Abstract
Group III metabotropic glutamate receptors (mGluR4,7,8) are widely distributed in the basal ganglia. Injection of group III mGluR agonists into the striatopallidal complex alleviates parkinsonian symptoms in 6-hydroxydopamine-treated rats. In vitro rodent studies have suggested that this may be partly due to modulation of synaptic transmission at striatopallidal and corticostriatal synapses through mGluR4 activation. However, the in vivo electrophysiological effects of group III mGluRs activation upon basal ganglia neurons activity in nonhuman primates remain unknown. Thus, in order to examine the anatomical substrates and physiological effects of group III mGluRs activation upon striatal and pallidal neurons in monkeys, we used electron microscopy immunohistochemistry to localize mGluR4, combined with local administration of the group III mGluR agonist L-AP4, or the mGluR4 positive allosteric modulator VU0155041, to assess the effects of group III mGluR activation on the firing rate and pattern of striatal and pallidal neurons in 1-methyl 4-phenyl 1,2,3,6-tetrahydropyridine (MPTP)-treated parkinsonian monkeys.
At the ultrastructural level, striatal mGluR4 immunoreactivity was localized in pre- (60%) and post-synaptic (30%) elements, while in the GPe, mGluR4 was mainly expressed presynaptically (90%). In the putamen, terminals expressing mGluR4 were evenly split between putative excitatory and inhibitory terminals, while in the GPe, most labeled terminals displayed the ultrastructural features of striatal-like inhibitory terminals, though putative excitatory boutons were also labeled. No significant difference was found between normal and parkinsonian monkeys. Extracellular recordings in awake MPTP-treated monkeys revealed that local microinjections of small volumes of L-AP4 resulted in increased firing rates in one half of striatal cells and one third of pallidal cells, while a significant number of neurons in both structures showed either opposite effects, or did not display any significant rate changes following L-AP4 application. VU0155041 administration had little effect on firing rates. Both compounds also had subtle effects on bursting and oscillatory properties, acting to increase the irregularity of firing. The occurrence of pauses in firing was reduced in the majority (80%) of GPe neurons after L-AP4 injection. Our findings indicate that glutamate can mediate multifarious physiological effects upon striatal and pallidal neurons through activation of pre-synaptic group III mGluRs at inhibitory and excitatory synapses in parkinsonian monkeys.
1. Introduction
Drugs that antagonize glutamate transmission have been of interest for years as potential treatments for Parkinson’s disease (PD), as they may reverse some of the major pathophysiologic hallmarks of the disease, specifically the increased glutamatergic corticostriatal and subthalamofugal transmission (Blandini et al., 1996; Chase et al., 2003; Greenamyre, 2001). However, early studies that focused on antagonizing ionotropic glutamate receptors as treatments for Parkinson’s disease and other conditions were generally disappointing, largely due to the occurrence of debilitating side effects, which may have resulted from unwanted drug actions outside of the intended targets (Blandini and Greenamyre, 1998; Hughes, 1997; Smith et al., 2012). In contrast, because of their modulatory effects and more restricted regional distributions, the G-protein coupled metabotropic glutamate receptors (mGluRs) have become attractive targets for glutamate-based pharmacotherapies in Parkinson’s disease (Breysse et al., 2003; Johnson et al., 2009; Lopez et al., 2007; Marino and Conn, 2006; Ossowska et al., 2007; Smith et al., 2012). These receptors are divided into three major groups based on their structure, pharmacology, and coupling to second messenger systems. Group I mGluRs (mGluR1 and mGluR5) are mainly expressed postsynaptically and increase neuronal excitability when activated, while group II (mGluR2 and mGluR3) and group III (mGluR4, mGluR6, mGluR7, and mGluR8) receptors are commonly found in presynaptic neuronal elements, where they decrease neurotransmitter release by inhibition of adenylyl cyclase.
In light of anatomical, electrophysiological and behavioral studies in rodents, it has become clear that activation of mGluR4 may represent a useful approach to normalize basal ganglia circuit activity, and to relieve PD motor symptoms (Beurrier et al., 2009; Johnson et al., 2009; Lopez et al., 2007; Lopez et al., 2008; Marino et al., 2003; Smith et al., 2012; Valenti et al., 2003). In rats, mGluR4 is strongly expressed in the globus pallidus (GP), where it is localized predominantly in putative striatopallidal GABAergic terminals (Bradley et al., 1999; Corti et al., 2002). Activation of mGluR4 attenuates inhibitory postsynaptic currents induced by striatal stimulation in rat GP slices (Beurrier et al., 2009; Matsui and Kita, 2003; Valenti et al., 2003). It has been suggested that, through decreasing striatopallidal GABAergic transmission, mGluR4 activation may disinhibit GP neurons, thereby increasing the pallidal inhibitory drive to the abnormally overactive subthalamic nucleus (STN) and potentially correcting aberrant basal ganglia circuit activity in the parkinsonian state (Johnson et al., 2009; Marino and Conn, 2006; Niswender and Conn, 2010). Parkinsonian motor signs are, indeed, improved in rodent models of PD following intracerebral administration of group III mGluR agonists or mGluR4 positive allosteric modulators (Beurrier et al., 2009; Cuomo et al., 2009; Konieczny et al., 2007; Lopez et al., 2007; MacInnes et al., 2004; Marino et al., 2005; Marino et al., 2003; Valenti et al., 2003). The recent development of mGluR4 potentiators with reliable pharmacokinetic properties and efficient brain penetration has set the stage for trials of mGluR4-related PD pharmacotherapeutics (Engers et al., 2011; Niswender and Conn, 2010; Smith et al., 2012).
In light of the promising rodent data, we aimed to characterize the target sites and physiological effects of mGluR4-related drugs in the primate basal ganglia, using a combination of light and electron microscopic immunohistochemistry, local application of group III mGluR-related drugs in the striatopallidal complex, and in vivo single unit recordings of striatal and pallidal neurons in awake parkinsonian rhesus monkeys. Some of the findings of this study have been presented in abstract forms (Bogenpohl et al., 2010, 2011).
2. Material and Methods
2.1 Animals
Eleven adult rhesus macaques (7 males, 4 females; 2–9 years old) were used in this study. All experiments were performed in accordance with the National Institutes of Health’s Guide for the Care and Use of Laboratory Animals and the United States Public Health Service Policy on Humane Care and Use of Laboratory Animals. Five of these monkeys were treated with weekly systemic injections of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP; 0.2–0.8 mg/kg/week; Sigma, St. Louis, MO; total cumulative doses ranged from 14 to 33 mg/kg, total treatment time ranged from 8 to 16 months) until moderate parkinsonian motor signs were observed.
The severity of parkinsonism was assessed as previously described (Kliem et al., 2010; Wichmann et al., 2001). Briefly, animals were transferred to an observation cage, in which locomotor behavior was measured by counting infrared beam breaks, and by direct visual quantification of the number of movements made by different body parts. A modified PD rating scale was also used to quantify parkinsonism. Following the development of moderate parkinsonian motor signs that remained stable for a period of at least 6–8 weeks after MPTP administration, two of the parkinsonian monkeys and three normal, untreated monkeys were trained to sit calmly in a restraint chair before being chronically implanted with transcranial recording chambers for electrophysiological experiments.
