group of mice, stimulated with LemA and the level of intracellular IFN-γ determined by flow cytometry. Consistent with their more activated surface phenotype, D7+SAP+/+ T cells produced significantly more intracellular IFN-γ upon stimulation with LemA as compared to D7+SAP−/− CD8+ T cells (Figure 6B). These results indicate an important role for SAP not only in the development of pre-activated surface phenotype, but also in the enhanced effector functions characteristic of the innate phenotype of M3-restricted CD8+ T cells.
SAP-deficient M3-restricted CD8+ T cells have significantly reduced expression of specific transcription factors linked to the acquisition of innate phenotype
While many of the signaling pathways downstream of SAP have yet to be characterized, members of the T-box family of transcription factors are involved in the terminal differentiation and acquisition of effector function in a number of innate T cell subsets including iNKT cells (32) and M3-restricted CD8+ T cells (12). Given that we have previously demonstrated the preferential up-regulation of T-box family transcription factors in M3-restricted CD8+ T cells selected on HC (as compared to those selected on TEC) (12) and T-bet has been demonstrated to play a role in iNKT cell maturation (32), we were interested in comparing the levels of T-bet expression in M3-restricted CD8+ T cells selected in the presence or absence of SAP.
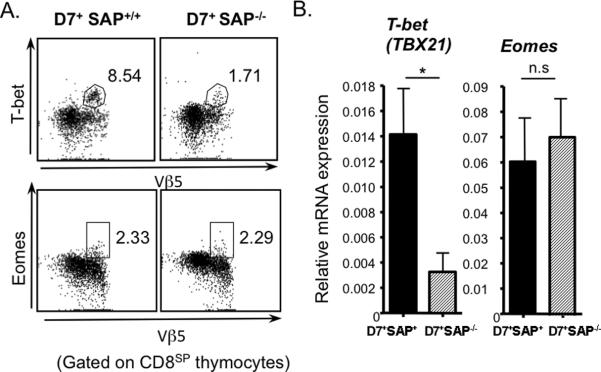
We isolated thymocytes from D7+SAP+/+ and D7+SAP−/− mice and assessed intracellular T-bet and eomesodermin expression in mature CD8+CD4− (CD8SP) thymocytes by flow cytometry (Figure 7A). We also isolated total RNA from HSAhi- depleted CD8SP thymocytes harvested from D7+SAP+/+ and D7+SAP−/− mice and analyzed the relative expression of T-bet and Eomes by real-time RT-PCR (Figure 7B). In keeping with their decreased levels of pre-activation and diminished effector functions, D7+SAP−/− thymocytes expressed significantly lower levels of T-bet than D7+SAP+/+ thymocytes, suggesting a role for this transcription factor in the SAP-mediated development of innate T cell phenotype. Interestingly, despite the fact that we have previously demonstrated that eomesodermin is involved in the development of innate phenotype in M3-restricted T cells selected on HC (12), there was no significant difference in SAP expression between D7+SAP−/− and D7+SAP+/+ thymocytes.
Figure 7. SAP−/− D7 Tg T cells express significantly lower levels of T-bet than SAP+ D7 Tg T cells.
(A) Thymocytes were isolated from D7+SAP+/+ and D7+SAP−/− mice and intracellular levels of T-bet and eomesodermin (Eomes) assessed via flow cytometry. Numbers represent percentage of CD8+ thymocytes that are expressing T-bet (upper panel) or Eomes (lower panel). Data are representative of three independent experiments. (B) Total RNA isolated from purified CD8SP thymocytes from D7+SAP+/+ and D7+SAP−/− mice were subjected to Real-time RT-PCR using primers specific to T-bet or Eomes. Bars represent the mean ± SEM of triplicate determinants of Tbet and Eomes expression normalized to GAPDH expression levels from multiple experiments. Data are representative of two independent experiments.
