Abstract
Neurons of the ventral tegmental area (VTA) are critical in the rewarding and reinforcing properties of drugs of abuse. Desensitization of VTA neurons to moderate extracellular concentrations of dopamine (DA) is dependent on protein kinase C (PKC) and intracellular calcium levels. This desensitization is called DA inhibition reversal (DIR), as it requires concurrent activation of D2 and D1-like receptors; activation of D2 receptors alone does not result in desensitization. Activation of other G-protein linked receptors can substitute for D1 activation. Like D2 receptors, GABAB receptors in the VTA are coupled to G-protein-linked potassium channels. In the present study, we examined interactions between a GABAB agonist, baclofen, and dopamine agonists, dopamine and quinpirole, to determine whether there was some interaction in the processes of desensitization of GABAB and D2 responses. Long-duration administration of baclofen alone produced reversal of the baclofen-induced inhibition indicative of desensitization, and this desensitization persisted for at least 60 min after baclofen washout. Desensitization to baclofen was dependent on protein kinase C. Dopamine inhibition was reduced for 30 min after baclofen-induced desensitization and conversely, the magnitude of baclofen inhibition was reduced for 30 min by long-duration application of dopamine, but not quinpirole. These results indicate that D2 and GABAB receptors share some protein kinase C-dependent mechanisms of receptor desensitization.
Keywords: Desensitization, quinpirole, baclofen, Gö6976, protein kinase C
Introduction
Ventral tegmental area (VTA) neurons are involved in mediating the rewarding and reinforcing effects of environmental stimuli including food, sex and drugs of abuse (Di Chiara and Imperato, 1988; Wise, 1996; Mirenowicz and Schultz, 1996). Dopaminergic (DAergic) VTA neurons fire action potentials spontaneously in vivo (Bunney, et al., 1973) and in vitro (Brodie and Dunwiddie, 1987). In addition, the firing activity of DAergic neurons of the VTA is subject to regulation by a number of neurotransmitters released by intrinsic and projection neurons. In addition to auto-regulation by the release of dopamine from neurons within the VTA (Ackerman, et al., 1993), DAergic neurons receive innervations from both local GABA-containing neurons (Steffensen, et al., 1998) and GABA-containing projection neurons from areas such as the nucleus accumbens (Kalivas, et al., 1993). DAergic VTA neurons also receive other neurotransmitter inputs, including glutamate, serotonin, and peptides such as neurotensin, and corticotrophin releasing factor (Kalivas, 1993; Tagliaferro and Morales, 2008).
There has been controversy about the exact electrophysiological profile of dopamine-containing neurons of the VTA (DA VTA neurons) (Margolis, et al., 2006; Chieng, et al., 2011), in general, dopamine-containing mesolimbic neurons are consistently inhibited by dopamine acting on D2 autoreceptors, an observation that has been reported by many laboratories (Brodie and Dunwiddie, 1990; Lacey, et al., 1987). Histochemical studies have demonstrated the presence of D2 receptors on DA VTA neurons (Bouthenet, et al., 1991); D1-like receptors (D1 and D5 receptors) also have been identified in the VTA. The DAergic neurons of the VTA possess high densities of D5 receptors (Ciliax, et al., 2000; Khan, et al., 2000), and D1 receptors are located presynaptically to DA VTA neurons on glutamate terminals (Caille, et al., 1996). We recently demonstrated that prolonged elevation of dopamine results in a time- and concentration-dependent decrease in the magnitude of dopamine-induced inhibition called “dopamine inhibition reversal” or DIR (Nimitvilai and Brodie, 2010). DIR is produced by concurrent stimulation of D2 and D1-like receptors, develops over 10–40 min, and persists for up to 90 min (Nimitvilai and Brodie, 2010). DIR is mediated by activation of phospholipase C (PLC) and conventional protein kinase C (cPKC), without an involvement of adenylyl cyclase, cyclic AMP and protein kinase A (Nimitvilai, et al., 2012a). Reversal of inhibition produced by D2 agonist quinpirole is induced only with stimulation of D1-like receptors, or with concurrent stimulation of some other receptors linked to the G-protein Gq, like neurotensin (Nimitvilai, et al., 2012b).
As D2 receptors are linked to activation of G-protein-linked potassium channels, we assessed whether activation of another receptor linked to these channels, namely GABAB receptors, would show characteristics similar to DIR. In addition, we assessed whether there was an interaction between D2 and GABAB receptors in this phenomenon.
Experimental Procedures
Animals
Fischer 344 (F344; adult rats, 4–6 weeks old, 90 – 150 g) used in these studies were obtained from Harlan Sprague-Dawley (Indianapolis, IN). All rats were treated in strict accordance with the NIH Guide for the Care and Use of Laboratory Animals and all experimental methods were approved by the Animal Care Committee of the University of Illinois at Chicago.
Preparation of brain slices
Brain slices containing the ventral tegmental area (VTA) were prepared from the subject animals as previously described (Brodie, et al., 1999). Briefly, following brief isoflurane anesthesia and rapid removal of the brain, the tissue was blocked coronally to contain the VTA and substantia nigra; the cerebral cortices and a portion of the dorsal mesencephalon were removed. The tissue block was mounted in the vibratome and submerged in chilled cutting solution to cut coronal sections (400 μm thick). Each slice was placed onto a mesh platform in the recording chamber and was totally submerged in aCSF maintained at a flow rate of 2 ml/min; the temperature in the recording chamber was kept at 35° C. The composition of the aCSF in these experiments was (in mM): NaCl 126, KCl 2.5, NaH2PO4 1.24, CaCl2 2.4, MgSO4 1.3, NaHCO3 26, glucose 11. The composition of the cutting solution was (in mM): KCl 2.5, CaCl2 2.4, MgSO4 1.3, NaHCO3 26, glucose 11, and sucrose 220. Both solutions were saturated with 95% O2/ 5% CO2 (pH=7.4). Equilibration time of at least one hour was allowed after placement of tissue in the recording chamber before electrodes were placed in the tissue.
Cell identification
The VTA was clearly visible in the fresh tissue as a grey area medial to the darker substantia nigra, and separated from the nigra by white matter. Recording electrodes were placed in the VTA under visual control. Putative dopaminergic (pDAergic) neurons have been shown to have distinctive electrophysiological characteristics (Grace and Bunney, 1984; Lacey, et al., 1989). Only those neurons that were anatomically located within the VTA and that conformed to the criteria for pDAergic neurons established in the literature and in this laboratory (Lacey, et al., 1989; Mueller and Brodie, 1989; Chieng, et al., 2011) were studied. These criteria include broad action potentials (2.5 msec or greater, measured as the duration of the bi- or tri-phasic waveform at the baseline), slow spontaneous firing rate (0.5 – 5 Hz), and a regular interspike interval. Cells were not tested with opiate agonists as has been done by other groups to further characterize and categorize VTA neurons (Margolis, et al., 2006; Chieng, et al., 2011). A recent study indicates that the majority of neurons with the electrophysiological characteristics associated with DA VTA neurons are, in fact, DA containing (Chieng, et al., 2011).
Additional characterization, such as determining the projection target of our cells of study (Margolis, et al., 2008) would have been difficult as we have used extracellular recording to insure high quality, long duration recordings. The long-duration, low frequency action potentials which characterized the cells from which we recorded are associated with DA-sensitive, DA-containing neurons projecting to the nucleus accumbens, and DA sensitivity also is associated with DA VTA neurons projecting to prefrontal cortex (Margolis, et al., 2008). One consequence of differential initial sensitivity to dopamine inhibition among groups of neurons projecting to different brain areas (Margolis, et al., 2008; Lammel, et al., 2008) would be different amounts of dopamine inhibition reversal (Nimitvilai and Brodie, 2010), resulting in a greater relative change in neurons more sensitive to dopamine inhibition.
Drug Administration
In most experiments, drugs were added to the aCSF by means of a calibrated infusion pump from stock solutions 100 to 1000 times the desired final concentrations. The addition of drug solutions to the aCSF was performed in such a way as to permit the drug solution to mix completely with aCSF before this mixture reached the recording chamber. Final concentrations were calculated from aCSF flow rate, pump infusion rate and concentration of drug stock solution. The small volume chamber (about 300 μl) used in these studies permitted the rapid application and washout of drug solutions. Typically drugs reach equilibrium in the tissue after 2 to 3 minutes of application.
