Abstract
We have previously shown that NKG2C+ NK cells from CMV naïve umbilical cord blood (UCB) grafts expand preferentially in recipients after CMV reactivation, representing a primary NK cell response after hematopoietic cell transplantation (HCT). In this study, recipients of adult donor HCT were assessed to evaluate the role of donor/recipient CMV serostatus on the expression and function of NKG2C+ NK cells in order to determine responses to secondary CMV events. Expansion of NKG2C+ NK cells was seen following clinical CMV reactivation. However, they also expanded in the absence of detectable CMV viremia when both the donor and recipient were CMV seropositive. Upregulation of NKG2C was observed in NK cells from CMV positive recipients receiving grafts from CMV seropositive or seronegative donors. These in vivo expanded NKG2C+ NK cells had an increased capacity for target cell induced cytokine production, expressed an inhibitory KIR for self HLA and preferentially acquired CD57. Most importantly, NKG2C+ NK cells transplanted from seropositive donors exhibit heightened function in response to a secondary CMV event compared to NKG2C+ NK cells from seronegative donors. We conclude that NKG2C+ memory-like NK cells are transplantable and require active or latent (subclinical) expression of CMV antigen in the recipient for clonal expansion of NK cells previously exposed to CMV in the donor.
Introduction
Natural killer cells, comprising approximately 10% of all circulating lymphocytes are important effectors in the elimination of virally infected and transformed cells. NK cells can potentially express a wide range of diverse receptors that transmit inhibitory or activating signals that ultimately regulate NK cell function(1, 2). Unlike B cells or T cells, NK cells do no express germline rearranged receptors and instead display a variety of receptors that are clonally distributed on NK cell subpopulations which may account for diverse NK cell functions. The best characterized NK-associated receptors include the killer immunoglobulin-like receptors (KIR) 3 and the C-type lectin-like families, of which both activating and inhibitory forms exist. Inhibitory KIR recognize allelic epitopes present on certain HLA-A, -B and –C alleles(3, 4), whereas ligands for activating KIR are less well characterized. The inhibitory C-type lectin-like receptor NKG2A recognizes the non-classical class I allele, HLA-E(5) and the activating receptor, NKG2C has also been shown to recognize HLA-E, albeit with lower affinity than its inhibitory counterpart(6). Using these receptors NK cells monitor changes in the expression of self-MHC class I associated with viral infection or transformation and lyse these cells, a phenomenon known as the ‘missing self’ hypothesis(7, 8).
NK cells have been shown to play a critical role in the host’s immune response to viral infections(9, 10). Infection with CMV, a herpes virus that remains latent in hosts for life, is usually asymptomatic, but can be a serious complication in solid organ- or hematopoietic cell transplantation recipients or for patients infected with HIV(11). CMV infection shapes the NK cell receptor repertoire resulting in an increase in NK cells expressing NKG2C(12). This increase in NKG2C+ NK cells persists throughout life, while in contrast, the proportions of NK cells expressing NKG2C remains low in individuals who have never encountered CMV. NK cells expressing NKG2C have also been shown to expand following co-culture with infected fibroblasts(13) and during CMV reactivation in recipients of solid organ(14) and umbilical cord blood (UCB) (15) transplantation. In addition, NKG2C+ cells expand in CMV-exposed individuals who experience acute infections with Hantavirus,(16) Chikungunya virus(17) or in those with HIV infection(18). Moreover, high percentages of NKG2C+ NK cells have been associated with lower viral loads and long term HIV persistence without progression to AIDS(19). The mechanism by which CMV drives the expression of an NKG2C expressing subpopulation is unknown, and in the context of CMV infection, the ligand for NKG2C remains elusive. NKG2C may recognize HLA-E, HLA-E loaded with a particular CMV peptide, or an unknown ligand of either viral or host origin.
We have reported that following CMV reactivation in recipients of CMV naïve UCB grafts, some of the reconstituting NK cells upregulate NKG2C cell surface density and expand and they persist long after viral clearance(15). These in vivo expanding NK cells lack NKG2A, express an inhibitory KIR specific for self HLA, are potent producers of IFNγ, and preferentially acquire CD57. Furthermore, recipients who reactivated CMV had increased IFNγ and T-bet mRNA transcripts. In this setting of “new” CMV infection of transplanted UCB donor graft cells seen in UCB transplantation, it is unclear what effect donor or recipient CMV serostatus has on the kinetics and function of NK cells in recipients of adult allogeneic HCT.
Methods and Materials
Patients and Samples
We analyzed PBMCs from 70 donor and recipient pairs including allogeneic transplants facilitated by the National Marrow Donor Program or at the University of Minnesota. Twenty-five patients with hematologic malignancies received unrelated adult donor (URD) HLA-matched unmanipulated (T-cell replete) bone marrow or peripheral blood stem cells (enrolled in the Blood and Marrow Transplant Clinical Trials Network [BMT CTN] Protocol 0201) using standardized cyclosporine or tacrolimus-based graft versus host disease (GvHD) prophylaxis [https://web.emmes.com/study/bmt2/protocol/0201_protocol.html]. Forty-five patients received unmanipulated sibling donor grafts using similar conventional GvHD prophylaxis.
Patients were routinely monitored for CMV reactivation by standard clinical testing at each center. Twenty-two patients developed detectable CMV in the blood 19 to 73 days after transplant. We collected pre-HCT donor samples and recipient samples at 3 months and 6 months and 1 year post-HCT. High resolution HLA typing was performed and NK ligand status was assigned based upon Bw4, HLA-C1 and HLA-C2 group ligands. Samples were obtained after informed consent and approval from the National Marrow Donor Program and University of Minnesota Institutional Review Boards.
PBMCs were isolated from each sample by density centrifugation and cryopreserved. Before analysis for production of intracellular cytokines, the thawed cells were incubated overnight at 37°C in complete media without exogenous cytokines (DMEM (Cellgro) supplemented with 20% human AB serum (Valley Biomedical), 30% Ham’s F-12 medium (Cellgro), 100 U/mL penicillin (Invitrogen), 100 U/mL streptomycin (Invitrogen), 24 μM 2-beta-mercaptaethanol, 50 μM ethanolamine, 20 mg/L ascorbic acid, and 50 μg/L sodium selanate).