The remaining three MPTP-treated parkinsonian monkeys and three untreated animals were deeply anesthetized with pentobarbital (100 mg/kg i.v.) and transcardially perfused with cold oxygenated Ringer’s solution, followed by a fixative containing 4% paraformaldehyde and 0.1% glutaraldehyde in a phosphate buffer (PB) solution, for immunohistochemistry. After perfusion, brains were removed from the skull, sliced coronally into thick (~1 cm) blocks, and post-fixed overnight in 4% paraformaldehyde. These blocks were cut into 60 μm-thick coronal sections using a vibrating microtome and stored at −20° C in an anti-freeze solution, containing 30% ethylene glycol and 30% glycerol in PB, until ready for immunohistochemistry.
2.2 Immunohistochemistry
2.2.1 Pre-embedding immunoperoxidase for light microscopy
The localization of mGluR4 was achieved using a highly specific polyclonal antibody (Invitrogen, Carlsbad, CA; Catalog # 51-3100; 1:200 dilution) raised in rabbit against a 200 amino acid C-terminal fragment of the rat mGluR4 protein. This antibody is specific for the mGluR4a splice variant. Reactivity with other related proteins has not been detected on immunoblots of transfected cells expressing other mGluR subtypes (Invitrogen). This antibody does not stain brain tissue from mGluR4 knockout mice (unpublished data). Depletion of the dopaminergic nigrostriatal system in MPTP-treated animals was confirmed in 3 of the 5 animals in this study (the 2 remaining animals are still alive at the time of this report) by staining sections at the level of the striatum and the substantia nigra with mouse anti-tyrosine hydroxylase (TH) antibodies (1:1000, Millipore; not shown).
Brain sections taken from various antero-posterior levels of the basal ganglia (see also below) in 2 normal and 2 MPTP-treated monkeys were processed for light microscopy (LM) immunohistochemical localization of mGluR4. Prior to immunohistochemical (IHC) processing, brain sections were washed with phosphate buffered saline (PBS; 0.01M, pH 7.4), treated with a 1% sodium borohydride solution for 20 minutes, and washed in PBS once more.
Sections were incubated for 1 hour in PBS containing 10% normal goat serum (NGS), 1% bovine serum albumin (BSA), and 0.3% Triton X-100, followed by incubation in the primary antibody solution containing 1% NGS, 1% BSA, and 0.3% Triton X-100 in PBS for 48 hours at 4°C. Sections were then rinsed three times in PBS and incubated in the secondary antibody solution containing 1% NGS, 1% BSA, 0.3% Triton X-100, and biotinylated goat anti-rabbit IgGs (Vector Laboratories, Burlingame, CA) at a dilution of 1:200 for 90 minutes at room temperature. After three rinses in PBS, sections were incubated for 90 minutes in avidin-biotin peroxidase complex (ABC) solution at a dilution of 1:100 (Vectastain standard ABC kit, Vector Laboratories) including 1% BSA.
To reveal the antigenic sites, sections were first rinsed with PBS and Tris buffer (50 mM; pH 7.6), then incubated in a solution containing 0.025% 3,3′-diaminobenzidine tetrahydrochloride (DAB; Sigma), 10 mM imidazole, and 0.005% hydrogen peroxide in Tris buffer for 10 minutes. Sections were then washed several times in PBS, mounted on gelatin-coated glass slides, dehydrated, and coverslipped with Permount™.
The slides were scanned at 20X using a ScanScope CS scanning light microscope system (Aperio Technologies, Vista, CA). Digital representations of the slides were saved and analyzed using ImageScope software (Aperio Technologies).
2.2.2 Pre-embedding immunoperoxidase for electron microscopy
Sections containing the striatopallidal complex from 3 normal and 3 MPTP-treated monkeys were transferred to a cryoprotectant solution containing 25% sucrose and 10% glycerol in PB (0.05M, pH 7.4) for 20 minutes and then placed in a −80°C freezer for 20 min to permeabilize cell membranes. They were then thawed through washes in decreasing concentrations of cryoprotectant solution until being washed in PBS. The subsequent tissue processing was identical to that used for light microscopy, up to the point of DAB revelation, with two important differences: Triton X-100 was omitted from all solutions, and sections were incubated in the primary antibody solution for 48 hours at 4°C.
After DAB revelation, the tissue was rinsed in PB (0.1M, pH 7.4) and treated with 1% osmium tetroxide for 20 minutes. It was then rinsed with PB and dehydrated with increasing concentrations of ethanol, up to 100%. Uranyl acetate (1%) was added to the 70% EtOH dehydration solution and incubated for 35 minutes in order to increase the contrast of membranes in the electron microscope. After alcohol dehydration, sections were treated with propylene oxide, embedded in epoxy resin (Durcupan ACM; Fluka, Buchs, Switzerland) for at least 12 hours, mounted onto slides, and placed in a 60°C oven for 48 hours to cure the resin.
2.2.3 EM observations and analysis
Small blocks of tissue from the dorsolateral putamen or GPe (12 – 15 mm anterior to the interaural coronal plane; Paxinos et al., 2000) were cut out from the embedded sections and glued onto resin blocks for ultrathin sectioning with an ultramicrotome (Leica Ultracut T2). Sixty-nanometer-thick sections were collected from the surface of the tissue block to ensure that antibody penetration was optimal in the tissue analyzed in the EM. Sections were mounted on single-slot pioloform-coated copper grids, stained for 5 minutes with lead citrate, and examined with a Zeiss EM-10C transmission electron microscope. Electron micrographs were captured and saved with a CCD camera (DualView 300W; Gatan, Inc., Pleasanton, CA) controlled by DigitalMicrograph software (version 3.11.1; Gatan, Inc.). Fifty micrographs of randomly-encountered mGluR4-labeled neuronal elements were captured at 25,000X in the GPe and putamen of each monkey, giving a total of 581 μm2 of tissue analyzed per structure per animal. Immunoperoxidase labeling for mGluR4 could be identified as a dark, amorphous deposit in the cytoplasm of labeled neuronal elements. Immunoreactive elements were classified based on ultrastructural features (Peters et al., 1991), quantified, and plotted as distribution histograms.
2.3 Electrophysiology
2.3.1 Surgical procedure
Two of the MPTP-treated parkinsonian monkeys and three normal monkeys were implanted with bilateral chronic recording chambers. Under aseptic conditions and isofluorane anesthesia (1–3%), recording chambers were stereotaxically positioned over trephine holes in the skull to provide chronic access to striatum and GP, using a lateral coronal approach (36° angle from the vertical). The chambers and a head post receptacle were attached to the animal’s skull with dental acrylic and stainless steel bone screws. After surgery, monkeys were allowed to recover for at least one week.