In some experiments, drugs were added to the microelectrode filling solution (0.9% NaCl) at a concentration about 10 times greater than that which would have been used in the extracellular medium. To allow time for the drug to diffuse from the pipette to the cell, the effects of bath-applied drugs were tested no less than 20 min after initiating the recording; this pipette-application method has produced comparable results to the administration of drugs through the extracellular medium in the cases in which both methods were tested (data not shown), with the advantage of more localized application and reduced expense. For the present study, long recording times were needed, and the presence of the antagonist throughout the experiment was required; as such, micorpressure ejection of antagonist was not appropriate for this study. Such local delivery of drugs through recording pipettes has been used by our lab and others (Sokolov and Kleschevnikov, 1995; Pesavento, et al., 2000; Nimitvilai, et al., 2012a). One disadvantage of this method is that the exact concentration of drug received by the neurons from which we recorded is unknown.
Quinpirole, dopamine, baclofen, and most of the salts used to prepare the extracellular media were purchased from Sigma (St. Louis, MO). Gö6976 (5,6,7,13-tetrahydro-13-methyl-5-oxo-12H-indolo[2,3-a]pyrrolo[3,4c]carbazole-12-propanenitrile), CGP35348 ((3-aminopropyl)(diethoxymethyl)phosphinic acid)), and muscimol (5-aminomethyl-3-hydroxylsoxazole) were purchased from Tocris (Ellisville, MO).
Extracellular recording
Extracellular recording was chosen for these studies as this method permits the recordings to be of long duration and allows us to assess the effects of extended exposure (>60 minutes) to drugs. The limitation of only measuring spontaneous action potential frequency (rather than membrane potential or other electrophysiological parameters) is counterbalanced by the advantage of being able to determine the time course of drug actions and interactions. Extracellular recording electrodes were made from 1.5 mm diameter glass tubing with filament and were filled with 0.9% NaCl. Tip resistance of the microelectrodes ranged from 2 – 5 MΩ A Fintronics amplifier was used in conjunction with an IBM-PC-based data acquisition system (ADInstruments Inc., Colorado Springs, CO). Offline analysis was used to calculate, display and store the frequency of firing in 1-minute intervals. Additional software was used to calculate the firing rate over 5-second intervals. Firing rate was determined before and during drug application. Firing rate was calculated over 1-minute intervals prior to administration of drugs and during the drug effect; peak drug-induced changes in firing rate were expressed as the percentage change from the control firing rate, which controls for small changes in firing rate that may occur over time. Peak excitation produced by the drug (e.g., DA) was defined as the peak increase in firing rate over pre-drug baseline. Inhibition was defined as the lowest firing rate below the pre-drug baseline. Inhibition reversal was identified as a statistically significant reduction in the inhibition during the period of drug application.
Data collection
For comparison of the time course of effects on firing rate, the data were normalized and averaged. Firing rates over one minute intervals were calculated and normalized to the one-minute interval immediately prior to DA administration. These normalized data were averaged by synchronizing the data to the DA administration period, and graphs of the averaged data were made.
Statistical analysis
Averaged numerical values were expressed as the mean ± the standard error of the mean (S.E.M.). Mean response graphs are shown as change in firing rate (%). Statistical significance of data from different drug conditions and among firing rates during the long drug administration intervals in these studies were assessed with a Student’s t-test or an appropriate one-way or two-way ANOVA; degrees of freedom and statistical error terms are shown as subscripts to df or F in the text (Kenakin, 1987). Statistical analyses were performed with OriginPro 8.5 (OriginLab Corp. Northampton, MA.).
Results
VTA neuron characteristics
A total of 58 VTA neurons were examined. Their firing rate in normal extracellular medium ranged from 0.65 to 4.66 Hz, with a mean of 2.25 ± 0.13 Hz. All neurons had regular firing rates and were inhibited by dopamine agonists, dopamine or quinpirole, and/or baclofen. The concentrations of agonists were adjusted in each neuron so that inhibition exceeded 50%, as dopamine inhibition that was less than 50% was not reliably reversed (Nimitvilai and Brodie, 2010). This method of adjusting the concentration of inhibitory agonist controlled for differences in sensitivity between neurons, so that a similar response was achieved, rather than using a fixed concentration. Cells which did not return to at least 70% of their pre-agonist firing rate during this washout were not used. Three out of 58 (about 5%) of the cells did not return to 70% of their pre-agonist baseline firing rate. One benefit of the extracellular recording method used in these studies is that long duration recordings can be made reliably; the average recording duration was 144.1 ± 6.3 minutes, with a range of 90 to 190 minutes.
In the course of performing the some of the experiments described below, we used a protein kinase C inhibitor, Gö6976, delivered either via the recording pipette; in those experiments, 20 min of recording was performed before any drugs were added to the superfusion medium, to allow the Gö6976 to have its effect (Nimitvilai, et al., 2012a).
Desensitization of baclofen-induced inhibition
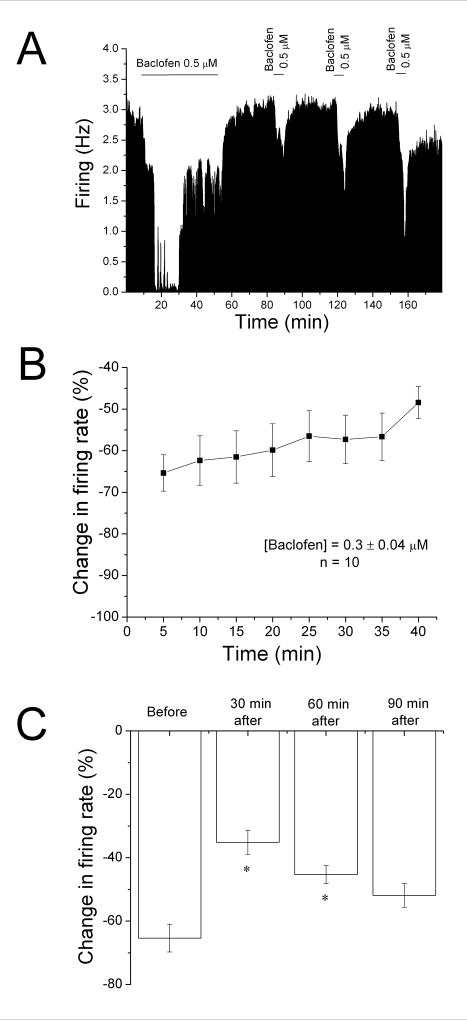
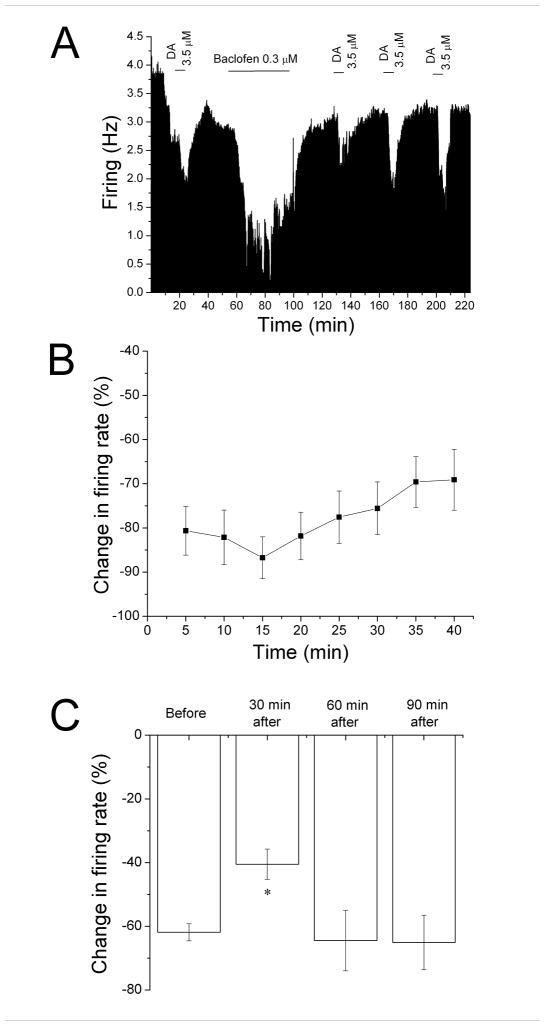
Using a protocol similar to that used to induce DIR (Nimitvilai and Brodie, 2010), we tested whether the response to baclofen would decrease over time. In most experiments, baclofen was added to the superfusate at a low concentration (0.2–0.5 μM); if greater than 50% inhibition was not achieved, the concentration of baclofen was increased stepwise until greater than 50% inhibition was obtained, and then that concentration of baclofen was added to the superfusate for a total of 40 min. An example of a 40 min application of baclofen (0.5 μM) is shown in Figure 1A. Note that near complete inhibition was produced by baclofen in the first 10 min, and that the firing rate of this VTA neuron increased in the continued presence of baclofen. Upon washout of baclofen, the firing rate increased to a level similar to the pre-baclofen control.