DISCUSSION
As prevailing knowledge of the innate and adaptive branches of the immune system has grown, the temporal and functional gaps between the cells that make up these two groups have shrunk. Innate T cells have been discovered as an important bridge between innate and adaptive immune responses, and several studies have focused on understanding the mechanisms that regulate the development and function of these cells. One area of particular interest is the relationship between HC-mediated selection and the development of innate T cell phenotype. Although the mechanism of HC-mediated selection is still not fully understood, SAP has been identified as being an important component of this process and a number of studies have described links between SAP and the selection of iNKT cells (33). Though SAP has been shown to play a role in conventional CD8+ T cell cytotoxicity during EBV infection (possibly through the polarization of the lytic machinery) (34), studies of SAP-deficient mice have revealed that conventional T cells develop normally in the absence of SAP and are capable of normal proliferation and cytokine production upon stimulation (34). Contrastingly, SAP is central to the development of iNKT cells (35). In the absence of SAP, iNKT cell precursors are unable to differentiate beyond the double positive stage of thymocyte development, where iNKT cell precursors would normally undergo CD1d-dependent positive selection and diverge from the conventional T cell developmental pathway (35). Interestingly, although iNKT cells are absent in SAP-deficient mice, expression of an invariant Vα14Jα18 TCR transgene in SAP-deficient mice results in the development of a sizeable population of iNKT cells (36). These cells however, failed to produce appreciable levels of IFN-γ and IL-4 as is typically observed in NKT cells (36), suggesting that SAP is not only involved in mediating positive selection, but is also responsible for mediating the acquisition of iNKT cell phenotype and function (37).
Besides iNKT cells, SAP has also been demonstrated to be involved in the selection of other innate T cell populations (21, 22, 38). Unlike iNKT cells however, most of these innate T cell populations have been identified in genetically modified models (including Tec-family kinase deficient Itk−/− as well as DNA binding protein Id3−/− mice) (30, 31, 38, 39), raising questions about their physiological relevance. By taking advantage of the fact that M3- (and possibly other MHC Ib-) restricted CD8+ T cells are selected on both HC and TEC, resulting in two phenotypically distinct populations, we have developed a unique and physiologically relevant model to study the role of SAP not only in the selection of innate T cells, but also in the acquisition of innate T cell phenotypes.
In this study, we demonstrate that SAP is required for HC-mediated selection of M3-restricted CD8+ T cells, since SAP−/−→ M3−/− chimeric mice (in which M3 expression is limited to SAP-deficient HC) are almost completely devoid of M3-restricted CD8+ T cells. Conversely, SAP does not appear to be necessary for TEC-mediated selection of M3-restricted CD8+ T cells since M3−/−→SAP−/− chimeric mice (in which M3 expression is limited to SAP-deficient TEC) exhibited numbers of M3-restricted CD8+ T cells similar to those found in wildtype mice. This result is compatible with previous reports demonstrating that SAP expression is restricted to HC (13–15). We have also been able to demonstrate that SAP is also responsible for the development of the innate phenotype characteristic of these unique CD8+ T cells. By analyzing both polyclonal and monoclonal M3-restricted CD8+ T cell populations that developed in the presence or absence of SAP, we illustrate that unlike iNKT cells, M3-restricted CD8+ T cells do develop in SAP−/− mice, however SAP−/− M3-restricted CD8+ T cells express much lower levels of T-bet and exhibit a severely impaired innate phenotype with diminished pre-activation and less potent effector functions compared to SAP-sufficient M3-restricted CD8+ T cells. Surprisingly, although we have previously demonstrated that the T-box transcription factor eomesodermin (Eomes) is also involved in the development of innate phenotype in M3-restricted T cells selected on HC (12), SAP deficiency did not appear to impact Eomes expression to the same extent as it decreased T-bet expression, suggesting that other SAP-independent signals derived from HC may be involved in the regulation of Eomes expression.
The kinetics of M3-restricted CD8+ T cell responses are also of significant interest, since the ability of these cells to expand faster than conventional CD8+ T cells is crucial to their ability to effectively bridge innate and adaptive immune responses. Based on our results, the differences between M3- and Kb-restricted CD8+ T cell responses reflect both differences in naïve precursor frequency as well as differences in intrinsic activation phenotype. The difference in magnitude between M3- and Kb-restricted responses at early time points post-infection appears to be a direct result of the larger number of M3-restricted naïve precursors, however the rate of T cell expansion is most likely due to the SAP-dependent differences in intrinsic T cell phenotype.