2.3.2 Electrophysiological mapping of brain structures
All recordings were performed in fully awake monkeys (determined by video surveillance showing open eyes and occasional movements). The animals were sitting in a restraint chair with the head immobilized and the body and limbs free to move. In each session, the dura was pierced with a guide tube, and a tungsten microelectrode (Z = 0.5–1.0 MΩ at 1 kHz; FHC, Bowdoinham, ME) was lowered into the brain using a microdrive (MO-95B; Narishige, Tokyo, Japan), using pre-determined anteroposterior and mediolateral positions. Extracellular neuronal electrical signals were recorded, amplified (DAM-80 amplifier; WPI, Sarasota, FL), filtered (400–6,000 Hz; Krohn-Hite, Brockton, MA), displayed on a digital oscilloscope (DL1540; Yokogawa, Tokyo, Japan), and made audible via an audio amplifier. Neuronal signals were digitized (sampling rate 25 kHz) and stored on a hard drive using a data acquisition interface (Power1401; CED, Cambridge, UK) and commercial software (Spike2, CED) for offline analysis. The actual locations of brain structures below the chambers were delineated by electrophysiological mapping. The putamen region was characterized by a mixture of neurons, some showing slow background firing rate, while others presented sustained spontaneous activity and regular discharge (see below for further details on these two neuronal types). GPe cells were identified by their location in relation to the overlying putamen and their characteristic high frequency discharge, interspersed with pauses (DeLong, 1971; Elias et al., 2007).
2.3.3 Intracerebral injections
Intracerebral microinjections were performed in two MPTP-treated monkeys with a custom-built device (“injectrode”), consisting of a tungsten microelectrode which was fused to a thin silica tube, as described previously (Galvan et al., 2010; Galvan et al., 2005; Kliem and Wichmann, 2004). The injection tubing was connected to a 1 ml gas-tight syringe (CMA Microdialysis, Solna, Sweden), driven by a computer-controlled infusion pump (Model 102, CMA).
The injectrode was lowered into the putamen or GPe using the microdrive, and positioned to isolate single neurons for recording. If two or more neurons were present near the tip of the injectrode (as determined by visual inspection of the recorded waveforms on the oscilloscope), the injectrode was moved slightly up or down to isolate a single neuron. If adjustments could be made to obtain a difference in spike amplitude of approximately two-fold, recording and injection commenced with focus on the larger-amplitude cell. In these cases, spikes from the two recorded cells were separated in the spike sorting step (see below).
Spontaneous baseline activity of each cell was recorded for at least 60 seconds before drug infusion. Recording continued through the drug injection (0.4 μL at 0.2 μL/min) and for at least 5 min after the end of the infusion. In some cases, more than one injection was performed along the same tract. In these cases, the recording/injection sites were separated by at least 1 mm, and a time lapse of at least 30 min was allowed to pass before the next recording could begin, to minimize residual effects of the previous injection.
2.3.4 Drugs
The group III mGluR agonist L-AP4 (20 or 50 mM, corresponding to 8 or 20 nmol/injection; Tocris Bioscience, Bristol, UK), and the mGluR4 allosteric potentiator VU0155041 (4 mM, corresponding to 1.6 nmol/injection; generous gift of Niswender, Conn et al., Vanderbilt University) were used (Engers et al., 2011, 2010, 2009). The choice of optimal concentrations was based on previous rodent data showing antiparkinsonian efficacy (Cuomo et al., 2009; Lopez et al., 2007). Due to the limited solubility of VU0155041 in aqueous solutions, 4mM was the highest concentration achieved for the intracerebral injections. The drugs were dissolved in artificial cerebrospinal fluid (aCSF, comprised of (in mM) 143 NaCl, 2.8 KCl, 1.2 CaCl2, 1.2 MgCl2, 1 Na2HPO4), and the pH adjusted to 7.2–7.4. Before being loaded into the injection syringe, all solutions were filtered with a 0.2 μm pore size nylon membrane (Fisher Scientific, Hampton, NH). Artificial CSF was used for control injections.
2.3.5 Analysis of electrophysiological data
Spike sorting was performed off-line with a waveform matching algorithm, followed by principal component analysis (Spike2). Inter-spike interval (ISI) distribution histograms were generated and examined for each cell to verify the quality of the recording and spike sorting. All subsequent steps of the analysis were done in Matlab (Mathworks, Natick, MA).
ISI data were used to generate second-by-second readouts of firing rates (FRs) and coefficients of variance (CV; ISI standard deviation/mean ISI; an index of the irregularity of neuronal firing) in 20 sec bins, which were subsequently smoothed using a sliding 20-point moving average. These readouts were plotted and used to select a ‘baseline segment’ (at least 60 sec immediately preceding drug or aCSF infusion) and a drug ‘effect segment’. Drug effect segments were selected by determining if the firing rate or CV passed beyond the 90th or below the 10th percentile of the baseline segment data, continuously for at least 90 sec. Drug effects had to begin within an ‘effect window’ beginning 30 sec after the beginning of drug infusion, and ending 120 sec after the end of the drug infusion. A 60 sec ‘effect segment’ containing the peak of the drug effect was selected for further analysis. For cells which did not fulfill the above requirements to define a drug effect, a 60 sec segment of firing rate and CV data was randomly selected from the effect window for further analysis. The effects lasted, on average, 180 sec (the maximal duration was 580 sec), based on 50 experiments in which responding neurons could be recorded long enough after the injections to observe recovery of the basal firing rate.
The magnitude of each drug effect was calculated as the mean firing rate or CV during the effect segment, expressed as a ratio (mean of effect segment/mean of baseline segment, termed FR ratios, or CV ratios, respectively). For the experiments in which aCSF was infused, the 90th and 10th percentiles of FR ratios and CV ratios of the recorded cells were calculated (Figs. 6, 7; dashed horizontal lines). For experiments in which L-AP4 or VU0155041 were infused, recorded cells whose FR ratios or CV ratios were above the 90th percentile or below the 10th percentile of the aCSF group were classified as having significantly increased or decreased their firing rate or CV values in response to the drug infusion, respectively. Otherwise, cells were classified as having no effect in response to drug infusion.
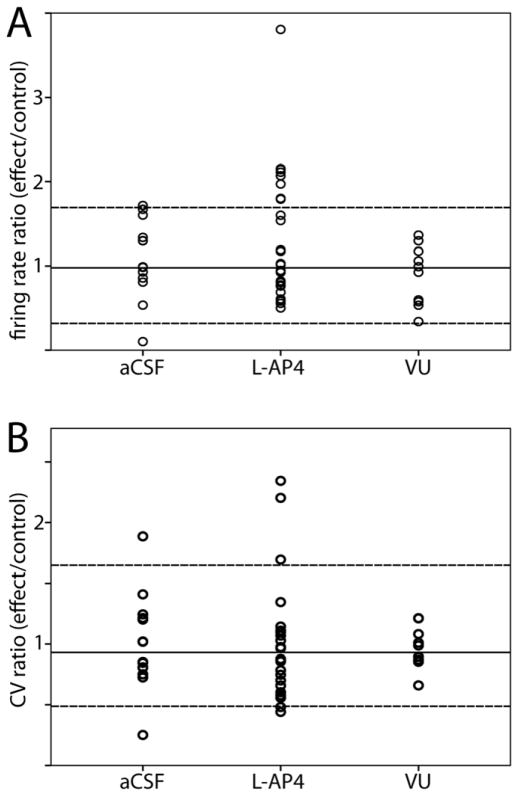
Figure 6.