Figure 1. Reversal of baclofen inhibition and persistence of desensitization.
A. Mean ratemeter graph of the effects of baclofen (0.5 μM) on the firing rate of a single DA VTA neuron. Vertical bars indicate the firing rate over 5 sec intervals. Horizontal bars indicate the duration of baclofen application (concentrations indicated above bar). Baclofen initially produced a decrease in firing rate of 84% at 5 min, and a maximum inhibition of 86%. By the end of the 40 min baclofen administration, the inhibition of firing rate subsided so that the firing rate was 50%. Then, Baclofen was washed out for 30 min. Following this washout period, baclofen was administered for 5 min at 30 min intervals, producing inhibitions of 27% at 30 min, 49% at 60 min, and 69% at 90 min after the long baclofen administration period.
B. The mean firing rate of 10 DA VTA neurons during the 40 min baclofen administration in experiments similar to the one depicted in Figure 1A. Baclofen concentration was adjusted so that greater than 50% inhibition was produced; the mean concentration of baclofen that was administered was 0.3 ± 0.04 μM. There was a statistically significant reduction in baclofen-induced inhibition (One-way repeated measures ANOVA, F6,73 = 2.91, p < 0.05).
C. The effect of 5 min application of baclofen during and after the 40-min application period. The inhibitory effect of baclofen was significantly reduced at 30 and 60 min after the 40 min baclofen administration period compared to the effect of baclofen at 5 min during that initial baclofen exposure (One-way ANOVA, F3,29 = 12.01, p < 0.05).
The mean response of ten neurons to a 40 min application of baclofen (0.3 ± 0.04 μM) is shown in Figure 1B. Note that there was a steady reduction of inhibition over time (shown as an increase in firing rate from the 5 min time point). There was a significant reduction in the inhibitory effect of baclofen at the 40 min time point compared to the 5 min time point (One-way repeated measures ANOVA, F7,63 = 2.91, p < 0.05).
Also in the experiment illustrated in Figure 1A, brief (5 min) applications of baclofen were administered at 30 min intervals following the 40 min application (Figure 1A). A reduction of the response to baclofen was observed at the 30 min and 60 min time point, but the response to brief baclofen administration was greater at the 90 min time point. The mean data from 10 experiments is shown in Figure 1C. Note that the graph illustrates the inhibitory effect of baclofen at the 5 min time point during the 40-min application (Before), and at 30 min intervals after the cessation of the 40 min application. There was a significant difference at the 30 and 60 min time points from the control (One-way ANOVA, F3,29 = 12.01, p < 0.05).
Desensitization of baclofen-induced inhibition was blocked by CGP35348
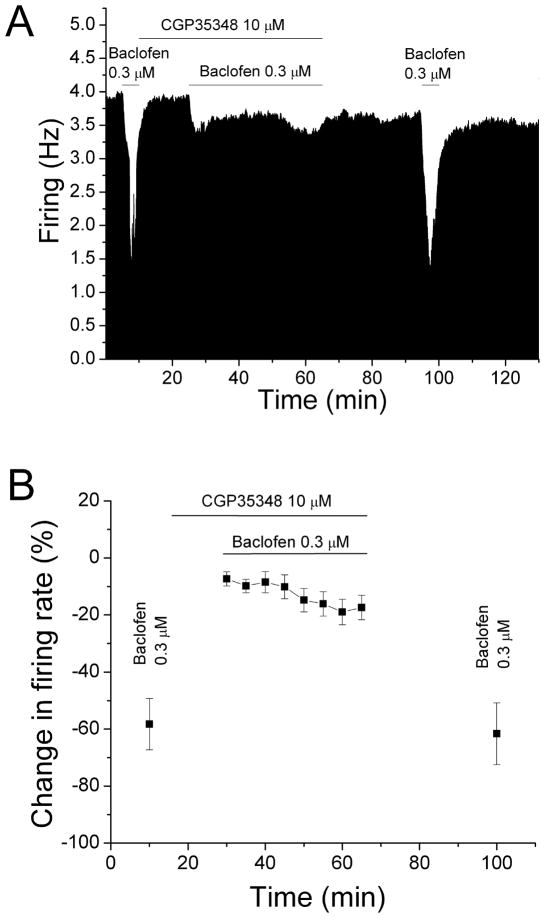
We confirmed the specificity of baclofen by determining whether the GABAB receptor antagonist CGP35348 could prevent the reduction of baclofen inhibition produced by long-term baclofen administration. The mean concentration of baclofen used in the previous experiment (0.3 μM) was tested before and after long-duration application of baclofen in the presence of CGP35348 (10 μM). An example from these experiments is shown in Figure 2A, and the mean response of six neurons is shown in Figure 2B.
Figure 2. Baclofen inhibition reversal blocked by CGP35348.
A. Mean ratemeter graph of the effects of baclofen and CGP35348 on the firing rate of a single DA VTA. Vertical bars indicate the firing rate over 5 sec intervals. Horizontal bars indicate the duration of baclofen (0.3 μM) or baclofen+CGP35348 (10 μM) application. Initially, a 5 min baclofen (0.3 μM) administration produced inhibition of 53.0%. During the 40 min application of baclofen and CGP35348, there was a decrease in firing rate of 10% at 5 min that further decreased to 13% by the end of the 40 min period. Baclofen was administered for 5 min after the cessation of baclofen and CGP35348 treatment, producing an inhibition of 60.9% at 30 min.
B. The mean firing rate of DA VTA neurons in response to baclofen and CGP35348 in experiments similar to the one depicted in Figure 2A. The mean concentration of baclofen at 0.3 μM from experiments such as that shown in Figure 1 was used. The 5 min application of baclofen initially produced an inhibition in firing rate of 58.3%. In the presence of CGP35348 (10 μM), baclofen produced only 7% decrease in firing rate at 5 min, and this inhibition was further increased to 17% at 40 min. After the cessation of baclofen and CGP35348 treatment, baclofen produced an inhibition in firing rate of 61.6%. There was no significant reduction in baclofen-induced inhibition (paired t-test, p > 0.05).
In these experiments, baclofen (0.3 μM) was administered for 5 min, producing a mean change in firing rate of −58.3 ± 9.0%. Then baclofen was washed out and CGP35348 (10 μM) was added to the superfusate. After CGP35348 was administered for 15 min, baclofen was applied for 40 min in the continued presence of CGP35348. In the presence of CGP35348, baclofen produced only 7.3 ± 2.4% decrease in firing rate during the first five min; there was a small but significant decrease in firing rate over time (one-way repeated measures ANOVA, F(7,35) = 4.6, p <0.05). Upon washout of baclofen and CGP35348 for 30 min, the effect of baclofen was tested again for 5 min and it produced a change in firing rate of −61.6 ± 10.8%. No significant reduction in the inhibitory effect of baclofen was observed when compared to the first brief baclofen application (paired t-test, p > 0.05). Therefore, CGP35348 blocked the desensitization by baclofen of baclofen-induced inhibition.
Long-duration administration of muscimol did not produce desensitization of muscimol-induced inhibition
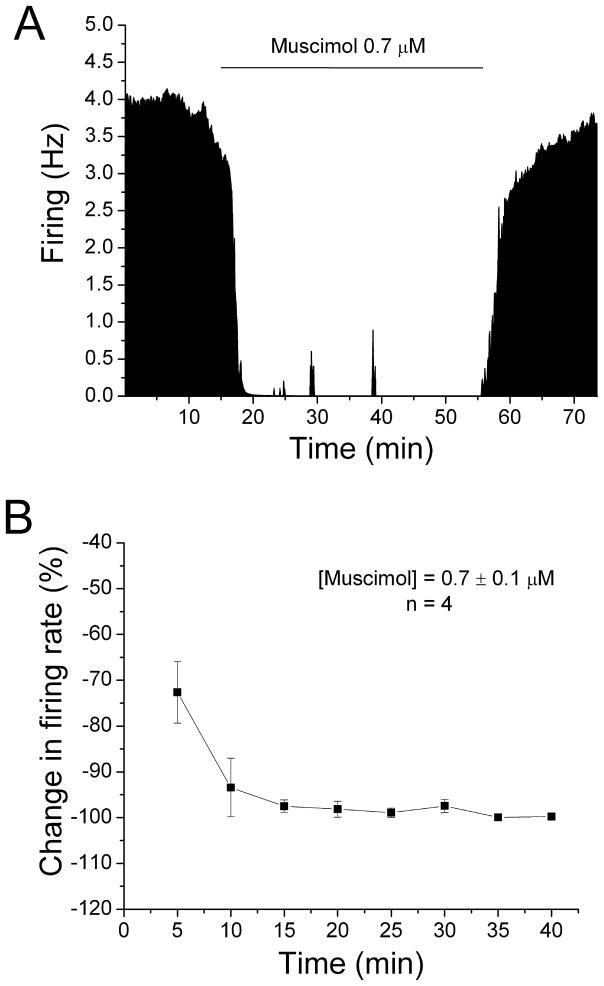
We also examined whether long-duration application of GABAA agonist muscimol would desensitize DA VTA neurons to muscimol-induced inhibition. Concentrations of muscimol were added in a stepwise fashion until inhibition of 50% or greater was achieved, and then these concentrations were sustained for 40 min. Figure 3A is the ratemeter graph of a single dopamine neuron, and Figure 3B is the mean response of four neurons to a 40 min application of muscimol (0.7 ± 0.1 μM). Muscimol produced an inhibition in firing rate to 72.64 ± 6.72% at 5 min and the inhibition increased significantly to 99.78 ± 0.19% at 40 min with no reversal over the time course (One-way repeated measures ANOVA, F(7,21) = 7.16, p < 0.05).