The results of this study suggest that while M3-restricted CD8+ T cells are normally selected by both TEC and HC, in the absence of SAP, M3-restricted CD8+ T cells are almost exclusively selected on TEC. As a result these M3-restricted CD8+ T cells develop a more “conventional” T cell phenotype. In this model it is reasonable to think of SLAM receptors as co-stimulatory receptors, providing the requisite stimulation to promote T cell selection in the absence of the more specialized receptors found on TEC (40). This role of SLAM as a co-stimulatory receptor is not entirely novel and agrees with previous studies demonstrating a role for SLAM in the regulation of T cell activation (41, 42). It is however interesting to note that, unlike some SAP-dependent T cell subsets (30, 31), SAP appears to be acting directly on M3-restricted CD8+ T cells. The fact that the phenotype of T cells from D7 Tg mice (on a Rag-2 deficient background) have a SAP-dependent innate phenotype (Figure 6), coupled with the fact that IL-4 deficiency has no effect on the phenotype of M3-restricted CD8+ T cells (Figure 2A) strongly suggests that SAP is acting directly on M3-restricted CD8+ T cells. The most significant support for this conclusion however, is provided by the fact that two subsets of M3-restricted thymocytes differing only in their expression of SAP, show significant differences in their capacity for HC-mediated selection when developed in the same microenvironment (Figure 5A, B). In presenting this data, we acknowledge that our results do not eliminate the possibility that SAP deficiency might affect the migration of M3-restricted CD8+ T cells to the periphery. However, considering that we have previously demonstrated that the relative number and phenotype of HC and TEC- selected M3-restricted CD8+ T cells isolated from peripheral organs are closely analogous to the relative number and phenotype of cells isolated from the thymus of an M3/LemA transgenic mouse (12), as well as the fact that we have shown that M3-restricted CD8+ T cells isolated from the spleens of SAP+ or SAP−/− M3-restricted transgenic mice reflect the phenotypes of cells isolated from the thymus, it is reasonable to conclude that numeric and phenotypic differences observed in the periphery are most likely a reflection of differences in thymic selection and not due to differences in migration. Further evidence against a migration defect is provided by the fact that there are comparable numbers of SAP-sufficient and deficient M3-restricted CD8+ T cells in the peripheral organs of immunized mixed bone marrow chimeric mice generated by transferring a mixture of CD45.1 SAP+ and CD45.2 SAP−/− BM into irradiated wildtype mice (Figure 5C).
Interestingly, although the requirement for SAP is not unique to M3-restricted CD8+ T cells and is shared by other innate T cell subtypes, the signaling and transcriptional regulation pathways downstream of SAP are not identical between different cell types. While it is well established that development of iNKT cells is dependent on the up-regulation of the transcription factor PLZF (44), we have been unable to detect any PLZF expression in M3-restricted CD8+ T cells (12). Furthermore, despite the fact that the innate phenotype of CD8+ T cells isolated from KLF2- and Id3- deficient animals has been shown to be regulated by IL-4 produced by a subset of PLZF+ T cells (30, 31), a lack of IL-4 had no discernible effect on the phenotype of M3-restricted CD8+ T cells. This would argue that M3-restricted CD8+ T cells are not dependent on PLZF+ cells. Similarly, while we demonstrated that T-bet is up-regulated in a SAP-dependent fashion (most likely during selection on HC) in M3-restricted CD8+ T cells, T-bet plays a minimal role in T-CD4+ T cell ontology (22). These results would appear to indicate that while SAP-dependence is conserved among most innate T cell types, SAP is capable of signaling through multiple pathways, enabling different innate T cell populations to occupy different functional niches.