Target Cells
The human erythroleukemia cell line K562 was maintained in IMDM (Invitrogen) supplemented with 10% FBS (Invitrogen) and 100 U/mL penicillin and 100 U/mL streptomycin (both Invitrogen).
Intracellular Production of IFNγ
Intracellular production of IFNγ was measured as previously reported(20). Briefly, PBMC were incubated in media alone or with K562 cells at an effector to target ratio of 2:1 for 5 hours. Brefeldin A (BD Biosciences) was added after 1 hour. The following antibodies were used: PeCy5.5-conjugated anti-CD158a (clone EB6, Beckman Coulter), APC-conjugated anti-CD158b (clone GL183, Beckman Coulter), Alexa Fluor 700-conjugated anti-KIR3DL1 (clone DX9, Biolegend), PE-conjugated anti-NKG2C (clone 134591, R&D Systems), APC-conjugated anti-NKG2A (clone z199, Beckman Coulter), PeCy7-conjugated anti-CD56 (clone HCD56, Biolegend), FITC-conjugated anti-CD57 (clone HNK-1, BD Biosciences), ECD-conjugated anti-CD3 (clone UCHT1, Beckman Coulter), APC-Cy7-conjugated anti-CD16 (clone 3G8, Biolegend) and Pacific Blue-conjugated anti-IFNγ (clone 4S.B3, Biolegend). Data was analysed on a LSRII 11-color flow cytometer (BD Biosciences) and with FlowJo 9.3.2 software (TreeStar). Gating strategies are outlined in supplemental Figure 1.
Statistical Analysis
Data were summarized with mean and standard error (mean ± SEM). For comparisons between independent samples, Student’s T-test was used. For comparisons of matched samples, paired T-test was used. Statistical significance was indicated as NS: P>0.05; *P≤0.05; **P<0.01; ***P<0.001. Statistical analyses were performed using SAS version 9.2 (SAS Institute, Cary, NC).
Results
Expansion of NKG2C+ NK cells from CMV seropositive donors
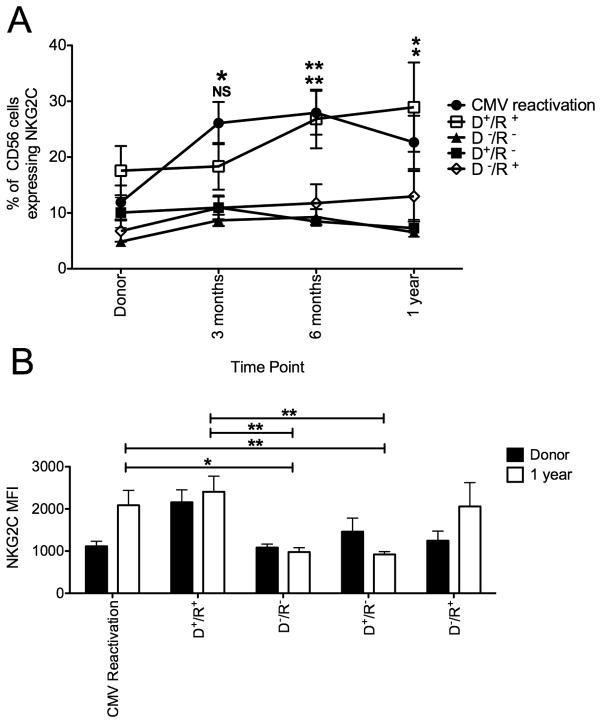
NKG2C expression was measured on PBMC isolated from 70 donor/recipient pairs who received transplants from either adult URD or sibling fully HLA-matched donors (Figure 1A). Each transplant was divided based on whether recipient CMV reactivation occurred post transplant (n=22) regardless of the donor CMV serostatus. Those that did not reactivate CMV (n=48) were stratified based on the donor and recipient CMV serostatus determined pre-transplant (e.g. CMV seropositive donor/CMV seropositive recipient [D+/R+]). There was minimal expansion of NKG2C expressing NK cells in donor/recipient pairs who were CMV seronegative (n=12). In recipients who reactivated CMV, NK cells expressing NKG2C increased significantly at 3 months and 6 months post transplant compared with the pre-HCT donor sample (3 months: 25%±4% v 12%±3%, p=0.005; 6 months: 28%±4% v 12%±3%, p=0.002) and were significantly higher than the percentage of NKG2C+ NK cells in D−/R− pairs at 1 year post transplant (CMV reactivation recipients: 23%±5% v D−/R−: 6%±0.7%, p=0.01). Beyond 6 months the percentages of NKG2C+ NK cells did not increase further. NKG2C cell surface density measured as median fluorescence intensity (MFI) on gated NKG2C+ NK cells also increased over time following CMV reactivation and by 1 year post transplant was significantly higher compared to D−/R− pairs (2088±352 v 978±102, p=0.01) (Figure 1B).
Figure 1. Receptor density and percentage of NKG2C+ NK cells increases following CMV reactivation or in the presence of recipient CMV antigen after allogeneic HSCT.
(A) NKG2C expression was measured on CD56+ CD3− NK cells from donor/recipient pairs who reactivated CMV (closed circles, n=22). The remaining donor (D)/recipient ® pairs did not reactivate CMV and were stratified by CMV serostatus established prior to transplantation ([D+/R+ open squares, n=18], [D−/R− closed triangles, n=12], [D+/R− closed squares, n=8] and [D−/R+ open diamonds, n=10]). Recipient samples were analyzed at 3 months, 6 months and 1 year post transplant. Points represent the mean ± SEM. D+/R+ pairs were compared with D−/R− and D+/R− using the student’s T test. Statistical significance is indicated as NS: P>0.05; *P≤0.05; **P<0.01. (B) NKG2C surface density was measured on PBMC from the donor (black bars) and recipient (white bars) at 1 year post transplant from donor/recipient pairs who reactivated CMV and those with no detectable CMV viremia (D+/R+, D−/R−, D+/R− and D−/R+). Bars represent the mean ± SEM. Recipients at 1 year post transplant were compared using the Student’s T test. Statistical significance is indicated as *P≤0.05; **P<0.01.