Figure 3. No reversal of muscimol-induced inhibition.
A. Mean ratemeter graph of the effects of muscimol (0.7 μM) on the firing rate of a single DA VTA neuron. Vertical bars indicate the firing rate over 5 sec intervals. Horizontal bars indicate the duration of muscimol application (concentrations indicated above bar). Muscimol initially produced a decrease in firing rate of 63.53% at 5 min, and the inhibition increase further so that by the end of the 40 min muscimol administration, the inhibition of firing rate was 99.96%.
B. The mean firing rate of 4 DA VTA neurons during the 40 min muscimol administration in experiments similar to the one depicted in Figure 3A. Muscimol concentration was adjusted so that greater than 50% inhibition was produced at 5 min; the mean concentration of muscimol that was administered was 0.7 ± 0.1 μM. Muscimol produced an inhibition in firing rate of 72.6% at 5 min and the inhibition increased significantly over time (one-way repeated measures ANOVA, F(7,21) = 7.16, p < 0.05).
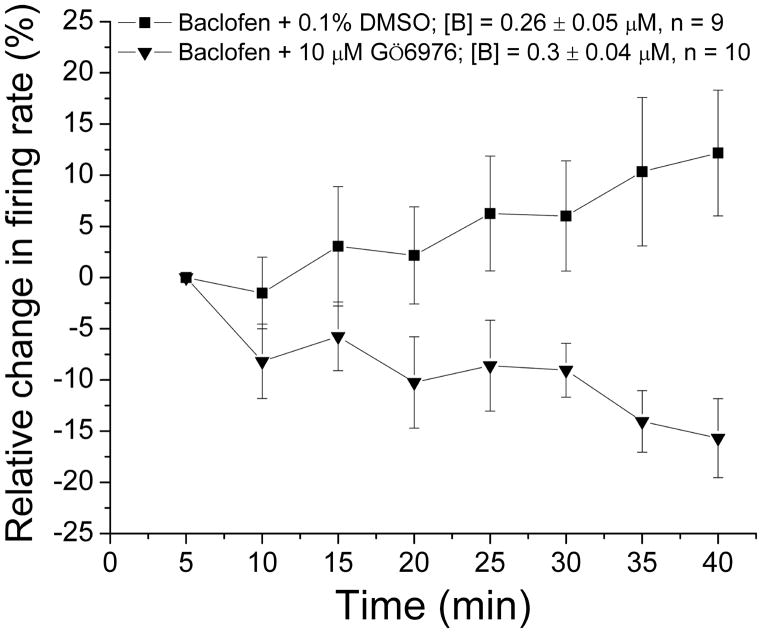
Desensitization to baclofen-induced inhibition was blocked by Gö6976
We have demonstrated that DIR was blocked by an inhibitor of conventional PKC, Gö6976 (Keenan, et al., 1997; Martiny-Baron, et al., 1993; Nimitvilai, et al., 2012a). In the present study, we tested whether PKC also was responsible for desensitization to baclofen inhibition. For these experiments, the recording electrode contained saline with the addition of either DMSO (0.1%) or Gö6976 (10 μM). Baclofen was administered for 40 min, and the firing rate was monitored. With saline/DMSO in the pipette, the slow desensitization to baclofen ([baclofen] = 0.26 ± 0.05 μM) was observed (Figure 4), similar to the effect seen in Figure 1B. When Gö6976 was included in the pipette, no decrease in baclofen inhibition was observed ([baclofen] = 0.3 ± 0.04 μM) (Figure 4). There was a significant difference in the response to baclofen over time for the two groups (Two-way ANOVA, F2,222 = 53.16, p < 0.05). Gö6976 alone caused a change in firing rate of −0.04 ± 3.37%, which was not significantly different from the initial baseline firing rate (paired t-test, p > 0.05). This indicates that blocking conventional PKC also blocks desensitization to baclofen inhibition.
Figure 4. Baclofen inhibition reversal blocked by Gö6976.
The mean firing rate of DA VTA neurons during the 40 min baclofen administration in experiments (similar to Figure 1B). In these experiments, the recording pipette included either DMSO vehicle (0.1%) or 10 μM Gö6976. Baclofen concentration was adjusted so that greater than 50% inhibition was produced. For recordings in which DMSO vehicle (0.1%) was included in the recording pipette, the mean concentration of baclofen that was administered was 0.3 ± 0.04 μM (■, n=9); for recordings in which 10 μM Gö6976 was included in the recording pipette, the mean concentration of baclofen that was administered was 0.26 ± 0.05 μM (▼, n=10). There was a significant difference in the response to baclofen over time for the two groups (Two-way ANOVA, F2,222 = 53.16, p < 0.05).
Cross-desensitization between baclofen and dopamine: baclofen-induced desensitization of dopamine inhibition
As the effects on firing rate of both dopamine and baclofen exhibit time-dependent desensitization, we examined whether desensitization to one of these would produce a muted response to the other. An experiment examining the effect of baclofen-induced desensitization on dopamine-induced inhibition is shown in Figure 5A. Dopamine (3.5 μM) was administered for 5 min, and then washed out for 30 min. Then, baclofen (0.3 μM) was administered for 40 min; as shown in Figure 5A, the baclofen-induced inhibition was reduced over time, and the firing rate after the cessation of baclofen administration returned to pre-baclofen baseline. Short applications of dopamine were tested at 30 min intervals following the end of the baclofen administration. As shown in Figure 3A, there was a slight decrease in the effect of dopamine at 30 min after baclofen administration, but the inhibitory effect of dopamine increased at 60 and 90 min after baclofen. Note that although the baseline firing rate was reduced after the initial dopamine administration, the subsequent baseline was stable, and that the response to dopamine after baclofen increased on this stable baseline. Figure 5B shows the effect of baclofen over time in a pool of experiments similar to the one shown in Figure 5A. There was a significant reduction in the inhibitory effect of baclofen at the last two time points compared to the 5 min time point (One-way repeated measures ANOVA, F7,42 = 5.23, p < 0.05). Figure 5C shown the mean effects of dopamine (4.64 ± 1.03 μM) in this paradigm; there was a significant reduction in dopamine inhibition at 30 min after baclofen application (One-way repeated measures ANOVA, F3,23 = 4.63, p < 0.05), but this reduction returned to baseline levels within 60 min.
Figure 5. Desensitization to dopamine inhibition following reversal of baclofen inhibition.
A. Mean ratemeter graph of the effects of dopamine (3.5 μM) and baclofen (0.3 μM) on the firing rate of a single DA VTA neuron. Vertical bars indicate the firing rate over 5 sec intervals. Horizontal bars indicate the duration of dopamine or baclofen application (concentrations indicated above bar). Initially, a 5 min dopamine administration produced inhibition of 59%. During the 40 min application, baclofen produced a decrease in firing rate of 73% at 5 min, with a maximum inhibition of 84%, and inhibition of only 57% by the end of the 40 min period. During the 30 min washout period, the baseline stabilized at a new, lower level. Dopamine was administered for 5 min at 30 min intervals after the cessation of baclofen treatment, producing inhibitions of 29% at 30 min, 43% at 60 min, and 45% at 90 min.
B. The mean firing rate of DA VTA neurons during the 40 min baclofen administration in experiments similar to the one depicted in Figure 3A. Baclofen concentration was adjusted so that greater than 50% inhibition was produced; the mean concentration of baclofen that was administered was 0.29 ± 0.04 μM. There was a statistically significant reduction in baclofen-induced inhibition over time (One-way repeated measures ANOVA, F7,42 = 4.63, p < 0.05).