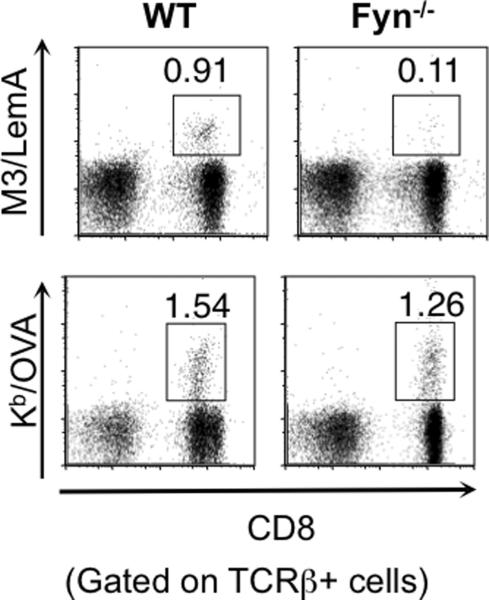
While SAP expression has been linked to a number of functional outcomes in several different cell types, the signaling intermediates downstream of SAP that mediate these outcomes remain largely unknown. One signaling intermediate that has been found to interact directly with SAP is the Src tyrosine kinase Fyn. Fyn has been shown to be important for NKT cell development (45) and maturation (35, 46). Interestingly, we found that similar to SAP−/− mice, Fyn−/− mice also exhibited impaired M3-restricted CD8+ T cell responses to bacterial infection (Figure 8), suggesting that the SAP-Fyn interaction may be needed for the proper function of M3-restricted CD8+ T cells. Another signaling molecule found to interact with SAP is protein kinase C-theta (PKC-θ) (24, 47), which is subsequently phosphorylated, leading to the activation of NF-κB, NFAT and AP-1 (48). While PKC-θ has been demonstrated to be critical in the development of functional iNKT cells and also plays an essential role in T cell activation and survival (49, 50), future studies will be needed to assess the nature of this interaction, and determine whether PKC-θ is necessary and/ or sufficient for the establishment of innate phenotype in M3-restricted CD8+ T cells.
Figure 8. Fyn−/− mice have impaired M3-restricted CD8+ T cell responses to LM infection.
B6 and Fyn−/− mice were infected with rLM-Ova. 7 days post-infection splenocytes were isolated from infected mice and the LemA and Ova-specific responses analyzed by flow cytometry. Percentages of M3/LemA- (upper panel) and Kb/Ova- (lower panel) specific T cells within the total T cell population are shown. Data are representative of three independent experiments with two mice per genotype.
Studies of SAP-deficient humans and mice have underscored the immense importance of SAP-mediated signaling in the development of certain immune cells and the critical functions performed by these different cell types. Innate T cells form a large proportion of these SAP-dependent immune cells and the ability of these cells to bridge innate and adaptive immunity makes them very attractive targets for a number of immune interventions. Up to this point however, the only naturally occurring innate T cells that have been demonstrated to have a dependence on SAP are iNKT cells. The absence of these cells in SAP-deficient mice has to this point limited the study of how SAP mediates the development of innate T cell phenotype. Our results demonstrating that SAP-dependent selection on HC is crucial to the development of innate phenotype in M3-restricted CD8+ T cells not only provides further evidence for the physiological relevance of SAP-dependent T cell populations, but also provides a more suitable model in which to study the mechanism by which T cells acquire innate phenotypes. By comparing M3-restricted T cells selected by SAP-dependent and independent pathways it will be possible to begin to further characterize the mechanism by which SAP mediates the development of innate T cell phenotype. A better understanding of innate T cell development has the potential to not only enable the development of new therapeutic interventions that more efficiently harness the unique abilities of these T cells, but may also allow for more extensive characterization of MHC class Ib-restricted CD8+T cells. While we acknowledge that M3-restricted CD8+ T cells may not be reflective of all MHC Ib-restricted CD8+ T cells, the majority of residual CD8+ T cells in MHC Ia−/−M3−/− mice appear to also possess innate phenotype (51), suggesting that SAP may be central to our understanding of MHC class Ib-restricted CD8+ innate T cell development and function.
ACKNOWLEDGEMENTS
We thank the National Institutes of Health tetramer core facility for M3/LemA and M3/Fr38 tetramers; Sharmila Shanmuganad, Rostyslav Kupriyenko, Kunik Lee and Brittany Zhang for technical assistance; Northwestern Flow Cytometry Core Facility for cell sorting service; and Dr. L. Lefrancois for providing rLM-Ova.