The frequency of NK cells expressing NKG2C also expanded when both the donor and recipient were CMV seropositive (D+/R+, n=18) but in the absence of detectable CMV viremia. This expansion did not occur in D+/R− pairs (n=8), and indeed the percentage of NK cells expressing NKG2C gradually declined in this group during the first year after transplant compared to recipients who reactivated CMV (7%±2% v 23%±5%, p=0.01) or in D+/R+ transplant pairs (7%±2% v 29%±8%, p=0.03). By 1 year post-transplant, there was a significantly higher proportion of NKG2C+ NK cells in D+/R+ transplants compared with and D−/R− (29%±8% v 6%±0.7%, p=0.02) or D+/R− transplants suggesting that post-transplant NKG2C+ NK cells from CMV seropositive donors continue to persist and expand in CMV seropositive recipients in the absence of clinically detectable CMV viremia. A modest increase in NKG2C cell surface density was observed over time in this group and was significantly higher compared to D−/R− pairs (2405±370 v 978±102, p=0.0098) at 1 year post transplant.
At 1 year post transplant compared to D−/R− pairs, cell surface density of NKG2C was higher in recipients who reactivated CMV (920±67 v 2088±352, p=0.01) or who did not reactivate but were in the D+/R+ transplant group (920±67 v 2405±370, p=0.008). This suggests that previous CMV exposure in the recipient contributes to the expansion of these cells post-transplant and that latent CMV antigen is sufficient to provide low level chronic stimulation to maintain expansion and a higher surface density of NKG2C. In D−/R+ pairs (n=10) there was a modest, but gradual increase in the percentage of NK cells expressing NKG2C, which did not differ significantly between D−/R− and D+/R− pairs and probably represents a weak primary NK cell response to low level CMV. However, cell surface density did increase over time and by 1 year post transplant was comparable (2059±564) with both recipients who reactivated CMV and D+/R+ pairs indicating that latent recipient CMV antigen does result in the upregulation of the receptor, even in cells derived from CMV seronegative donors.
Additionally, not only was an increase in both the percentage of NK cells expressing NKG2C and cell surface density observed following CMV reactivation, absolute counts of NKG2C+ NK cells also increased (Figure 2). At 6 months post-HCT, recipients who reactivated CMV or in CMV+ recipients there were significantly more absolute NKG2C+ NK cells compared with CMV seronegative recipients. A higher proportion of NKG2C+ NK cells was also observed at 1 year post-transplant, therefore in both recipients who reactivated CMV and seropositive recipients in the absence of detectable CMV viremia, NK cells expressing NKG2C expand and upregulate cell surface density.
Figure 2. Absolute NKG2C+ NK cells increase following CMV reactivation.
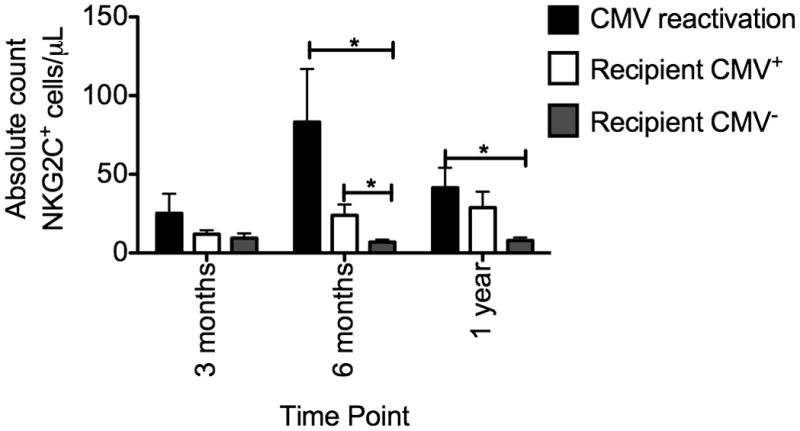
NKG2C expression was measured on CD56+ CD3− NK cells from recipients who reactivated CMV (black bars), recipients who were CMV seropositive (white bars) and recipients who were CMV seronegative (grey bars) and the absolute number of NKG2C+ NK cells/μL was calculated. Bars represent the mean ± SEM. Each group was compared using the student’s T test. Statistical significance is indicated as *P≤0.05; **P<0.01.
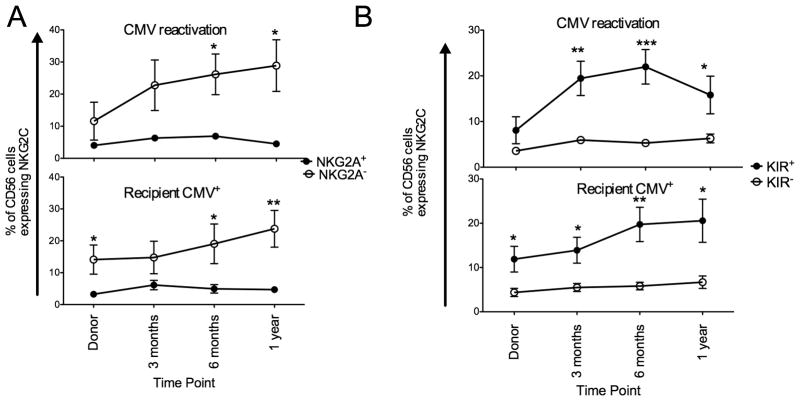
Expanding NKG2C+ NK cells lack NKG2A and express a KIR specific for self
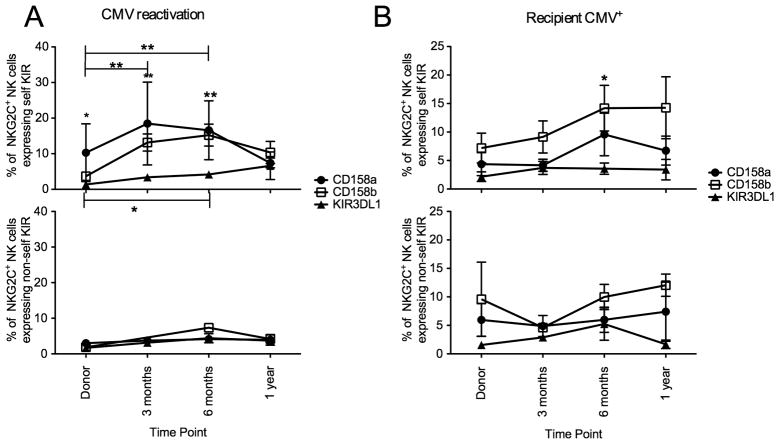
As NKG2C+ NK cells expand and upregulate surface expression following CMV viremia and in the presence of latent CMV antigen we were next interested in the expression of KIR and NKG2A on these expanding cells. We examined NKG2A and KIR (based on a cocktail of anti-CD158a/h, CD158b/j and CD158e) expression in both groups (Figure 3). Both in patients with CMV reactivation and in CMV seropositive recipients, expanding NKG2C+ NK cells lacked NKG2A and there was no change in the proportion of NKG2C+ NK cells co-expressing NKG2A over time (Figure 3A). Expanding NKG2C+ NK cells also expressed KIR, both in CMV reactivation and in recipients who were CMV seropositive with no change in the proportion of NKG2C+ NK cells lacking KIR. As all expanding NKG2C+ NK cells express KIR we were interested if individual KIR were equally represented and if there was a preference for KIR that recognized self HLA which would imply that the principals of NK licensing(21) applied under these circumstances. NKG2C+ NK cells expressing CD158a, CD158b or KIR3DL1 were divided into two groups based on whether each KIR recognized self HLA (Figure 4). Both following CMV reactivation and in CMV positive recipients, expanding NKG2C+ NK cells preferentially expressed either self-CD158a or CD158b and the proportion of NKG2C+ NK cells expressing self-KIR3DL1 was stable during the first year after transplant.