C. The effect of 5 min application of dopamine during and after the 40-min baclofen application period. The inhibitory effect of dopamine was significantly reduced only at 30 min after the 40 min baclofen administration period compared to the effect of dopamine before the baclofen treatment (One-way ANOVA, F3,29 = 12.01, p < 0.05).
Cross-desensitization between baclofen and dopamine: dopamine-induced desensitization of baclofen inhibition
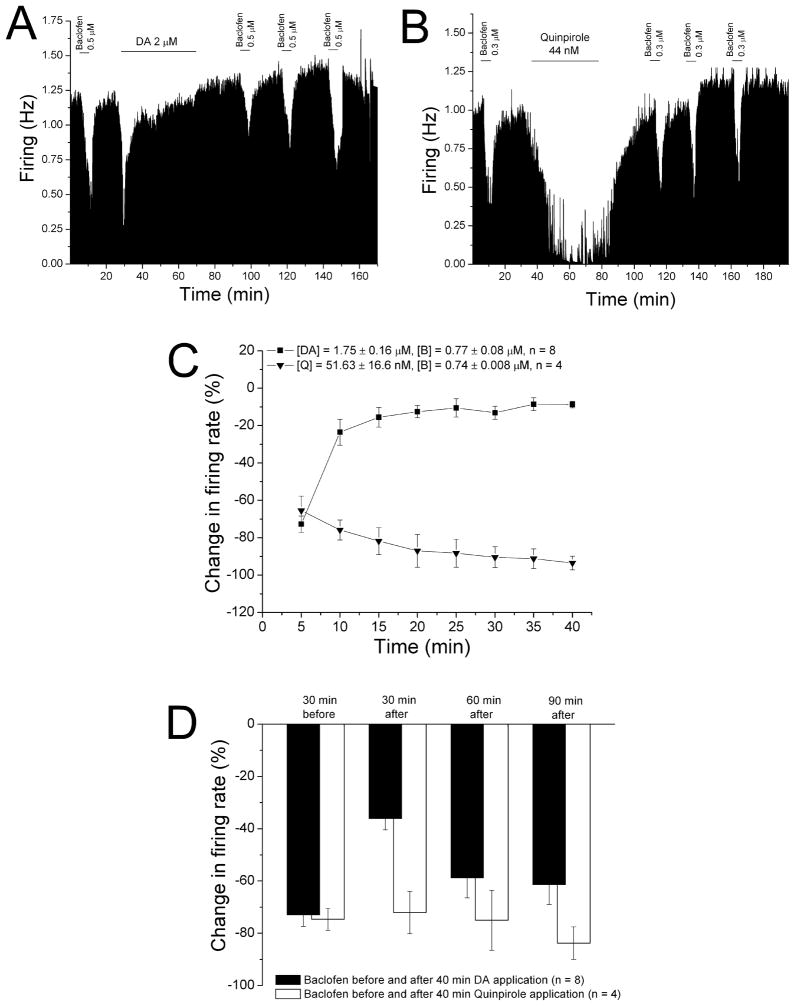
We also tested whether DIR produced a change in sensitivity to the GABAB agonist baclofen. A typical experiment testing the effect of DIR on baclofen sensitivity is shown in Figure 6A. Baclofen (0.5 μM) was applied in the superfusion medium for 5 min, followed by a 30 min washout. Then dopamine (2 μM) was administered for 40 min; note that the inhibition produced by dopamine subsided over time; when the dopamine was washed out. Baclofen (0.5 μM) was again tested at 30 min intervals after the end of the dopamine application. The effect of baclofen was reduced at 30 min following the dopamine application, but increased at 60 min, and at 90 min was similar to the pre-dopamine effect of baclofen.
Figure 6. Desensitization to baclofen inhibition following reversal of dopamine inhibition.
A. Mean ratemeter graph of the effects of baclofen (0.5 μM) and dopamine (2 μM) on the firing rate of a single DA VTA neuron. Vertical bars indicate the firing rate over 5 sec intervals. Horizontal bars indicate the duration of baclofen or dopamine application (concentrations indicated above bar). Initially, a 5 min baclofen administration produced inhibition of 68%. During the 40 min application, dopamine produced a decrease in firing rate of 62% at 5 min (which was the maximum), and inhibition of only 7% by the end of the 40 min period. Baclofen was administered for 5 min at 30 min intervals after the cessation of dopamine treatment, producing inhibitions of 31% at 30 min, 52% at 60 min, and 51% at 90 min.
B. Mean ratemeter graph of the effects of baclofen (0.3 μM) and quinpirole (44nM) on the firing rate of a single DA VTA neuron. Vertical bars indicate the firing rate over 5 sec intervals. Horizontal bars indicate the duration of baclofen or quinpirole application (concentrations indicated above bar). Initially, a 5 min baclofen administration produced inhibition of 67%. During the 40 min application, quinpirole produced a decrease in firing rate of 63% at 5 min, and a maximum inhibition of 95%; inhibition at the end of the 40 min period was 87%. Baclofen was administered for 5 min at 30 min intervals after the cessation of quinpirole treatment, producing inhibitions of 55% at 30 min, 43% at 60 min, and 69% at 90 min.
C. The mean firing rate of DA VTA neurons during the 40 min administration of dopamine (■, n=8) or quinpirole (▼, n=4) in experiments similar to the ones depicted in Figures 4A and 4B. Concentrations were adjusted so that greater than 50% inhibition was produced; the mean concentration of dopamine that was administered was 1.75 ± 0.16 μM; mean quinpirole concentration was 51.6 ± 16.6 nM.
D. The effect of 5 min application of baclofen during and after the 40-min period of application of dopamine (Filled bars) or quinpirole (open bars). The inhibitory effect of baclofen was significantly reduced only at 30 min after the 40 min dopamine administration period compared to prior to the dopamine treatment (One-way ANOVA, F3,26 = 7.08, p < 0.05); there was no difference in baclofen-induced inhibition before and after the quinpirole treatment (One-way ANOVA, F3,9 = 0.67, p > 0.05).
While long-term administration of dopamine produces a reduction in dopamine inhibition, long-term application of the selective D2 agonist quinpirole does not result in desensitization of the D2 receptor (Nimitvilai and Brodie, 2010). We have determined that desensitization of D2 receptors in DA VTA neurons requires activation of both D1-like and D2 receptors (Nimitvilai and Brodie, 2010). We tested whether D2 activation alone could reduce the response to baclofen. An experiment using quinpirole is shown in Figure 6B. Baclofen (0.3 μM) was applied for 5 min and then washed out for 30 min, then quinpirole (44 nM) was administered for 40 min, and then washed out. Note that there was no reversal of quinpirole inhibition during the 40 min application period. After quinpirole was washed out, baclofen was tested at 30 min intervals. There was no reduction in balcofen-induced inhibition after quinpirole administration. The mean effect of dopamine and quinpirole in a series of experiments similar to those shown in Figure 6A and B is shown in Figure 6C. Note that there was a significant reduction in dopamine inhibition from −72.8 ± 4.4% at 5 min to −8.8 ± 1.8% at 40 min ([DA] = 1.8 ± 0.2 μM) (One-way repeated measures ANOVA, F7,49 = 49.23, p < 0.05). In contrast, quinpirole inhibition at 5 min was −65.5 ± 7.8 % and the inhibition increased significantly to −93.5 ± 3.6% at 40 min ([quinpirole] = 51.6 ± 16.6 nM) (One-way repeated measures ANOVA, F7,21 = 6.63, p < 0.05). The effect of baclofen before and after the administration of the dopamine agonists is shown in Figure 6D. There was a significant reduction of baclofen-induced inhibition 30 min after the dopamine administration ([baclofen] = 0.8 ± 0.08 μM) (One-way ANOVA, F3,26 = 7.08, p < 0.05), and the response to baclofen was not significantly different from the pre-dopamine response at the 60 and 90 min time points. The 40 min application of quinpirole had no significant effect on the response to baclofen ([baclofen] = 0.7 ± 0.01 μM) (One-way ANOVA, F3,9 = 0.67, p > 0.05).