Abbreviations used in this paper:
- BMDC
(bone marrow-derived dendritic cell)
- (B6)
C57BL/6
- (HKLM)
Heat-killed Listeria monocytogenes
- (iNKT cell)
Invariant NKT cell
- (LM)
Listeria monocytogenes
- PLZF
promyelocytic zinc finger
- SLAM
signaling lymphocytic activation molecule
- SAP
SLAM-associated protein
- Id3
inhibitor of DNA-binding factor 3
- KLF 2
Kruppel-like factor 2
- TEC
thymic epithelial cells
- HC
hematopoietic cells
Footnotes
This work was supported by National Institute of Health grants AI40310 and AI57460 (to C.-R.W.).
REFERENCES
- 1.Rodgers JR, Cook RG. MHC class Ib molecules bridge innate and acquired immunity. Nat. Rev. Immunol. 2005;5:459–471. doi: 10.1038/nri1635. [DOI] [PubMed] [Google Scholar]
- 2.Bendelac A, Rivera MN, Park SH, Roark JH. Mouse CD1-specific NK1+ T cells: development, specificity, and function. Annu. Rev. Immunol. 1997;15:535–562. doi: 10.1146/annurev.immunol.15.1.535. [DOI] [PubMed] [Google Scholar]
- 3.Chen YH, Chiu NM, Mandal M, Wang N, Wang CR. Impaired NK1+ T cell development and early IL-4 production in CD1-deficient mice. Immunity. 1997;6:459–467. doi: 10.1016/s1074-7613(00)80289-7. [DOI] [PubMed] [Google Scholar]
- 4.Pamer EG, Wang CR, Flaherty L, Lindahl KF, Bevan MJ. H2-M3 presents a Listeria monocytogenes peptide to cytotoxic T lymphocytes. Cell. 1992;70:215–223. doi: 10.1016/0092-8674(92)90097-v. [DOI] [PubMed] [Google Scholar]
- 5.Loveland B, Wang CR, Yonekawa H, Hermel E, Lindahl KF. Maternally transmitted histocompatibility antigen of mice: a hydrophobic peptide of a mitochondrially encoded protein. Cell. 1990;60:971–980. doi: 10.1016/0092-8674(90)90345-f. [DOI] [PubMed] [Google Scholar]
- 6.Seaman MS, Wang CR, Forman J. MHC class Ib-restricted CTL provide protection against primary and secondary Listeria monocytogenes infection. J. Immunol. 2000;165:5192–5201. doi: 10.4049/jimmunol.165.9.5192. [DOI] [PubMed] [Google Scholar]
- 7.Das G, Sheridan S, Janeway CA., Jr. The source of early IFN-γ that plays a role in Th1 priming. J. Immunol. 2001;167:2004–2010. doi: 10.4049/jimmunol.167.4.2004. [DOI] [PubMed] [Google Scholar]
- 8.Ploss A, Leiner I, Pamer EG. Distinct regulation of H2-M3-restricted memory T cell responses in lymph node and spleen. J. Immunol. 2005;175:5998–6005. doi: 10.4049/jimmunol.175.9.5998. [DOI] [PubMed] [Google Scholar]
- 9.Xu H, Chun T, Choi HJ, Wang B, Wang CR. Impaired response to Listeria in H2-M3-deficient mice reveals a nonredundant role of MHC class Ib-specific T cells in host defense. J. Exp. Med. 2006;203:449–459. doi: 10.1084/jem.20051866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anderson G, Owen JJ, Moore NC, Jenkinson EJ. Thymic epithelial cells provide unique signals for positive selection of CD4+CD8+ thymocytes in vitro. J. Exp. Med. 1994;179:2027–2031. doi: 10.1084/jem.179.6.2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bendelac A. Positive selection of mouse NK1+ T cells by CD1-expressing cortical thymocytes. J. Exp. Med. 1995;182:2091–2096. doi: 10.1084/jem.182.6.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cho H, Bediako Y, Xu H, Choi HJ, Wang CR. Positive selecting cell type determines the phenotype of MHC class Ib-restricted CD8+ T cells. Proc. Natl. Acad. Sci. USA. 2011;32:13241–246. doi: 10.1073/pnas.1105118108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Veillette A, Dong Z, Latour S. Consequence of the SLAM-SAP signaling pathway in innate-like and conventional lymphocytes. Immunity. 2007;27:698–710. doi: 10.1016/j.immuni.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 14.Ma CS, Nichols KE, Tangye SG. Regulation of cellular and humoral immune responses by the SLAM and SAP families of molecules. Annu. Rev. Immunol. 2007;25:337–379. doi: 10.1146/annurev.immunol.25.022106.141651. [DOI] [PubMed] [Google Scholar]