Figure 3. Expanding NKG2C+ NK cells express KIR and lack NKG2A.
(A) NKG2C+ NK cells from donor/recipients pairs who reactivated CMV (upper panel) or from donor/recipient pairs where the recipient was CMV seropositive (lower panel) were gated as being either NKG2A+ (closed circles) or NKG2A− (open circles). Points represent the mean ± SEM. NKG2A+ and NKG2A− NK cells were compared using the paired T test. Statistical significance is indicated as *P≤0.05; **P<0.01; ***P<0.001. (B) NKG2C+ NK cells from donor/recipients pairs who reactivated CMV (upper panel) or from donor/recipient pairs where the recipient was CMV seropositive (lower panel) were gated as being either KIR+ (closed circles) or KIR− (open circles) based on a cocktail of anti-CD158a, anit-CD158b and anti-KIR3DL. Points represent the mean ± SEM. KIR+ and KIR− NK cells were compared using the paired T test. Statistical significance is indicated as *P≤0.05; **P<0.01; ***P<0.001.
Figure 4. Expanding NKG2C+ NK cells preferentially express KIR for self.
NKG2C+ NK cells from donor/recipients pairs who reactivated CMV (A) or from donor/recipient pairs where the recipient was CMV seropositive (B) were stained with CD158a, CD158b and KIR3DL1 and divided into self KIR (upper panel) and non-self KIR (lower panel) based on donor/recipient HLA class I. Points represent the mean ± SEM. NKG2C+ NK cells expressing self CD158b and KIR3DL1 were compared at each time point and across time points using the student’s T test. Statistical significance is indicated as *P≤0.05; **P<0.01.
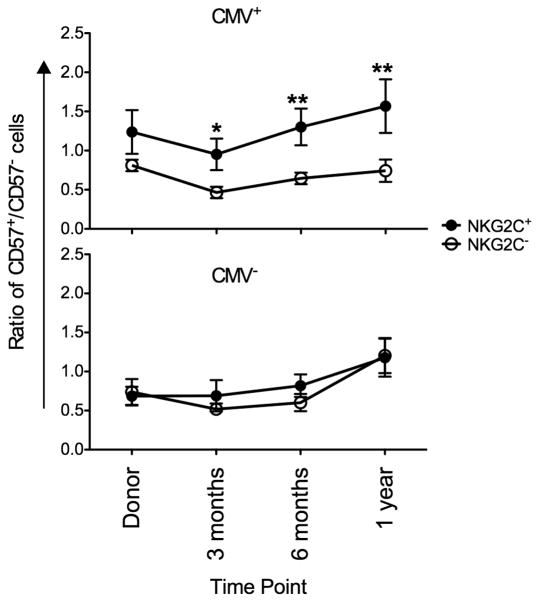
Preferential acquisition of CD57 in the presence of CMV antigen
Following CMV reactivation in recipients of solid organ(14) and UCB(15) transplants, expanding NKG2C+ NK cells preferentially acquired CD57 over time, potentially representing a memory-like population of human NK cells. Furthermore, NKG2C+ NK cells frequently co-express CD57 in healthy CMV seropositive donors(14). CD57 expression is acquired by NK cells over time and represents a marker of NK cell maturity regardless of CMV viremia. We therefore compared the ratio of CD57+ NKG2C+ to CD57+NKG2C− NK cells during the first year after HCT. In the presence of CMV antigen (either through CMV reactivation or presumed low level viremia associated with latency in seropositive recipients) NKG2C+ NK cells preferentially acquired CD57 expression over time whereas no difference was observed in recipients who were CMV seronegative (Figure 5).
Figure 5. NKG2C+ preferential acquire CD57 in the presence of recipient CMV antigen or CMV reactivation.
CD57 co-expression was measured on CD56+ CD3− NKG2C+ (closed circles) and NKG2C− (open squares) NK cells from donor/recipients pairs who reactivated CMV combined with donor/recipient pairs where the recipient was CMV seropositive (upper panel) and with donor/recipient pairs where the recipient was CMV seronegative (lower panel). The ratio of CD57+/CD57− was plotted at the indicated time points. Points represent the mean ± SEM. NKG2C+ cells were compared with NKG2C− NK cells using the paired T test. Statistical significance is indicated as *P≤0.05; **P<0.01.