Responses of GPe neurons in MPTP-treated monkeys to infusion of aCSF, L-AP4, or VU0155041 (VU). A) Changes in firing rate (expressed as firing rate ratios, effect/baseline) of GPe neurons in response to drug infusion. The proportions of cells showing increased, decreased, or no change in firing rate were statistically different between the L-AP4 and aCSF groups (chi-squared, p=0.02). B) Changes in firing pattern (expressed as coefficient of variance ratios) of GPe neurons in response to drug infusion. Solid horizontal line represents the mean of aCSF data. Upper and lower dashed lines represent 90th and 10th percentiles of aCSF data, respectively. aCSF n=14, L-AP4 n=27, VU n=10.
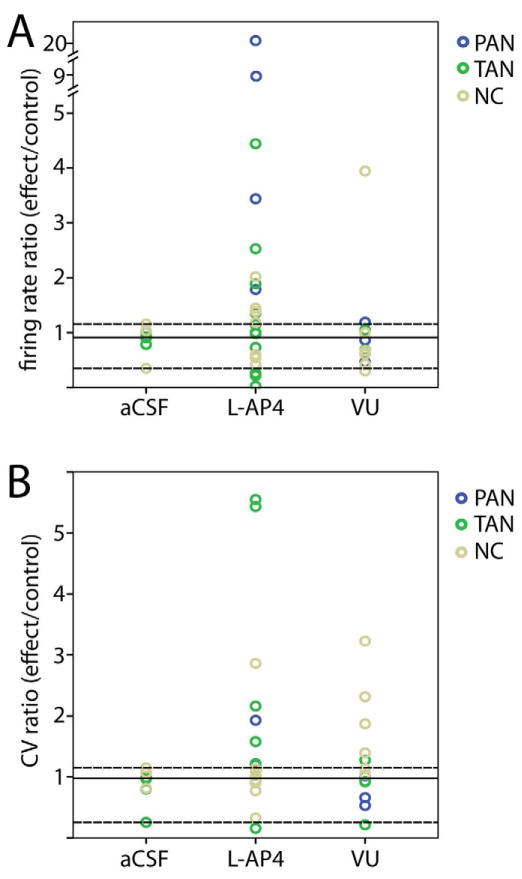
Figure 7.
Responses of striatal neurons in MPTP-treated monkeys to infusion of aCSF, L-AP4, or VU0155041 (VU). A) Changes in firing rate (expressed as firing rate ratios, effect/baseline) of striatal neurons in response to drug infusion. B) Changes in firing pattern (expressed as coefficient of variance ratios) of striatal neurons in response to drug infusion. Blue circles represent PANs, green circles represent TANs, and tan circles represent striatal cells that were not classified (NC). Solid horizontal line represents the mean of aCSF data. Upper and lower dashed lines represent 90th and 10th percentiles of aCSF data, respectively. aCSF n=9, L-AP4 n=24, VU n=13.
The Legendy and Salcman burst detection method was used to calculate burst indices (Legendy and Salcman, 1985; Wichmann and Soares, 2006). The method described by Elias et al. (2007), a variant of the Legendy-Salcman algoritm, was used to define deceleration of neuronal firing. A ‘surprise’ value of 3 was used for bursting or deceleration events. Burst and deceleration indices were defined as the proportion of spikes in the bursts or decelerations compared to the total number of spikes, or they were based on the proportion of time the cell spent in bursts or decelerations. Bursts were further classified as ‘rebound bursts’ if the ISI immediately preceding each burst was at least three times longer than the mean ISI for that neuron. Finally, pauses in firing were defined as cessations of neuronal activity of 500 ms or longer.
To examine oscillatory properties of neuronal discharge, a power spectral analysis was performed using the Neurospec 2.0 Matlab functions for frequency domain analyses, written by David Halliday (Halliday et al., 1995; Nielsen et al., 2005). For each neuron, the raw spectra were integrated in the 1–3 Hz, 3–8 Hz, 8–13 Hz, 13–30 Hz, and 30–100 Hz ranges, and the resulting values were expressed as fractions of the power in the entire 1–100 Hz band (for a similar approach see (Soares et al., 2004).
2.3.6 Characterization of striatal neurons
The classification of striatal neurons was based on their basal firing rates and CV of ISIs, as described in previous studies (Kimura, 1986; Aosaki et al., 1994; Blazquez et al., 2002; Nanda et al., 2009). Thus, cells were classified as phasically active neurons (PANs, likely medium spiny neurons) if they had a FR ≤ 2.5 spikes/s and an ISI-CV ≥ 1; and as tonically active neurons (TANs, likely cholinergic interneurons) if they had a FR > 2 and < 12, and a CV <1. Cells that did not fulfill these criteria remained “unclassified” and may represent other types of striatal interneurons, or PANs and TANs with uncharacteristic firing properties.
2.3.7 Data from normal monkeys
For comparisons of basic neuronal firing characteristics of normal and MPTP-treated monkeys, we used data previously collected from normal monkeys, in the GPe (Galvan et al., 2005) and striatum (n=3 monkeys). The recording methods and analysis of data from normal monkeys was identical to that described above for MPTP-treated monkeys.