Discussion
Extended exposure of DAergic VTA neurons to moderate concentrations of dopamine results in a time- and concentration-dependent decrease in dopamine-induced inhibition, which persists for up to 90 min (Nimitvilai and Brodie, 2010). This decrease in dopamine sensitivity requires concurrent stimulation of D2 and D1/D5 receptors so that it is neither homologous- nor heterologous-desensitization, and we termed this phenomenon “dopamine inhibition reversal” or DIR. Even high quinpirole concentrations (3 μM) fail to mediate D2 desensitization in the presence of a D1/D5 antagonist (Nimitvilai and Brodie, 2010). DIR is mediated by PLC and phorbol ester-sensitive PKC, and is dependent on extracellular calcium concentration and intracellular calcium release, and is blocked by the selective PKC antagonist Gö6976; this profile suggests an involvement of conventional PKC, rather than novel- or atypical- PKC (Nimitvilai, et al., 2012a). In the present study, we demonstrated the GABAB selective agonist baclofen shares some characteristics with dopamine, in that extended administration of moderate concentrations of baclofen produces a desensitization to the inhibitory action of baclofen, and this desensitization lasts for a long time (up to 60 min). In addition, desensitization of baclofen responses is blocked by Gö6976, suggesting an involvement of conventional protein kinase C in the desensitization. Unlike desensitization of the D2 receptor, baclofen alone caused desensitization of the GABAB responses, indicating a homologous desensitization. We did not block other GABA receptor subtypes, nor did we antagonize other of the numerous non-GABA receptors in the preparation, so we cannot say for sure that there is not a necessary cooperation of GABAB activation with receptors activated by endogenous neurotransmitters. Such a cooperation is apparently the case with D2 activation, as high concentrations of quinpirole can cause desensitization (Bartlett, et al., 2005) only if endogenous dopamine can act at D1/D5 receptors (Nimitvilai and Brodie, 2010).
As these desensitization phenomena were similar, we examined whether there was a cross-desensitization between dopamine and baclofen, and the results above indicate that cross-desensitization did occur. One point of note is that the cross-desensitization was of shorter duration (30 min but not 60 min) than the desensitization to baclofen by baclofen (Figure 1), or dopamine following DIR (Nimitvilai and Brodie, 2010). This difference in the time-course suggests that while there is some overlap between the two mechanisms, there is also selectivity in the duration of the effects of dopamine and baclofen on responses to their respective agonists. Additional studies would be needed to determine whether these time-course differences are due to spatial differences between the dopamine and GABAB receptors, or whether there are differences in the molecular mechanisms.
Both D2 receptors and GABAB receptors are G-protein coupled receptors (GPCRs), like the adrenergic β receptor. The mechanism of desensitization is generally related to phosphorylation of the GPCR, either at the C-terminal or within the third intracellular loop during agonist occupancy, which results in de-coupling of the G-protein from its receptor, decreasing function of the receptor (Cho, et al., 2006; Namkung and Sibley, 2004). A role of PKC in desensitization of D2 receptors has been reported in many systems such as HEK293 cells, striatal and hippocampal neurons (Rogue, et al., 1990; Bofill-Cardona, et al., 2000; Namkung and Sibley, 2004; Thibault, et al., 2011). In contrast, it has been shown that desensitization of GABAB receptors can occur through a phosphorylation-independent mechanism via GRK4 in HEK293 cells and cerebellar granule cells (Perroy, et al., 2003). Phosphorylation of D2 receptors by PKC results in desensitization and internalization of those receptors through β-arrestins (Namkung and Sibley, 2004; Thibault, et al., 2011), whereas it has been shown that baclofen stimulation of GABAB receptors does not result in internalization of that receptor, nor did it result in interaction with β-arrestins even in the presence of GRK4 (Sudo, et al., 2012). Despite the cross-desensitization between dopamine and baclofen in the present study, the mechanisms for desensitization for each receptor may be different.
As these experiments were performed in brain slices, it would be interesting to examine the effect of sustained or intense activation of GABAB receptors in the VTA in vivo. There is evidence that GABAB receptors are found on DA VTA neurons (Westerink, et al., 1998) and on glutamatergic terminals to GABA interneurons within the VTA (Bonci and Malenka, 1999; Steffensen, et al., 2000). We do not know the cellular location of the GABAB receptors with which baclofen interacted in our study. In vivo studies could more precisely investigate the influence of endogenous GABAergic inputs to VTA DA neurons, and whether endogenous activation of GABAergic inputs would result in desensitization of GABAB or D2 receptors.
The desensitization of responses to GABAB or D2 agonists is blocked by selective PKC inhibitor Gö6976 (Keenan, et al., 1997; Martiny-Baron, et al., 1993), suggesting a phosphorylation mechanism underlying the reversal of inhibition by dopamine (Nimitvilai, et al., 2012a) and baclofen in the DAergic VTA neurons. Others have shown that activation of GPCRs like NK1 can result in reduction of GABAB responsiveness of VTA neurons via PKC (Xia, et al., 2010). The present report is the first to show that such interaction (in this case between GPCRs that both activate GIRKs), can be long-lasting (30 min). Activation of PKC by either dopamine or baclofen may result in promiscuous phosphorylation of GPCRs, or of other intracellular elements (like GRK4) that mediate the desensitization. Note that as the PKC inhibitor Gö6976 was delivered via the recording pipette, PKC-containing neurons and processes in the vicinity of the pipette tip would receive Gö6976 as well, so a polysynaptic interaction of PKC and receptor desensitization mechanisms is possible. Additional detailed experiments would be necessary to determine which specific intracellular proteins undergo phosphorylation, and whether the modification of GPCRs is a local phenomenon, or extends to diverse cellular structures once PKC is activated.
Drugs of abuse like cocaine, which blocks dopamine reuptake, cause an increase in DAergic transmission in the reward system in a sustained manner (Di Chiara, et al., 2004), and adaptation in the reward system to this prolonged increase in dopamine transmission may be related to the craving and seeking for drugs even in the presence of negative consequences. Cocaine causes an increase in dopamine concentration in the VTA (Einhorn, et al., 1988; Brodie and Dunwiddie, 1990), as well as in target regions (White, 1986). We have previously demonstrated that dopamine inhibition reversal can occur at much lower concentrations of dopamine when cocaine is present (Nimitvilai, et al., 2012a). Cocaine may increase the likelihood that dopamine in the VTA can activate PKC, and subsequently decrease sensitivity to not only D2 agonists, but also to GABAB agonists. Alteration in GABAB signaling in the VTA has been shown to occur after chronic cocaine and chronic morphine, but this was linked to cAMP protein kinase A (Bonci and Williams, 1996; Bonci and Williams, 1997), which is not the case for DIR, which is dependent on PKC but not PKA (Nimitvilai, et al., 2012a). Similarly, functional desensitization of GABAB receptors in the nucleus accumbens was observed after repeated cocaine treatment (Xi, et al., 2003). Increases or decreases in GABA transmission in the VTA during withdrawal from drugs of abuse could occur due to the desensitization of GABAB receptor sensitivity as a result of increased extracellular dopamine concentration.
As noted in the Introduction, both GABAB receptors and D2 dopamine receptors are coupled to G-protein-linked inwardly rectifying potassium channels (GIRKs) (Kim, et al., 1997; Lacey, et al., 1988). It may be that GIRKs are the common point of action of GABAB receptors and dopamine D2 receptors, and that desensitization of the effect of baclofen and dopamine are produced by a reduction of GIRK responsiveness. This possibility is supported by the observation that in vivo treatment with one or five injections of cocaine produces desensitization of baclofen-induced inhibition of DA VTA neurons, and this desensitization is dependent on D2 receptors (Arora, et al., 2011). Furthermore, the desensitization of GABAB receptor GIRK currents was correlated with a reduction of Girk2 density, but not with a reduction in GABABR1 protein, in the dendrites of DA VTA neurons (Arora, et al., 2011). It may be that increased extracellular dopamine in the VTA as a result of cocaine treatment results in a reduction of GABAB function via a decrease in density of dendritic GIRKs. As to the interaction between D2 dopamine and GABAB responses in our study, there may be action of the two receptors at common GIRKs, or mediation by PKC of different mechanisms at the D2 and GABAB receptors. Our observation that cross-desensitization of responses was not as long-lasting as desensitization of each receptor by its preferred agonist suggests different pools of GIRKs linked to each type of receptor, or a more complicated mechanism not solely dependent on reduction of GIRK density. Determination of the mechanisms of cross-desensitization would require additional complex studies.
One of the functional consequences of desensitization of GABAB receptors as a result of increased dopamine concentration is that DA VTA neurons may exhibit increased excitability. Cocaine causes an enhancement of NMDA/AMPA glutamate transmission and can promote induction of LTP in the VTA (Ungless, et al., 2001; Borgland, et al., 2004); increased excitability of DA VTA neurons due to reduced DA autoinhibition and reduced GABAB inhibition may help promote such LTP. At the whole animal level, cocaine-induced seizures are attenuated by GABAB agonists and potentiated by GABAB agonists (Gasior, et al., 2004), suggesting an interaction between monoamine reuptake blockade and GABAB function. Understanding molecular mechanisms underlying the effects of sustained increases in dopamine or GABA concentration in the VTA may contribute to medication discovery for more effective treatment of disorders related to addiction.