Enhanced capacity of target cell induced IFNγ production in the presence of CMV antigen
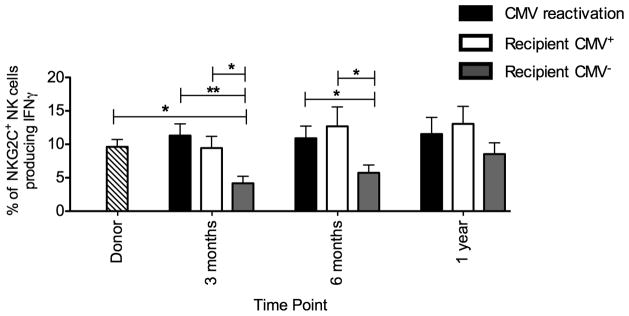
In recipients of HCT, target-cell induced IFNγ production is low early after transplant(20). PBMC from donor/recipient pairs who reactivated CMV, from CMV positive recipients and from CMV seronegative recipients were incubated with the class I negative cell line K562 to determine potential capacity to produce IFNγ (Figure 6). Both at 3 and 6 months post-transplant, significantly more IFNγ producing NKG2C+ NK cells were detected in recipients who either reactivated CMV or those who were CMV seropositive compared with CMV seronegative recipients. Furthermore, post-transplant IFNγ production was similar to the combined group of normal donors if the recipients reactivated CMV or were CMV seropositive. However, in the absence of documented CMV antigenemia in the recipient, NKG2C+ NK cell IFNγ production was substantially decreased compared with donors (3 months: 4%±1% v 10%±1%, p=0.01). This decrease was not specific to NKG2C+ NK cells, as total NK cell IFNγ production was significantly decreased in CMV seronegative recipients early after transplant compared with donor NK cells at both 3 months (4%±1% v 9%±1%, p=0.0052) and 6 months (5%±2% v 9%±1%, p=0.04). Collectively, these data demonstrate that exposure to recipient CMV antigen post-transplant, both in the context of CMV reactivation and latency, shapes NK cell immune responses to favor robust IFNγ production.
Figure 6. Capacity to produce IFNγ is increased in the presence of recipient CMV antigen.
PBMC from donors (all combined-stripped bar) and recipients who reactivated CMV (black bars), from donor/recipient pairs where the recipient was CMV seropositive (white bars) and from donor/recipient pairs where the recipient was CMV seronegative (grey bars) were incubated in either media alone (not shown) or with the class I negative cell line K562 for 5 hours. Intracellular production of IFNγ was measured on NKG2C+ NK cells. Bars represent the mean ± SEM. Each group was compared using the student’s T test. Statistical significance is indicated as *P≤0.05; **P<0.01.
Transfer of donor NKG2C+ NK results in memory-like function after a secondary CMV exposure in the recipient
Expansion of educated NKG2C+ NK cells following CMV reactivation with potent function and their continued persistence in the presence of antigen suggest that NK cells may exhibit memory-like properties, a characteristic usually restricted to adaptive immune responses. To address this, we compared the kinetics of NKG2C+ NK cell expansion and function following CMV reactivation based on donor CMV serostatus (Figure 7). While the proportion of NK cells expressing NKG2C was higher in recipients who received a seropositive donor graft, the kinetics of NKG2C+ NK cell expansion was similar between the two groups. NKG2C+ NK cells from seropositive donors had a significantly higher capacity for target cell induced IFNγ compared with seronegative donors (16%±3% v 8%±2%, p=0.04). This higher capacity to produce IFNγ was maintained post transplant and at 1 year, NKG2C+ NK cells from a seropositive donor graft produced significantly more IFNγ (20%±1% v 9%±2%, p=0.008). These results suggest that NKG2C+ NK cells transplanted from a seropositive donor expand and maintain their high capacity to produce IFNγ post-transplant and exhibit heightened function in response to a secondary CMV reactivation (the first event being in the donor) compared to NKG2C+ NK cells from seronegative donors experiencing primary CMV viremia, where the NK cell response is the first exposure.
Figure 7. Following CMV reactivation NKG2C+ NK cells from CMV seropositive donors have increased capacity to produce IFNγ.
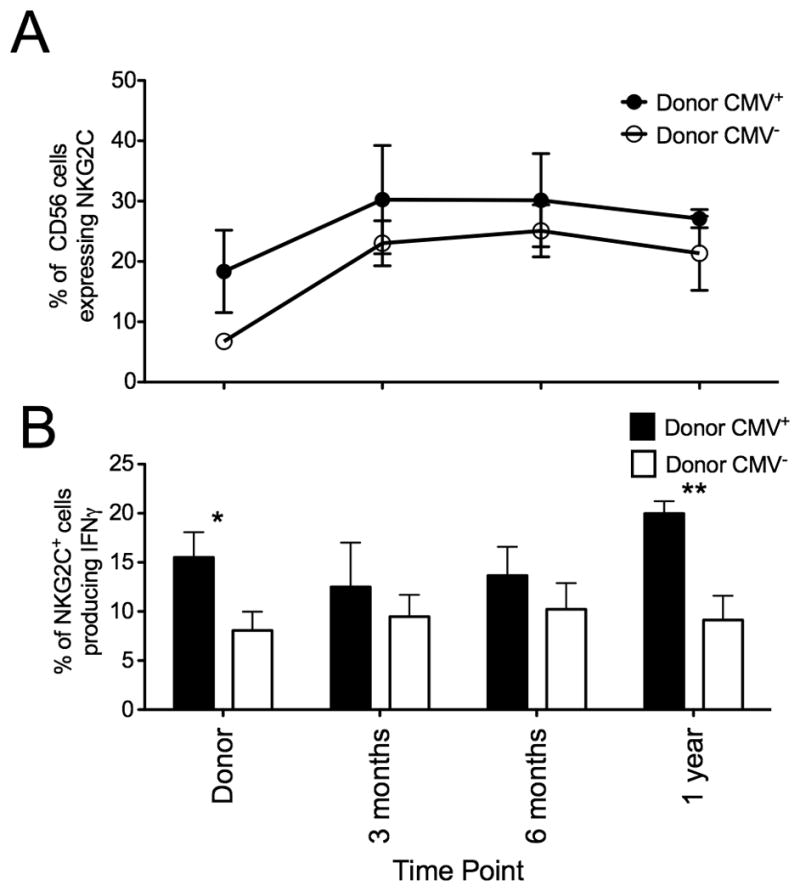
(A) NKG2C expression was measured on donor/recipient pairs who reactivated CMV were divided based on donor CMV serostatus (Donor CMV+ closed circles, n=6; Donor CMV− open circles, n=12). Points represent the mean ± SEM. (B) Intracellular IFNγ production was measured on NKG2C+ NK cells from donor/recipient pairs who reactivated CMV divided based on donor CMV serostatus (Donor CMV+ black bars; Donor CMV− white bars) after 5 hours incubation with K562 cells. Bars represent the mean ± SEM. CMV+ donors were compared with CMV− donors using the student’s T test. Statistical significance is indicated as *P≤0.05; **P<0.01.