3. Results
3.1 mGluR4 Localization in the Primate Basal Ganglia
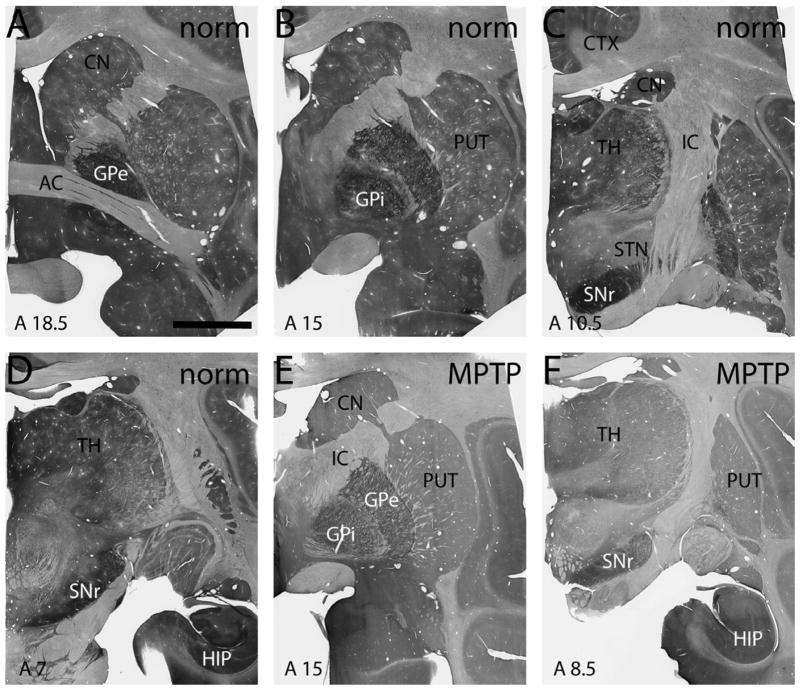
In order to characterize the potential sites through which mGluR4-related drugs could mediate their symptomatic antiparkinsonian effects in the primate brain, we examined the overall distribution of mGluR4 immunoreactivity in the basal ganglia nuclei of normal and parkinsonian monkeys. At the light microscopic level, mGluR4 immunoreactivity was expressed to varying degrees in the neuropil of cortical and subcortical forebrain regions, but the GPe and GPi were, by far, the two most strongly labeled forebrain regions (Fig. 1). The entire extent of GPe and GPi was invaded by a rich plexus of mGluR4-immunoreactive fibers. In contrast, significantly lighter labeling was found in the caudate nucleus, putamen, thalamus, hippocampus, and cerebral cortex which, in addition to modest neuropil immunoreactivity, also contained some immunoreactive perikarya. The SNr harbored dense mGluR4 neuropil immunoreactivity, while the SNc contained light to moderate perikaryal labeling with minimal neuropil immunoreactivity often associated with dendrite-like processes. STN neuronal cell bodies and proximal dendrites displayed light to moderate immunoreactivity. Overall, there was no obvious difference in the pattern or intensity of mGluR4 labeling between normal and MPTP-treated monkeys (Fig. 1).
Figure 1.
Light micrographs of coronal monkey brain sections showing immunostaining for mGluR4 at various levels of a normal (A-D) and an MPTP-treated monkey (E,F). The approximate interaural coordinate for each section is designated in the lower left of each panel. Scale bar in A equals 5 mm and applies to all panels. AC – anterior commissure, CN – caudate nucleus, CTX – cortex, GPe – external globus pallidus, GPi – internal globus pallidus, HIP – hippocampus, IC – internal capsule, PUT – putamen, SNc – substantia nigra pars compacta, SNr – substantia nigra pars reticulata, TH - thalamus.
3.2 Ultrastructural Analysis of mGluR4 in the Striatopallidal Complex
3.2.1 mGluR4 localization in the GPe
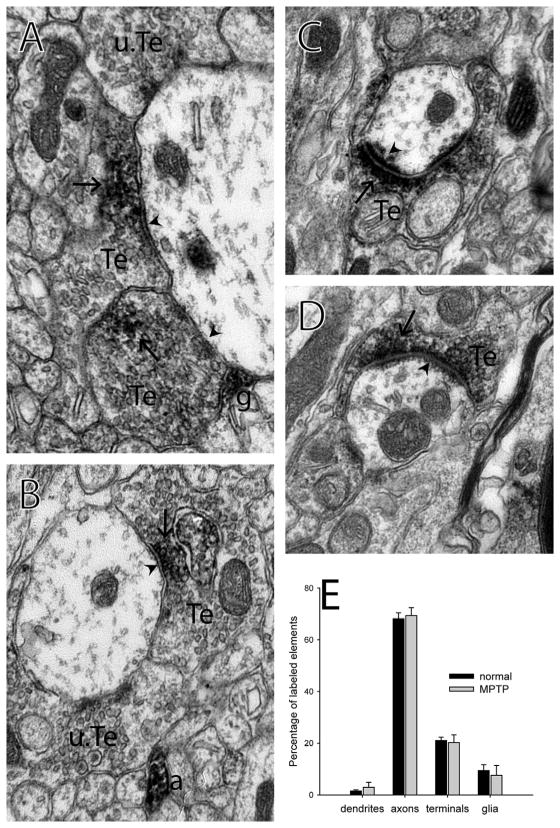
In the GPe of normal monkeys, mGluR4 immunoreactivity was primarily found in axons (68±2% of labeled elements, mean±SEM) and terminals (21±1%), with little postsynaptic (2±0.4%) or glial immunoreactivity (9±2%, Fig. 2). The relative prevalence of labeled elements in the GPe in normal monkeys was not different from that in MPTP-treated animals (Pearson chi-square = 2.508, p=0.474; Fig. 2E). Most of the mGluR4-immunoreactive terminals (122 of 128 in normal monkeys; 106 of 120 in MPTP-treated monkeys) in the GPe formed symmetric axo-dendritic synapses and had ultrastructural features of striatal GABAergic boutons (Shink and Smith, 1995; Smith et al., 1998), while the remainder formed asymmetric axo-dendritic synapses (Fig. 3A).
Figure 2.
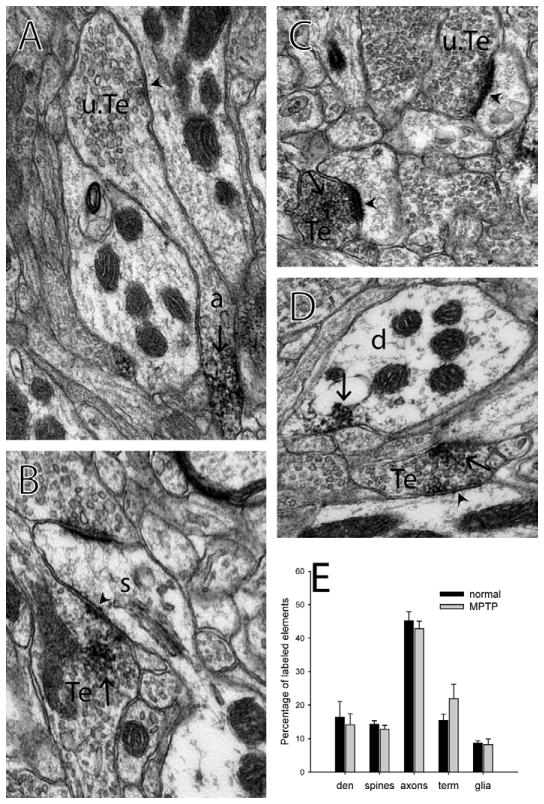
Immunolabeling for mGluR4 at the electron microscopic level in the monkey external globus pallidus. A, B) Labeled terminals form symmetric synapses on dendrites in the GPe. C, D) Labeled terminals form asymmetric synapses on dendrites in the GPe. E) Histogram showing the breakdown of the proportions of each type of labeled element found in the GPe of normal and MPTP-treated monkeys. n=3 normal, 3 MPTP-treated monkeys. Error bars represent SEM. No significant difference was found between normal and MPTP-treated monkeys. Synapses are identified with arrowheads. Immunoperoxidase labeling is identified with arrows. a – labeled axon, g – labeled glial process, Te – labeled terminal, u.Te – unlabeled terminal.