Highlights.
Desensitization of GABAB receptors & dopamine D2 receptors was studied in the VTA
GABAB agonist baclofen produced long-lasting desensitization of GABAB receptors
Baclofen also produced desensitization of dopamine D2 receptors
D2 receptor desensitization also desensitized GABAB receptors
Reciprocal desensitization of GABAB & D2 receptors may play a role in therapeutics
Acknowledgments
Grants: The authors gratefully acknowledge support from PHS Grant AA05846
Non-Standard abbreviations
- pDAergic
putative dopaminergic
- DAergic
dopaminergic
- VTA
ventral tegmental area
- LTP
long term potentiation
- NMDA
N-methyl-D-aspartate
- Gö6976
5,6,7,13-tetrahydro-13-methyl-5-oxo-12H-indolo[2,3-a]pyrrolo[3,4c]carbazole-12-propanenitrile
- GIRKs
G-protein-linked inwardly rectifying potassium channels
- GRK
G-protein coupled receptor kinase
Footnotes
Disclosures: None of the authors have any conflict of interest associated with the content of this report.
Author contributions:
Sudarat Nimitvilai: Performed experiments for this study and was involved in planning and design of the experiments and participated in the writing of the manuscript.
Devinder S. Arora: Performed experiments for this study and was involved in planning and design of the experiments and participated in the writing of the manuscript.
Maureen A. McElvain: Performed experiments for this study
Mark S. Brodie: Was involved in planning and design of the experiments and participated in the writing of the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Ackerman JM, Johansen PA, Clark D, White FJ. Electrophysiological effects of putative autoreceptor-selective dopamine agonists on A10 dopamine neurons. J Pharmacol Exp Ther. 1993;265:963–970. [PubMed] [Google Scholar]
- Arora D, Hearing M, Haluk DM, Mirkovic K, Fajardo-Serrano A, Wessendorf MW, Watanabe M, Lujan R, Wickman K. Acute cocaine exposure weakens GABA(B) receptor-dependent G-protein-gated inwardly rectifying K+ signaling in dopamine neurons of the ventral tegmental area. J Neurosci. 2011;31:12251–12257. doi: 10.1523/JNEUROSCI.0494-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett SE, Enquist J, Hopf FW, Lee JH, Gladher F, Kharazia V, Waldhoer M, Mailliard WS, Armstrong R, Bonci A, Whistler JL. Dopamine responsiveness is regulated by targeted sorting of D2 receptors. Proc Natl Acad Sci U S A. 2005;102:11521–11526. doi: 10.1073/pnas.0502418102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bofill-Cardona E, Kudlacek O, Yang Q, Ahorn H, Freissmuth M, Nanoff C. Binding of calmodulin to the D2-dopamine receptor reduces receptor signaling by arresting the G protein activation switch. J Biol Chem. 2000;275:32672–32680. doi: 10.1074/jbc.M002780200. [DOI] [PubMed] [Google Scholar]
- Bonci A, Malenka RC. Properties and plasticity of excitatory synapses on dopaminergic and GABAergic cells in the ventral tegmental area. J Neurosci. 1999;19:3723–3730. doi: 10.1523/JNEUROSCI.19-10-03723.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonci A, Williams JT. A common mechanism mediates long-term changes in synaptic transmission after chronic cocaine and morphine. Neuron. 1996;16:631–639. doi: 10.1016/s0896-6273(00)80082-3. [DOI] [PubMed] [Google Scholar]
- Bonci A, Williams JT. Increased probability of GABA release during withdrawal from morphine. J Neurosci. 1997;17:796–803. doi: 10.1523/JNEUROSCI.17-02-00796.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgland SL, Malenka RC, Bonci A. Acute and chronic cocaine-induced potentiation of synaptic strength in the ventral tegmental area: electrophysiological and behavioral correlates in individual rats. J Neurosci. 2004;24:7482–7490. doi: 10.1523/JNEUROSCI.1312-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouthenet M, Souil E, Martres M, Sokoloff P, Giros B, Schwartz J. Localization of dopamine D3 receptor mRNA in the rat brain using in situ hybridization histochemistry: comparison with dopamine D2 receptor mRNA. Brain Res. 1991;564:203–219. doi: 10.1016/0006-8993(91)91456-b. [DOI] [PubMed] [Google Scholar]
- Brodie MS, Dunwiddie TV. Cholecystokinin potentiates dopamine inhibition of mesencephalic dopamine neurons in vitro. Brain Res. 1987;425:106–113. doi: 10.1016/0006-8993(87)90488-4. [DOI] [PubMed] [Google Scholar]
- Brodie MS, Dunwiddie TV. Cocaine effects in the ventral tegmental area: Evidence for an indirect dopaminergic mechanism of action. Naunyn Schmiedebergs Arch Pharmacol. 1990;342:660–665. doi: 10.1007/BF00175709. [DOI] [PubMed] [Google Scholar]
- Brodie MS, McElvain MA, Bunney EB, Appel SB. Pharmacological reduction of small conductance calcium-activated potassium current (SK) potentiates the excitatory effect of ethanol on ventral tegmental area dopamine neurons. J Pharmacol Exp Ther. 1999;290:325–333. [PubMed] [Google Scholar]
- Bunney BS, Walters JR, Roth RH, Aghajanian GK. Dopaminergic neurons: Effects of antipsychotic drugs and amphetamine on single cell activity. J Pharmacol Exp Ther. 1973;185:560–571. [PubMed] [Google Scholar]
- Caille I, Dumartin B, Bloch B. Ultrastructural localization of D1 dopamine receptor immunoreactivity in rat striatonigral neurons and its relation with dopaminergic innervation. Brain Res. 1996;730:17–31. doi: 10.1016/0006-8993(96)00424-6. [DOI] [PubMed] [Google Scholar]
- Chieng B, Azriel Y, Mohammadi S, Christie MJ. Distinct cellular properties of identified dopaminergic and GABAergic neurons in the mouse ventral tegmental area. J Physiol. 2011;589:3775–3787. doi: 10.1113/jphysiol.2011.210807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho DI, Beom S, Van Tol HH, Caron MG, Kim KM. Characterization of the desensitization properties of five dopamine receptor subtypes and alternatively spliced variants of dopamine D2 and D4 receptors. Biochem Biophys Res Commun. 2006;350:634–640. doi: 10.1016/j.bbrc.2006.09.090. [DOI] [PubMed] [Google Scholar]
- Ciliax BJ, Nash N, Heilman C, Sunahara R, Hartney A, Tiberi M, Rye DB, Caron MG, Niznik HB, Levey AI. Dopamine D(5) receptor immunolocalization in rat and monkey brain. Synapse. 2000;37:125–145. doi: 10.1002/1098-2396(200008)37:2<125::AID-SYN7>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Bassareo V, Fenu S, De Luca MA, Spina L, Cadoni C, Acquas E, Carboni E, Valentini V, Lecca D. Dopamine and drug addiction: the nucleus accumbens shell connection. Neuropharmacology. 2004;47(Suppl 1):227–241. doi: 10.1016/j.neuropharm.2004.06.032. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic dopamine system of freely moving rats. Proc Natl Acad Sci U S A. 1988;85:5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einhorn LC, Johansen PA, White FJ. Electrophysiological effects of cocaine in the mesoaccumbens dopamine system: Studies in the ventral tegmental area. J Neurosci. 1988;8:100–112. doi: 10.1523/JNEUROSCI.08-01-00100.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasior M, Kaminski R, Witkin JM. Pharmacological modulation of GABA(B) receptors affects cocaine-induced seizures in mice. Psychopharmacology (Berl) 2004;174:211–219. doi: 10.1007/s00213-003-1743-0. [DOI] [PubMed] [Google Scholar]
- Grace AA, Bunney BS. The control of firing pattern in nigral dopamine neurons: single spike firing. J Neurosci. 1984;4:2866–2876. doi: 10.1523/JNEUROSCI.04-11-02866.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW. Neurotransmitter regulation of dopamine neurons in the ventral tegmental area. Brain Res Rev. 1993;18:75–113. doi: 10.1016/0165-0173(93)90008-n. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Churchill L, Klitenick MA. GABA and enkephalin projection from the nucleus accumbens and ventral pallidum to the ventral tegmental area. Neuroscience. 1993;57:1047–1060. doi: 10.1016/0306-4522(93)90048-k. [DOI] [PubMed] [Google Scholar]