Discussion
NKG2C+ NK cells expressing an inhibitory KIR for self preferentially expand following CMV reactivation in recipients of adult donor allogeneic HCT. These NK cells have a high capacity for target cell induced IFNγ production and preferentially acquire CD57 over time. NKG2C+ NK cells also expand in the absence of clinically detectable CMV viremia if both the donor and recipient were CMV seropositive. In contrast, NKG2C+ cells do not account for a significant fraction of NK cells if they are transferred from a CMV seropositive donor to CMV seronegative recipient. Collectively, these results demonstrate for the first time that in recipients of allogeneic HCT, latent CMV antigen is required for clonal expansion and maintenance of memory-like NKG2C+ NK cells.
The receptor profile, function and kinetics of NKG2C+ NK cells following CMV reactivation in recipients of adult grafts is similar to recipients of UCB grafts with a few important exceptions. We reported that NKG2C+ NK cells continued to persist in recipients of UCB HCT long after viral clearance(15). In contrast, in this study using adult donor grafts, high proportions of NK cells expressing NKG2C peaked early. This is reminiscent of the response of Ly49H+ NK cells that expand in mice infected with murine CMV(22) and those that respond after adoptive transfer and secondary CMV exposure. Since UCB is considered CMV naïve, CMV reactivation represents a primary infection from the perspective of the donor immune response. In contrast, following transplantation using adult grafts containing mature NK cells from CMV seropositive donors, post-transplant CMV reactivation represents a secondary expansion of CMV primed NK memory-like cells. Despite the differences in kinetics between UCB and adult donor HCT recipients, expanding NKG2C+ NK cells always lacked NKG2A and all expressed KIR. This KIR was specific for a self HLA, either C1 or C2, and there was very little expansion of cells expressing KIR3DL1 in Bw4-expressing recipients. This oligoclonal-like expansion of NK cells expressing an inhibitory receptor for self HLA-C has also been observed during acute infection with Hantavirus(16) and Chikungunya virus(17) with preferential expansion of KIR recognizing C1 (KIR2DL2 and KIR2DL3). Similar to what was observed in healthy CMV seropositive individuals(14), a higher proportion of NKG2C+ NK cells express CD57, a marker of terminally differentiated NK cells, than NKG2C− NK cells following CMV reactivation. The preferential acquisition of CD57 on NK cells expressing NKG2C following CMV reactivation suggests that NK cell activation through exposure to viral antigen may drive NK cell differentiation. Overall target cell induced IFNγ production was increased significantly following CMV reactivation compared with seronegative recipients and in the absence of CMV reactivation, CMV seropositive recipients had a higher capacity for target cell induced IFNγ production, regardless of donor serostatus. This suggests that latent CMV antigen or low level chronic activation results in the clonal expansion and persistence of NKG2C+ NK cells that produce IFNγ. These cells persist for up to a year clearly demonstrating that at least some NK cells are long-lived.
NKG2C expression is uniquely associated with CMV and CMV is the only virus known to date that shapes the human NK cell receptor repertoire(12). Not only does acute CMV infection result in the expansion of NK cells expressing NKG2C, it also induces an up regulation in cell surface density of the receptor. Co-culture with infected fibroblasts also results in the expansion of NKG2C+ NK cells(13). Our results demonstrate for the first time that NKG2C+ NK cells from CMV seropositive donors also expand post transplant in the absence of clinically detectable CMV viremia, but only when the recipient was also CMV seropositive and there was a source of latent antigen. Furthermore, recipient CMV antigen was required to induce the up regulation of NKG2C on NK cells from seronegative donors. These findings suggest that CMV is required to maintain NKG2C expression and in the absence of CMV, NK cells may down regulate their expression of NKG2C and/or fail to expand.
How CMV is involved in regulating NKG2C expression still remains unknown. HLA-E is the cognate ligand for NKG2C and while CMV infection results in the down regulation of class I HLA, HLA-E usually remains intact on the cell surface(23, 24). NKG2C+ NK cells have also been shown to expand in culture with cell lines transfected with HLA-E and IL-15(16), yet no direct correlation between CMV, HLA-E and NKG2C has been demonstrated. Infected cells may express HLA-E loaded with a viral peptide that drives NKG2C expansion or alternatively, CMV infected cells may encode a viral protein that binds to NKG2C. CMV remains latent in the host for life and chronic low-level levels of CMV may continually stimulate NK cells to express NKG2C. Alternatively, latently infected cells may constitutively express a ligand or present a viral peptide that regulates NKG2C expression. All of these possibilities warrant further study.
During acute CMV infection in mice, NK cells expressing the activating receptor Ly49H preferentially expand and respond more rapidly against subsequent challenges with CMV compared with naïve Ly49H+ NK cells(22). These findings suggest that NK cells are capable of immune memory, akin to memory CD8+ T cells. We find that NK cells expressing NKG2C may represent memory-like NK cells in humans. While primary CMV infection increases the capacity for cytokine production, which is maintained long after viral clearance, subsequent exposure to CMV further increases this capacity. This suggests that NKG2C+ NK cells have memory-like properties which retain their phenotype and function after transplantation into CMV naïve or seropositive recipients.
Although we see an expansion in both the percentage of NK cells expressing NKG2C and the absolute number of these cells, it still remains unclear what role these cells play in controlling CMV reactivation and viremia as CMV reactivation remains a common occurrence following HSCT. However, while not specifically investigating NK cell responses, Zhou and colleagues (25) demonstrated that D+/R+ transplants required less antiviral therapy then D−/R+ transplants which correlated with a more robust CD8+ T cell response. In addition, patients with chronic lymphocytic leukemia treated with the NK and T cell pan-lymphodepleting antibody Alemtuzumab (anti-CD52) develop an unexpected CMV reactivation (26, 27). These findings suggest that the immune response can modulate CMV but other factors such as antiviral therapy need to be taken into consideration. It is quite possible that perhaps these NKG2C+ NK cells do not necessarily play an important role in controlling CMV replication in the blood (which is what is routinely measured to determine CMV reactivation) but play a larger role in controlling the CMV disease in peripheral tissues that can be lethal. Further studies investigating the role of NK cell responses in recurrent CMV reactivation and in CMV disease are certainly warranted.