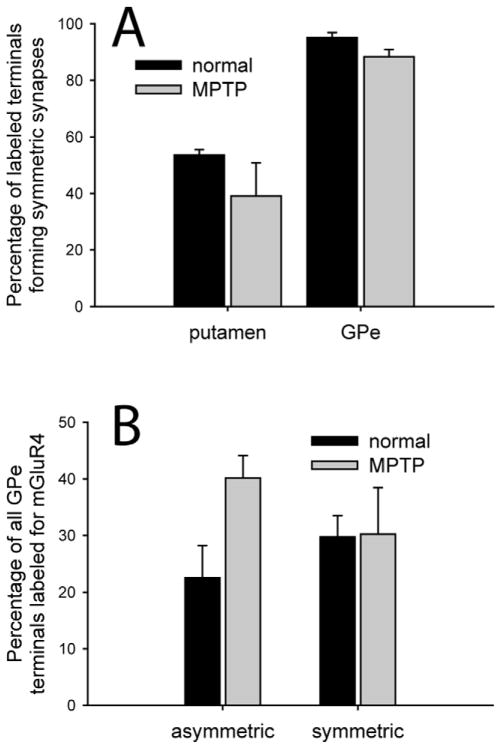
Figure 3.
Prevalence of mGluR4-labeled terminal subtypes in the putamen and GPe. A) The percentage of mGluR4-positive terminals that formed symmetric synapses in normal and MPTP-treated monkeys. (N total labeled terminals=Normal: 52 in putamen, 128 in GPe; MPTP: 56 in putamen, 120 in GPe) B) The percentage of the total population of GPe terminals, forming asymmetric or symmetric synapses, that contained mGluR4 immunoreactivity. (N total GPe terminals labeled and unlabeled=Normal: 26 asymmetric, 412 asymmetric; MPTP: 33 asymmetric, 351 symmetric) Error bars represent SEM. No significant difference was found between normal and MPTP-treated monkeys. (N monkeys=3 normal, 3 MPTP-treated).
Because GABAergic terminals account for almost 90% of the total population of axon terminals in the monkey GPe (Shink and Smith, 1995), the relative prevalence of the different categories of mGluR4-labeled terminals based on their type of synaptic specializations, must be interpreted cautiously. In order to provide a more accurate assessment of the preponderance of mGluR4-containing boutons relative to the size of the populations of putative GABAergic or glutamatergic terminals in the GPe, we quantified the percentages of the total populations of terminals forming symmetric or asymmetric synapses that displayed mGluR4 immunoreactivity (Fig 3B). We found that 30±4% of all GPe boutons involved in symmetric synapses contained mGluR4 labeling, while 23±6% of putative glutamatergic boutons forming asymmetric synapses did so in normal monkeys. No significant difference was found in the proportions of labeled terminals forming symmetric (t-test, p=0.96) or asymmetric synapses between normal and MPTP-treated monkeys, although there was a trend for a higher proportion of labeled asymmetric terminals in the GPe of MPTP-treated monkeys (40±4%; t-test, p=0.063).
3.2.2 mGluR4 localization in the putamen
Although the mGluR4 labeling in the putamen was mostly found in presynaptic elements (axons and terminals; 45±3% and 15±2% of labeled elements, respectively), labeling was also seen postsynaptically (dendrites and spines; 16±5% and 14±1%, respectively; Fig.4). Glial immunostaining was rare (9±1%). No significant difference was found in the relative prevalence of labeled elements in the putamen between normal and MPTP-treated monkeys (Pearson chi-square = 4.248, p=0.373; Fig. 4E).
Figure 4.
Immunolabeling for mGluR4 at the electron microscopic level in the monkey putamen. A) An unlabeled terminal arising from a labeled axon and forming a symmetric synapse on a dendrite. B) A labeled terminal forming a symmetric synapse on a spine. C) A labeled and an unlabeled terminal forming asymmetric synapses on spines. D) A labeled dendrite and a labeled terminal forming a symmetric synapse on a dendrite. E) Histogram showing the breakdown of the proportions of each type of labeled element found in the putamen of normal and MPTP-treated monkeys. n=3 normal, 3 MPTP-treated monkeys. Error bars represent SEM. No significant difference was found between normal and MPTP-treated monkeys. Synapses are identified with arrowheads. Immunoperoxidase labeling is identified with arrows. a – labeled axon, d – labeled dendrite, s - spine, Te – labeled terminal, u.Te – unlabeled terminal.
Of the 52 mGluR4-immunoreactive axon terminals with clear synaptic specializations seen in the putamen of normal monkeys, 28 formed symmetric synapses, preferentially onto dendritic shafts and rarely onto spines (Fig. 4B), and 24 formed asymmetric synapses, usually onto spines and rarely onto dendritic shafts, thereby suggesting that mGluR4 is localized to subserve presynaptic regulatory functions towards both glutamatergic and GABAergic transmission in the primate striatum. We obtained similar findings in the putamen of MPTP-treated monkeys, where 21 of 56 mGluR4-positive terminals formed symmetric synapses, and the remaining 35 formed asymmetric synapses.
3.3 Electrophysiological Effects of Group III mGluR Activation
3.3.1 Basic firing characteristics of GPe neurons in normal vs. MPTP-treated monkeys
To assess the electrophysiological effects of group III mGluRs activation on GPe neurons, single unit recordings were obtained from 51 GPe neurons in 2 MPTP-treated monkeys, in which the stability and isolation of single units was maintained before, during and after microinjections of aCSF or drugs in the vicinity of the recorded cells. Table 1 compares descriptors of neuronal firing recorded during the pre-injection (control) period in parkinsonian animals with values obtained under similar conditions in normal monkeys (data from normal monkeys obtained from (Galvan et al., 2011)). Although it has been described that striatal cells might show altered firing rates after dopaminergic denervation induced by MPTP treatment (Liang et al., 2008), we did not detect significant differences in the mean FR or CV of striatal cells recorded in normal or MPTP-treated monkeys (see Table 1). It is possible that the slow method of MPTP administration in our study induced adaptive processes that may have reduced the differences in striatal neuronal firing between normal and parkinsonian animals. We must also consider the fact that, even if differences in firing parameters were not found under resting conditions, such changes may become apparent during active movements.
Table 1.