- 15.Engel P, Eck MJ, Terhorst C. The SAP and SLAM families in immune responses and X-linked lymphoproliferative disease. Nat. Rev. Immunol. 2003;3:813–821. doi: 10.1038/nri1202. [DOI] [PubMed] [Google Scholar]
- 16.Latour S, Gish G, Helgason CD, Humphries RK, Pawson T, Veillette A. Regulation of SLAM-mediated signal transduction by SAP, the X-linked lymphoproliferative gene product. Nat. Immunol. 2001;2:681–690. doi: 10.1038/90615. [DOI] [PubMed] [Google Scholar]
- 17.Latour S, Roncagalli R, Chen R, Bakinowski M, Shi X, Schwartzberg PL, Davidson D, Veillette A. Binding of SAP SH2 domain to FynT SH3 domain reveals a novel mechanism of receptor signalling in immune regulation. Nat. Cell Biol. 2003;5:149–154. doi: 10.1038/ncb919. [DOI] [PubMed] [Google Scholar]
- 18.Chan B, Lanyi A, Song HK, Griesbach J, Simarro-Grande M, Poy F, Howie D, Sumegi J, Terhorst C, Eck MJ. SAP couples Fyn to SLAM immune receptors. Nat. Cell Biol. 2003;5:155–160. doi: 10.1038/ncb920. [DOI] [PubMed] [Google Scholar]
- 19.Coffey AJ, Brooksbank RA, Brandau O, Oohashi T, Howell GR, Bye JM, Cahn AP, Durham J, Heath P, Wray P, Pavitt R, Wilkinson J, Leversha M, Huckle E, Shaw-Smith CJ, Dunham A, Rhodes S, Schuster V, Porta G, Yin L, Serafini P, Sylla B, Zollo M, Franco B, Bolino A, Seri M, Lanyi A, Davis JR, Webster D, Harris A, Lenoir G, de St Basile G, Jones A, Behloradsky BH, Achatz H, Murken J, Fassler R, Sumegi J, Romeo G, Vaudin M, Ross MT, Meindl A, Bentley DR. Host response to EBV infection in X-linked lymphoproliferative disease results from mutations in an SH2-domain encoding gene. Nat. Genet. 1998;20:129–135. doi: 10.1038/2424. [DOI] [PubMed] [Google Scholar]
- 20.Nichols KE, Harkin DP, Levitz S, Krainer M, Kolquist KA, Genovese C, Bernard A, Ferguson M, Zuo L, Snyder E, Buckler AJ, Wise C, Ashley J, Lovett M, Valentine MB, Look AT, Gerald W, Housman DE, Haber DA. Inactivating mutations in an SH2 domain-encoding gene in X-linked lymphoproliferative syndrome. Proc. Natl. Acad. Sci. USA. 1998;95:13765–13770. doi: 10.1073/pnas.95.23.13765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nichols KE, Hom J, Gong SY, Ganguly A, Ma CS, Cannons JL, Tangye SG, Schwartzberg PL, Koretzky GA, Stein PL. Regulation of NK T cell development by SAP, the protein defective in XLP. Nat. Med. 2005;11:340–345. doi: 10.1038/nm1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li W, Sofi MH, Rietdijk S, Wang N, Terhorst C, Chang CH. The SLAM-associated protein signaling pathway is required for development of CD4+ T cells selected by homotypic thymocyte interaction. Immunity. 2007;27:763–774. doi: 10.1016/j.immuni.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chiu NM, Wang B, Kerksiek KM, Kurlander R, Pamer EG, Wang CR. The selection of M3-restricted T cells is dependent on M3 expression and presentation of N-formylated peptides in the thymus. J. Exp. Med. 1999;190:1869–1878. doi: 10.1084/jem.190.12.1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cannons JL, Yu LJ, Hill B, Mijares LA, Dombroski D, Nichols KE, Antonellis A, Koretzky GA, Gardner K, Schwartzberg PL. SAP regulates TH2 differentiation and PKC-θ -mediated activation of NF-κB1. Immunity. 