As the first cells to reconstitute following HCT, NK cells play a critical role in mediating the graft versus leukemia effect. However, NK cell functional responses are diminished early after transplant across all platforms of allogeneic HCT, with the greatest defects seen in target cell induced IFNγ production(20). IFNγ has been shown to play a critical role in tumor suppression(28–30) and in the control of viral infections(31). Therefore strategies to enhance NK cell function post HCT could be therapeutically advantageous. We have demonstrated that transfer of mature NK cells in the graft exhibit memory-like properties capable of enhanced responsiveness to subsequent CMV challenge. This CMV challenge is seen with high viral loads as seen with CMV viremia but also the low antigen load that persists in recipients with latent CMV. In contrast, high viral loads are needed to induce an NK cell response from a CMV negative donor. Using a unique cohort of allogeneic transplant samples, we have been able to show innate NK cell memory for the first time based on a secondary challenge in the recipient, the primary infection occurring in the adult transplant donor at some distant time from transplantation. We suggest that the term memory be reserved for cells that clonally expand upon secondary challenge with a minimum of at least one enhanced function, in our case IFNγ, and that human NK cells exhibit long-term memory for potent immunologic viruses like CMV. Selecting donor and recipient pairs, which can allow transplantation of highly differentiated potent and functionally educated NK cells may be a beneficial strategy to increase the anti-infective and GvL effect after allogeneic HCT, a strategy that will require clinical testing.
Supplementary Material
Acknowledgments
We acknowledge sample procurement and cell processing services from the Translational Therapy Core. We would also like to acknowledge the protocol committee and participants for collection of samples on the CTN 0201 clinical transplant study.
Footnotes
This work was supported in part by the National Institutes of Health Grant P01-CA65493 and P01-CA111412, P30-CA77598, the National Marrow Donor Program and Minnesota Masonic Charities. Support for the CTN study was provided by grant #U10HL069294 from the National Heart, Lung, and Blood Institute and the National Cancer Institute.
Abbreviations: KIR, Killer immunoglobulin-like receptor; HCT, hematopoietic cell transplantation; UCB, umbilical cord blood; URD, unrelated adult donor; GvHD, graft versus host disease
References
- 1.Bryceson YT, March ME, Ljunggren HG, Long EO. Activation, coactivation, and costimulation of resting human natural killer cells. Immunol Rev. 2006;214:73–91. doi: 10.1111/j.1600-065X.2006.00457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lanier LL. Up on the tightrope: natural killer cell activation and inhibition. Nat Immunol. 2008;9:495–502. doi: 10.1038/ni1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moretta A, Bottino C, Vitale M, Pende D, Biassoni R, Mingari MC, Moretta L. Receptors for HLA class-I molecules in human natural killer cells. Annu Rev Immunol. 1996;14:619–648. doi: 10.1146/annurev.immunol.14.1.619. [DOI] [PubMed] [Google Scholar]
- 4.Lanier LL. NK cell receptors. Annu Rev Immunol. 1998;16:359–393. doi: 10.1146/annurev.immunol.16.1.359. [DOI] [PubMed] [Google Scholar]
- 5.Braud VM, Allan DS, O’Callaghan CA, Soderstrom K, D’Andrea A, Ogg GS, Lazetic S, Young NT, Bell JI, Phillips JH, Lanier LL, McMichael AJ. HLA-E binds to natural killer cell receptors CD94/NKG2A, B and C. Nature. 1998;391:795–799. doi: 10.1038/35869. [DOI] [PubMed] [Google Scholar]
- 6.Vales-Gomez M, Reyburn HT, Erskine RA, Lopez-Botet M, Strominger JL. Kinetics and peptide dependency of the binding of the inhibitory NK receptor CD94/NKG2-A and the activating receptor CD94/NKG2-C to HLA-E. EMBO J. 1999;18:4250–4260. doi: 10.1093/emboj/18.15.4250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Karre K, Ljunggren HG, Piontek G, Kiessling R. Selective rejection of H-2-deficient lymphoma variants suggests alternative immune defence strategy. Nature. 1986;319:675–678. doi: 10.1038/319675a0. [DOI] [PubMed] [Google Scholar]
- 8.Ljunggren HG, Karre K. In search of the ‘missing self’: MHC molecules and NK cell recognition. Immunol Today. 1990;11:237–244. doi: 10.1016/0167-5699(90)90097-s. [DOI] [PubMed] [Google Scholar]
- 9.Biron CA, Byron KS, Sullivan JL. Severe herpesvirus infections in an adolescent without natural killer cells. N Engl J Med. 1989;320:1731–1735. doi: 10.1056/NEJM198906293202605. [DOI] [PubMed] [Google Scholar]
- 10.Orange JS. Human natural killer cell deficiencies and susceptibility to infection. Microbes Infect. 2002;4:1545–1558. doi: 10.1016/s1286-4579(02)00038-2. [DOI] [PubMed] [Google Scholar]
- 11.Crough T, Khanna R. Immunobiology of human cytomegalovirus: from bench to bedside. Clin Microbiol Rev. 2009;22:76–98. doi: 10.1128/CMR.00034-08. Table of Contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guma M, Angulo A, Vilches C, Gomez-Lozano N, Malats N, Lopez-Botet M. Imprint of human cytomegalovirus infection on the NK cell receptor repertoire. Blood. 2004;104:3664–3671. doi: 10.1182/blood-2004-05-2058. [DOI] [PubMed] [Google Scholar]
- 13.Guma M, Budt M, Saez A, Brckalo T, Hengel H, Angulo A, Lopez-Botet M. Expansion of CD94/NKG2C+ NK cells in response to human cytomegalovirus-infected fibroblasts. Blood. 2006;107:3624–3631. doi: 10.1182/blood-2005-09-3682. [DOI] [PubMed] [Google Scholar]