Descriptors of neuronal firing in the GPe; comparison of MPTP-treated monkeys with data from normal monkeys previously acquired (Galvan et al. 2011). Values are means ± standard deviation.
| Normal | MPTP | ||
|---|---|---|---|
| Number of neurons | 24 | 51 | |
| ISI CV | 1.6±1.6 | 1.3±0.6 | |
| Firing rate (spikes/s) | 65.1±34.1 | 55.2±22.0 | |
| Power spectral components (% within stated spectral bins) | 1–3 Hz | 2.8±2.4 | 2.18±1.56 |
| 3–8 Hz | 1.4±0.9 | 1.4±0.9 | |
| 8–13 Hz | 0.7±0.4 | 0.9±0.5* | |
| 13–30 Hz | 0.6±0.2 | 0.7±0.2* | |
| 30–100 Hz | 1.1±0.2 | 1.0±0.2 | |
| Burst index (spikes, %) | 26.0±22.1 | 29.4±17.6 | |
| Burst index (time, %) | 8.1±6.2 | 8.7±4.5 | |
| Deceleration index (spikes, %) | 3.6±2.8 | 4.3±2.6 | |
| Deceleration index (time %) | 14.1±14.7 | 14.3±10.7 | |
| Pause index (spikes %) | 0.4±0.5 | 0.1±0.2* | |
| Pause index (time %) | 9.5±8.7 | 3.2±3.5* | |
statistically different from normal; t-test, p<0.05
The pause indices (based on the proportion of spikes or the proportion of time within pauses) were significantly lower in MPTP-treated monkeys than in controls. In addition, we found higher oscillatory power in the 8–13 Hz and 13–30 Hz bands in the GPe of MPTP-treated monkeys than in controls (all differences assessed with t-test, p<0.05). The firing rate of GPe cells was lower in the MPTP-treated state, but this did not reach significance.
3.3.2 Group III mGluRs activation in the GPe
The effects of local injections of L-AP4 (20 and 50 mM) and VU0155041 (4 mM) on the firing rate and CV values of GPe cells are summarized in Table 2 and Fig. 6. Increases and decreases in firing rate or CV are considered as such if they were above the 90th or below the 10th percentile of the aCSF cases’ distribution (see Methods). No significant differences were found between cells treated with 20 and 50 mM L-AP4, so these data were pooled.
Table 2.
Summary of changes in firing rate (FR) and pattern (CV) of neurons in the GPe and striatum, in response to drug infusion.
| GPe cells | Number of neurons | FR below 10th percentile | No FR effect | FR above 90th percentile | CV below 10th percentile | No CV effect | CV above 90th percentile |
|---|---|---|---|---|---|---|---|
| aCSF | 14 | 1 | 12 | 1 | 1 | 12 | 1 |
| L-AP4 | 27 | 0 | 19 | 8 | 2 | 22 | 3 |
| VU0155041 | 10 | 0 | 10 | 0 | 0 | 10 | 0 |
| Striatal cells | Number of neurons | FR below 10th percentile | No FR effect | FR above 90th percentile | CV below 10th percentile | No CV effect | CV above 90th percentile |
|---|---|---|---|---|---|---|---|
| aCSF | 9 | 0 | 8 | 1 | 0 | 9 | 0 |
| L-AP4 | 24 | 3 | 9 | 12 | 1 | 14 | 9 |
| VU0155041 | 13 | 1 | 10 | 2 | 1 | 7 | 5 |
Eight of 27 GPe cells increased their firing rates after L-AP4 infusion (an example is shown in Fig. 5), while the remaining 19 cells showed no significant change in firing rate. The proportions of cells showing increased, decreased, or no change in firing rate were statistically different between the L-AP4 and aCSF groups (Chi-square test, p=0.02). VU0155041 (4 mM) had no significant effect on the firing rate of GPe neurons.
Figure 5.
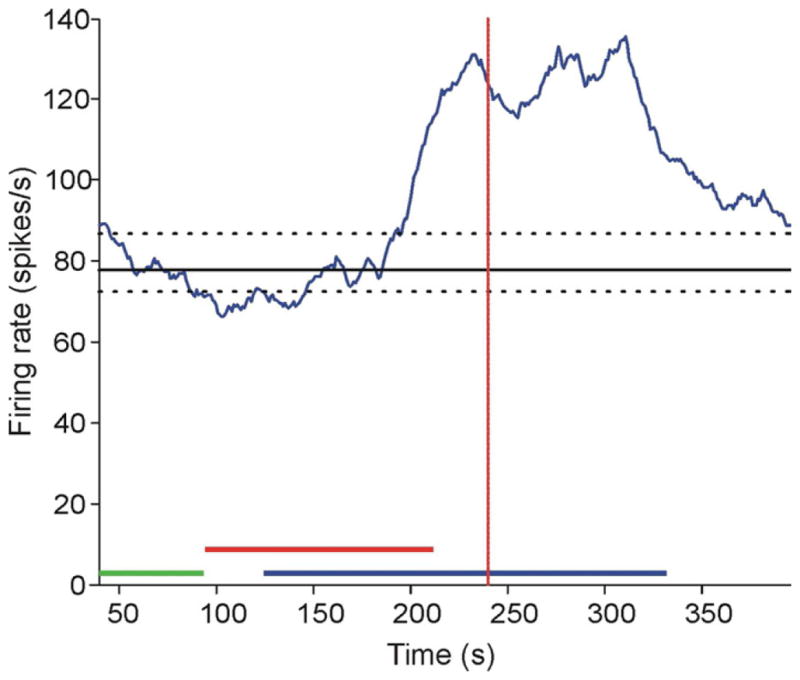
An example trace of a GPe neuron that increased its firing rate in response to L-AP4. The green bar represents the control period. The red bar represents the drug injection. The blue bar represents the window of time during which a drug effect may begin, in order to be considered as such. The solid horizontal line indicates the median firing rate at baseline, and the dashed lines show the 90th and 10th percentiles. The red vertical dotted line represents the center of the 60 sec period analyzed for drug effect.
As can be seen in Fig. 6B, most GPe neurons did not display significant changes in their firing patterns (as determined by CV values) after infusion of the group III mGluR agonists, as compared with aCSF. Only 3/27 GPe cells showed an increased CV in response to L-AP4, while 2/27 GPe cells showed a decreased CV. It is worth noting, however, that the characteristic pauses in firing that are commonly seen in GPe neurons under baseline conditions were completely abolished after infusion of L-AP4 in 11 out of 27 recorded cells. VU0155041 did not affect this parameter in the 10 recorded GPe neurons. Neither L-AP4 nor VU0155041 affected other parameters of firing, such as bursting or oscillatory activity.
None of the pallidal (or striatal) drug injections produced obvious behavioral changes. However, this study was not designed to assess behavioral drug effects, which would likely necessitate larger drug injection volumes.
3.3.3 Effect of MPTP on basic firing characteristics of striatal neurons
We recorded from 46 striatal neurons in 2 MPTP-treated monkeys, in which the stability and isolation of single units were maintained before, during and after microinjections of aCSF or drugs in the vicinity of the recorded cells. In Table 3, we show a comparison of different descriptors of the discharge of striatal neurons which were either recorded during the pre-injection (control) period in parkinsonian animals or under similar conditions in three normal monkeys. Striatal cells were divided into PANs, TANs, or not classified (NC), based on firing rates and CV values (see Methods). We found no statistical differences in these parameters between the groups of neurons recorded in the normal and MPTP-treated states, except in the firing rate of PANs, which was lower after MPTP treatment.
Table 3.