- Keenan C, Goode N, Pears C. Isoform specificity of activators and inhibitors of protein kinase C gamma and delta. FEBS Lett. 1997;415:101–108. doi: 10.1016/s0014-5793(97)01104-6. [DOI] [PubMed] [Google Scholar]
- Kenakin TP. Pharmacologic analysis of drug-receptor interaction. Raven Press; New York, New York: 1987. Analysis of dose-response data; pp. 129–162. [Google Scholar]
- Khan ZU, Gutierrez A, Martin R, Penafiel A, Rivera A, de la CA. Dopamine D5 receptors of rat and human brain. Neuroscience. 2000;100:689–699. doi: 10.1016/s0306-4522(00)00274-8. [DOI] [PubMed] [Google Scholar]
- Kim KM, Nakajima S, Nakajima Y. Dopamine and GABA receptors in cultured substantia nigra neurons: correlation of electrophysiology and immunocytochemistry. Neuroscience. 1997;78:759–769. doi: 10.1016/s0306-4522(96)00585-4. [DOI] [PubMed] [Google Scholar]
- Lacey MG, Mercuri NB, North RA. Dopamine acts on D2 receptors to increase potassium conductance in neurones of the rat substantia nigra zona compacta. J Physiol (Lond ) 1987;392:397–416. doi: 10.1113/jphysiol.1987.sp016787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacey MG, Mercuri NB, North RA. On the potassium conductance increase activated by GABAB and dopamine D2 receptors in rat substantia nigra neurones. J Physiol (Lond ) 1988;401:437–453. doi: 10.1113/jphysiol.1988.sp017171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacey MG, Mercuri NB, North RA. Two cell types in rat substantia nigra zona compacta distinguished by membrane properties and the actions of dopamine and opioids. J Neurosci. 1989;9:1233–1241. doi: 10.1523/JNEUROSCI.09-04-01233.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammel S, Hetzel A, Hackel O, Jones I, Liss B, Roeper J. Unique properties of mesoprefrontal neurons within a dual mesocorticolimbic dopamine system. Neuron. 2008;57:760–773. doi: 10.1016/j.neuron.2008.01.022. [DOI] [PubMed] [Google Scholar]
- Margolis EB, Lock H, Hjelmstad GO, Fields HL. The ventral tegmental area revisited: is there an electrophysiological marker for dopaminergic neurons? J Physiol. 2006;577:907–924. doi: 10.1113/jphysiol.2006.117069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis EB, Mitchell JM, Ishikawa J, Hjelmstad GO, Fields HL. Midbrain dopamine neurons: projection target determines action potential duration and dopamine D(2) receptor inhibition. J Neurosci. 2008;28:8908–8913. doi: 10.1523/JNEUROSCI.1526-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martiny-Baron G, Kazanietz MG, Mischak H, Blumberg PM, Kochs G, Hug H, Marme D, Schachtele C. Selective inhibition of protein kinase C isozymes by the indolocarbazole Go 6976. J Biol Chem. 1993;268:9194–9197. [PubMed] [Google Scholar]
- Mirenowicz J, Schultz W. Preferential activation of midbrain dopamine neurons by appetitive rather than aversive stimuli. Nature. 1996;379:449–451. doi: 10.1038/379449a0. [DOI] [PubMed] [Google Scholar]
- Mueller AL, Brodie MS. Intracellular recording from putative dopamine-containing neurons in the ventral tegmental area of Tsai in a brain slice preparation. J Neurosci Methods. 1989;28:15–22. doi: 10.1016/0165-0270(89)90005-8. [DOI] [PubMed] [Google Scholar]
- Namkung Y, Sibley DR. Protein kinase C mediates phosphorylation, desensitization, and trafficking of the D2 dopamine receptor. J Biol Chem. 2004;279:49533–49541. doi: 10.1074/jbc.M408319200. [DOI] [PubMed] [Google Scholar]
- Nimitvilai S, Arora DS, Brodie MS. Reversal of Dopamine Inhibition of Dopaminergic Neurons of the Ventral Tegmental Area is Mediated by Protein Kinase C. Neuropsychopharmacology. 2012a;37:543–556. doi: 10.1038/npp.2011.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimitvilai S, Brodie MS. Reversal of prolonged dopamine inhibition of dopaminergic neurons of the ventral tegmental area. J Pharmacol Exp Ther. 2010;333:555–563. doi: 10.1124/jpet.109.163931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimitvilai S, McElvain MA, Arora DS, Brodie MS. Reversal of quinpirole inhibition of ventral tegmental area neurons is linked to the phosphatidylinositol system and is induced by agonists linked to Gq. J Neurophysiol. 2012b doi: 10.1152/jn.01137.2011. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perroy J, Adam L, Qanbar R, Chenier S, Bouvier M. Phosphorylation-independent desensitization of GABA(B) receptor by GRK4. EMBO J. 2003;22:3816–3824. doi: 10.1093/emboj/cdg383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesavento E, Margotti E, Righi M, Cattaneo A, Domenici L. Blocking the NGF-TrkA interaction rescues the developmental loss of LTP in the rat visual cortex: role of the cholinergic system. Neuron. 2000;25:165–175. doi: 10.1016/s0896-6273(00)80880-6. [DOI] [PubMed] [Google Scholar]
- Rogue P, Zwiller J, Malviya AN, Vincendon G. Phosphorylation by protein kinase C modulates agonist binding to striatal dopamine D2 receptors. Biochem Int. 1990;22:575–582. [PubMed] [Google Scholar]
- Sokolov MV, Kleschevnikov AM. Atropine suppresses associative LTP in the CA1 region of rat hippocampal slices. Brain Res. 1995;672:281–284. doi: 10.1016/0006-8993(94)01376-s. [DOI] [PubMed] [Google Scholar]
- Steffensen SC, Nie Z, Criado JR, Siggins GR. Ethanol inhibition of N-methyl-D-aspartate responses involves presynaptic gamma-aminobutyric acid(B) receptors. J Pharmacol Exp Ther. 2000;294:637–647. [PubMed] [Google Scholar]
- Steffensen SC, Svingos AL, Pickel VM, Henriksen SJ. Electrophysiological characterization of GABAergic neurons in the ventral tegmental area. J Neurosci. 1998;18:8003–8015. doi: 10.1523/JNEUROSCI.18-19-08003.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudo Y, Hojo M, Ando Y, Takada M, Murata H, Kurata S, Nishida N, Uezono Y. GABA(B) receptors do not internalize after baclofen treatment, possibly due to a lack of beta-arrestin association: Study with a real-time visualizing assay. Synapse. 2012 doi: 10.1002/syn.21565. [DOI] [PubMed] [Google Scholar]
- Tagliaferro P, Morales M. Synapses between corticotropin-releasing factor-containing axon terminals and dopaminergic neurons in the ventral tegmental area are predominantly glutamatergic. J Comp Neurol. 2008;506:616–626. doi: 10.1002/cne.21576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibault D, Albert PR, Pineyro G, Trudeau LE. Neurotensin Triggers Dopamine D2 Receptor Desensitization through a Protein Kinase C and {beta}-Arrestin1-dependent Mechanism. J Biol Chem. 2011;286:9174–9184. doi: 10.1074/jbc.M110.166454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungless MA, Whistler JL, Malenka RC, Bonci A. Single cocaine exposure in vivo induces long-term potentiation in dopamine neurons. Nature. 2001;411:583–587. doi: 10.1038/35079077. [DOI] [PubMed] [Google Scholar]
- Westerink BH, Enrico P, Feimann J, De Vries JB. The pharmacology of mesocortical dopamine neurons: a dual-probe microdialysis study in the ventral tegmental area and prefrontal cortex of the rat brain. J Pharmacol Exp Ther. 1998;285:143–154. [PubMed] [Google Scholar]
- White FJ. Electrophysiological effects of cocaine in the mesoaccumbens dopamine system: Studies in the nucleus accumbens. Soc Neurosci Abstr. 1986;12:1516. doi: 10.1523/JNEUROSCI.08-01-00100.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA. Neurobiology of addiction. Curr Opin Neurobiol. 1996;6:243–251. doi: 10.1016/s0959-4388(96)80079-1. [DOI] [PubMed] [Google Scholar]
- Xi ZX, Ramamoorthy S, Shen H, Lake R, Samuvel DJ, Kalivas PW. GABA transmission in the nucleus accumbens is altered after withdrawal from repeated cocaine. J Neurosci. 2003;23:3498–3505. doi: 10.1523/JNEUROSCI.23-08-03498.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia YF, Margolis EB, Hjelmstad GO. Substance P inhibits GABAB receptor signalling in the ventral tegmental area. J Physiol. 2010;588:1541–1549. doi: 10.1113/jphysiol.2010.188367. [DOI] [PMC free article] [PubMed] [Google Scholar]