2004;21:693–706. doi: 10.1016/j.immuni.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 25.Moon JJ, Chu HH, Pepper M, McSorley SJ, Jameson SC, Kedl RM, Jenkins MK. Naive CD4+ T cell frequency varies for different epitopes and predicts repertoire diversity and response magnitude. Immunity. 2007;27:203–213. doi: 10.1016/j.immuni.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Obar JJ, Khanna KM, Lefrancois L. Endogenous naive CD8+ T cell precursor frequency regulates primary and memory responses to infection. Immunity. 2008;28:859–869. doi: 10.1016/j.immuni.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lenz LL, Dere B, Bevan MJ. Identification of an H2-M3-restricted Listeria epitope: implications for antigen presentation by M3. Immunity. 1996;5:63–72. doi: 10.1016/s1074-7613(00)80310-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hamilton SE, Jameson SC. The nature of the lymphopenic environment dictates protective function of homeostatic-memory CD8+ T cells. Proc. Natl. Acad. Sci. USA. 2008;105:18484–18489. doi: 10.1073/pnas.0806487105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haluszczak C, Akue AD, Hamilton SE, Johnson LD, Pujanauski L, Teodorovic L, Jameson SC, Kedl RM. The antigen-specific CD8+ T cell repertoire in unimmunized mice includes memory phenotype cells bearing markers of homeostatic expansion. J. Exp. Med. 2009;206:435–448. doi: 10.1084/jem.20081829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weinreich MA, Odumade OA, Jameson SC, Hogquist KA. T cells expressing the transcription factor PLZF regulate the development of memory-like CD8+ T cells. Nat. Immunol. 2010;11:709–716. doi: 10.1038/ni.1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Verykokakis M, Boos MD, Bendelac A, Kee BL. SAP protein-dependent natural killer T-like cells regulate the development of CD8+ T cells with innate lymphocyte characteristics. Immunity. 2010;33:203–215. doi: 10.1016/j.immuni.2010.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Townsend MJ, Weinmann AS, Matsuda JL, Salomon R, Farnham PJ, Biron CA, Gapin L, Glimcher LH. T-bet regulates the terminal maturation and homeostasis of NK and Vα14i NKT cells. Immunity. 2004;20:477–494. doi: 10.1016/s1074-7613(04)00076-7. [DOI] [PubMed] [Google Scholar]
- 33.Pasquier B, Yin L, Fondaneche MC, Relouzat F, Bloch-Queyrat C, Lambert N, Fischer A, de Saint-Basile G, Latour S. Defective NK T cell development in mice and humans lacking the adapter SAP, the X-linked lymphoproliferative syndrome gene product. J. Exp. Med. 2005;201:695–701. doi: 10.1084/jem.20042432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dupre L, Andolfi G, Tangye SG, Clementi R, Locatelli F, Arico M, Aiuti A, Roncarolo MG. SAP controls the cytolytic activity of CD8+ T cells against EBV-infected cells. Blood. 2005;105:4383–4389. doi: 10.1182/blood-2004-08-3269. [DOI] [PubMed] [Google Scholar]
- 35.Nunez-Cruz S, Yeo WC, Rothman J, Ojha P, Bassiri H, Juntilla M, Davidson D, Veillette A, Koretzky GA, Nichols KE. Differential requirement for the SAP-Fyn interaction during NK T cell development and function. J. Immunol. 2008;181:2311–2320. doi: 10.4049/jimmunol.181.4.2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cen O, Ueda A, Guzman L, Jain J, Bassiri H, Nichols KE, Stein PL. The adaptor molecule signaling lymphocytic activation molecule-associated protein (SAP) regulates IFN-gamma and IL-4 production in Vα14 transgenic NK T cells via effects on GATA-3 and T-bet expression. J. Immunol. 2009;182:1370–1378. doi: 10.4049/jimmunol.182.3.1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Godfrey DI, Berzins SP. Control points in NK T cell development. Nat. Rev. Immunol. 2007;7:505–518. doi: 10.1038/nri2116. [DOI] [PubMed] [Google Scholar]