- 14.Lopez-Verges S, Milush JM, Schwartz BS, Pando MJ, Jarjoura J, York VA, Houchins JP, Miller S, Kang SM, Norris PJ, Nixon DF, Lanier LL. Expansion of a unique CD57+NKG2Chi natural killer cell subset during acute human cytomegalovirus infection. Proc Natl Acad Sci U S A. 2011;108:14725–14732. doi: 10.1073/pnas.1110900108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Foley B, Cooley S, Verneris MR, Pitt M, Curtsinger J, Luo X, Lopez-Verges S, Lanier LL, Weisdorf D, Miller JS. Cytomegalovirus reactivation after allogeneic transplantation promotes a lasting increase in educated NKG2C+ natural killer cells with potent function. Blood. 2012;119:2665–2674. doi: 10.1182/blood-2011-10-386995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bjorkstrom NK, Lindgren T, Stoltz M, Fauriat C, Braun M, Evander M, Michaelsson J, Malmberg KJ, Klingstrom J, Ahlm C, Ljunggren HG. Rapid expansion and long-term persistence of elevated NK cell numbers in humans infected with hantavirus. J Exp Med. 2011;208:13–21. doi: 10.1084/jem.20100762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Petitdemange C, Becquart P, Wauquier N, Beziat V, Debre P, Leroy EM, Vieillard V. Unconventional Repertoire Profile Is Imprinted during Acute Chikungunya Infection for Natural Killer Cells Polarization toward Cytotoxicity. PLoS Pathog. 2011;7:e1002268. doi: 10.1371/journal.ppat.1002268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brunetta E, Fogli M, Varchetta S, Bozzo L, Hudspeth KL, Marcenaro E, Moretta A, Mavilio D. Chronic HIV-1 viremia reverses NKG2A/NKG2C ratio on natural killer cells in patients with human cytomegalovirus co-infection. AIDS. 2010;24:27–34. doi: 10.1097/QAD.0b013e3283328d1f. [DOI] [PubMed] [Google Scholar]
- 19.Thomas R, Low HZ, Kniesch K, Jacobs R, Schmidt RE, Witte T. NKG2C Deletion Is a Risk Factor of HIV Infection. AIDS Res Hum Retroviruses. 2012 doi: 10.1089/aid.2011.0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Foley B, Cooley S, Verneris MR, Curtsinger J, Luo X, Waller EK, Weisdorf DJ, Miller JS. NK cell education after allogeneic transplantation: dissociation between recovery of cytokine-producing and cytotoxic functions. Blood. 2011;118:2784–2792. doi: 10.1182/blood-2011-04-347070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim S, Poursine-Laurent J, Truscott SM, Lybarger L, Song YJ, Yang L, French AR, Sunwoo JB, Lemieux S, Hansen TH, Yokoyama WM. Licensing of natural killer cells by host major histocompatibility complex class I molecules. Nature. 2005;436:709–713. doi: 10.1038/nature03847. [DOI] [PubMed] [Google Scholar]
- 22.Sun JC, Beilke JN, Lanier LL. Adaptive immune features of natural killer cells. Nature. 2009;457:557–561. doi: 10.1038/nature07665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tomasec P, V, Braud M, Rickards C, Powell MB, McSharry BP, Gadola S, Cerundolo V, Borysiewicz LK, McMichael AJ, Wilkinson GW. Surface expression of HLA-E, an inhibitor of natural killer cells, enhanced by human cytomegalovirus gpUL40. Science. 2000;287:1031. doi: 10.1126/science.287.5455.1031. [DOI] [PubMed] [Google Scholar]
- 24.Llano M, Guma M, Ortega M, Angulo A, Lopez-Botet M. Differential effects of US2, US6 and US11 human cytomegalovirus proteins on HLA class Ia and HLA-E expression: impact on target susceptibility to NK cell subsets. Eur J Immunol. 2003;33:2744–2754. doi: 10.1002/eji.200324182. [DOI] [PubMed] [Google Scholar]
- 25.Zhou W, Longmate J, Lacey SF, Palmer JM, Gallez-Hawkins G, Thao L, Spielberger R, Nakamura R, Forman SJ, Zaia JA, Diamond DJ. Impact of donor CMV status on viral infection and reconstitution of multifunction CMV-specific T cells in CMV-positive transplant recipients. Blood. 2009;113:6465–6476. doi: 10.1182/blood-2009-02-203307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lundin J, Kimby E, Bjorkholm M, Broliden PA, Celsing F, Hjalmar V, Mollgard L, Rebello P, Hale G, Waldmann H, Mellstedt H, Osterborg A. Phase II trial of subcutaneous anti-CD52 monoclonal antibody alemtuzumab (Campath-1H) as first-line treatment for patients with B-cell chronic lymphocytic leukemia (B-CLL) Blood. 2002;100:768–773. doi: 10.1182/blood-2002-01-0159. [DOI] [PubMed] [Google Scholar]
- 27.Hillmen P, Skotnicki AB, Robak T, Jaksic B, Dmoszynska A, Wu J, Sirard C, Mayer J. Alemtuzumab compared with chlorambucil as first-line therapy for chronic lymphocytic leukemia. J Clin Oncol. 2007;25:5616–5623. doi: 10.1200/JCO.2007.12.9098. [DOI] [PubMed] [Google Scholar]
- 28.Ikeda H, Old LJ, Schreiber RD. The roles of IFN gamma in protection against tumor development and cancer immunoediting. Cytokine Growth Factor Rev. 2002;13:95–109. doi: 10.1016/s1359-6101(01)00038-7. [DOI] [PubMed] [Google Scholar]
- 29.Shankaran V, Ikeda H, Bruce AT, White JM, Swanson PE, Old LJ, Schreiber RD. IFNgamma and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature. 2001;410:1107–1111. doi: 10.1038/35074122. [DOI] [PubMed] [Google Scholar]
- 30.Smyth MJ, Hayakawa Y, Takeda K, Yagita H. New aspects of natural-killer-cell surveillance and therapy of cancer. Nat Rev Cancer. 2002;2:850–861. doi: 10.1038/nrc928. [DOI] [PubMed] [Google Scholar]
- 31.Biron CA, Nguyen KB, Pien GC, Cousens LP, Salazar-Mather TP. Natural killer cells in antiviral defense: function and regulation by innate cytokines. Annu Rev Immunol. 1999;17:189–220. doi: 10.1146/annurev.immunol.17.1.189. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.