- 38.Horai R, Mueller KL, Handon RA, Cannons JL, Anderson SM, Kirby MR, Schwartzberg PL. Requirements for selection of conventional and innate T lymphocyte lineages. Immunity. 2007;27:775–785. doi: 10.1016/j.immuni.2007.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Broussard C, Fleischacker C, Horai R, Chetana M, Venegas AM, Sharp LL, Hedrick SM, Fowlkes BJ, Schwartzberg PL. Altered development of CD8+ T cell lineages in mice deficient for the Tec kinases Itk and Rlk. Immunity. 2006;25:93–104. doi: 10.1016/j.immuni.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 40.Fink PJ, Bevan MJ. Positive selection of thymocytes. Adv. Immunol. 1995;59:99–133. doi: 10.1016/s0065-2776(08)60630-6. [DOI] [PubMed] [Google Scholar]
- 41.Cocks BG, Chang CC, Carballido JM, Yssel H, de Vries JE, Aversa G. A novel receptor involved in T cell activation. Nature. 1995;376:260–263. doi: 10.1038/376260a0. [DOI] [PubMed] [Google Scholar]
- 42.Mehrle S, Frank S, Schmidt J, Schmidt-Wolf IG, Marten A. SAP and SLAM expression in anti-CD3 activated lymphocytes correlates with cytotoxic activity. Immunol. Cell Biol. 2005;83:33–39. doi: 10.1111/j.1440-1711.2004.01302.x. [DOI] [PubMed] [Google Scholar]
- 43.Savage AK, Constantinides MG, Han J, Picard D, Martin E, Li B, Lantz O, Bendelac A. The transcription factor PLZF directs the effector program of the NK T cell lineage. Immunity. 2008;29:391–403. doi: 10.1016/j.immuni.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Eberl G, Lowin-Kropf B, MacDonald HR. Cutting edge: NK T cell development is selectively impaired in Fyn- deficient mice. J. Immunol. 1999;163:4091–4094. [PubMed] [Google Scholar]
- 45.Borowski C, Bendelac A. Signaling for NK T cell development: the SAP-FynT connection. J. Exp. Med. 2005;201:833–836. doi: 10.1084/jem.20050339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cannons JL, Wu JZ, Gomez-Rodriguez J, Zhang J, Dong B, Liu Y, Shaw S, Siminovitch KA, Schwartzberg PL. Biochemical and genetic evidence for a SAP-PKC-theta interaction contributing to IL-4 regulation. J. Immunol. 2010;185:2819–2827. doi: 10.4049/jimmunol.0902182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Arendt CW, Albrecht B, Soos TJ, Littman DR. PKC-θ: signaling from the center of the T-cell synapse. Curr. Opin. Immunol. 2002;14:323–330. doi: 10.1016/s0952-7915(02)00346-1. [DOI] [PubMed] [Google Scholar]
- 48.Coudronniere N, Villalba M, Englund N, Altman A. NF-kappa B activation induced by T cell receptor/CD28 costimulation is mediated by PKC-θ. Proc. Natl. Acad. Sci. USA. 2000;97:3394–3399. doi: 10.1073/pnas.060028097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Manicassamy S, Gupta S, Huang Z, Sun Z. P PKC-θ -mediated signals enhance CD4+ T cell survival by up-regulating Bcl-xL. J. Immunol. 2006;176:6709–6716. doi: 10.4049/jimmunol.176.11.6709. [DOI] [PubMed] [Google Scholar]
- 50.Cho H, Choi HJ, Xu H, Felio K, Wang CR. Nonconventional CD8+ T cell responses to Listeria infection in mice lacking MHC class Ia and H2-M3. J. Immunol. 2011;186:489–498. doi: 10.4049/jimmunol.1002639. [DOI] [PMC free article] [PubMed] [Google Scholar]