Descriptors of neuronal firing in the striatum; comparison of MPTP-treated monkeys with normal monkeys. Data reported as mean ± standard deviation.
| PANs | TANs | Not Classified | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Normal | MPTP | Normal | MPTP | Normal | MPTP | ||||||||
| Number of neurons | 22 | 8 | 27 | 21 | 18 | 17 | |||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | ||
| ISI CV | 1.77 | 0.60 | 1.75 | 0.60 | 0.66 | 0.17 | 0.68 | 0.18 | 1.62 | 0.72 | 1.53 | 0.32 | |
| Firing rate (spikes/s) | 1.14 | 0.76 | .5958* | 0.52 | 6.21 | 3.96 | 7.56 | 3.26 | 8.14 | 4.65 | 9.72 | 8.17 | |
| Power spectral components (% within stated spectral bins) | 1–3 Hz | 0.96 | 0.39 | 1.40 | N/A | 0.70 | 0.43 | 0.53 | 0.23 | 1.06 | 0.53 | 1.69 | 1.66 |
| 3–8 Hz | 1.15 | 0.13 | 1.44 | N/A | 1.12 | 0.23 | 1.05 | 0.23 | 1.40 | 0.67 | 1.57 | 0.90 | |
| 8–13Hz | 1.13 | 0.13 | 1.32 | N/A | 0.91 | 0.24 | 0.97 | 0.12 | 1.20 | 0.44 | 1.10 | 0.56 | |
| 13–30Hz | 1.06 | 0.11 | 1.03 | N/A | 0.98 | 0.11 | 0.99 | 0.05 | 1.08 | 0.25 | 0.94 | 0.27 | |
| 30–100 Hz | 0.98 | 0.03 | 0.94 | N/A | 1.03 | 0.04 | 1.03 | 0.02 | 0.95 | 0.13 | 0.96 | 0.14 | |
| Burst index (spikes %) | 51.35 | 15.59 | 51.96 | N/A | 8.93 | 7.50 | 8.53 | 6.30 | 35.52 | 21.46 | 45.27 | 12.06 | |
| Burst index (time, %) | 8.14 | 2.10 | 7.12 | N/A | 1.99 | 1.83 | 2.15 | 1.67 | 8.11 | 4.40 | 9.67 | 3.17 | |
statistically different from normal; t-test, p<0.05. Cases with <100 ISIs were not included in the power spectral and burst analysis (for those cells classified as PANs in the MPTP-treated monkeys, only one cell had >100 ISIs, and therefore the power spectral and burst analysis values are based on a single case).
3.3.4 Group III mGluR activation in the striatum
The effects of local injections of L-AP4 and VU0155041 on the firing rates and coefficients of variation of striatal cells are summarized in Table 2 and Fig. 7. Half of our sample of striatal cells (12/24) showed increased firing rates in response to L-AP4 infusion, while a few (3/24) significantly decreased their firing rates. The remaining 9 cells showed no significant change in firing rate. The proportions of cells showing increased, decreased, or no change in firing rate were statistically different between the L-AP4 and aCSF groups (chi-squared, p=0.02). After a local infusion of VU0155041, the recorded cells responded similarly to those recorded after local infusion of aCSF, except for a single cell that increased its firing rate (Fig. 7A).
In contrast to GPe injection results, many striatal cells showed increases in CV after L-AP4 (9/24) and VU0155041 (5/13), indicating less regular firing patterns following the drug infusion. The distribution of responses (increases, decreases, or no effect, based on the aCSF 10th/90th percentiles) showed a tendency towards a significant difference from the aCSF group (chi-squared; aCSF vs. L-AP4, p=0.058; aCSF vs. VU0155041, p=0.068). Other changes recorded in striatal neurons included an increase in the power of the 13 to 30 Hz band after L-AP4 injections, as well as an increase in the time spent in bursts after the VU0155041 injections.
4. Discussion
Our results demonstrate that mGluR4 is heavily expressed throughout the primate basal ganglia circuitry, and that its overall distribution is not significantly affected in the parkinsonian condition. In the striatopallidal complex, mGluR4 is located to subserve presynaptic regulation of glutamatergic and GABAergic transmission. Among responding cells, local intrastriatal or intrapallidal applications of the group III mGluRs agonist, L-AP4, induced predominantly increases in firing rates. L-AP4 application also altered striatopallidal firing patterns through the attenuation of the characteristic pauses in firing of GPe cells and increased CVs in the putamen. The striatal effects of L-AP4 were partly mimicked by local application of the GluR4 allosteric potentiator, VU0155041.
Together, these anatomical and electrophysiological effects suggest that the neural mechanisms underlying the possible antiparkinsonian effects of mGluR4-related drugs in primates may involve modulation of both GABAergic and glutamatergic synapses in GPe and striatum. Although not studied in great detail here, other structures such as the cerebral cortex, thalamus, or SNr may be involved as well, as mGluR4 expression is fairly widespread (see Fig. 1).
4.1 MGluR4 expression in the primate basal ganglia: Potential significance towards mGluR4-mediated antiparkinsonian effects
Our light and electron microscopic data from normal and MPTP-treated monkeys are consistent with those previously described in rodents, showing that striatal-like GABAergic terminals account for most of the mGluR4-immunoreactive elements in the GPe (Bradley et al., 1999; Corti et al., 2002). In addition, these findings are in line with slice electrophysiology data in which group III mGluRs activation reduced striatopallidal GABAergic transmission in normal rats (Beurrier et al., 2009; Marino et al., 2003; Matsui and Kita, 2003; Valenti et al., 2003). However, our results also demonstrate that mGluR4 is present in putative glutamatergic terminals in the monkey GPe. These observations, combined with our light microscopic evidence for perikaryal mGluR4 immunoreactivity in the STN, suggest that mGluR4 is expressed at the subthalamopallidal synapse. These receptors may thus regulate both GABAergic and glutamatergic transmission in the GPe of normal and parkinsonian monkeys. This possible dual regulatory role may be one of the reasons why group III mGluRs activation had relatively heterogeneous effects on the electrical activities of GPe neurons. Recent microdialysis data from 6-OHDA-treated parkinsonian rats showing that the local application of an orthosteric group III mGluRs agonist decreases extracellular GABA and glutamate levels in the GP are also in line with these observations (Deltheil et al., 2011). Expression of mGluR4 in STN neurons also suggests that modulation of glutamatergic transmission in the GPi/SNr may play an important role in the effects of mGluR4 activation as well.
Indeed, in addition to its strong expression in the striatum and GPe, mGluR4 was also found to be enriched in the neuropil of both GPi and SNr of normal and MPTP-treated monkeys, a pattern reminiscent to that described in a previous study in which striatal GABAergic and putative glutamatergic terminals immunoreactive for mGluR4 were identified in the SNr and entopeduncular nucleus of normal rats (Corti et al., 2002). Together with earlier data showing that both direct and indirect pathway GABAergic neurons in the striatum express mGluR4 mRNA (Kerner et al., 1997), these findings provide evidence for mGluR4-mediated presynaptic modulation of GABAergic and glutamatergic synapses in both the GPe and basal ganglia output nuclei.
Several previous studies have linked the antiparkinsonian effects of group III mGluR agonists specifically to the mGluR4-mediated modulation of GABA release, over other group III mGluR subtypes, in GPe (See Niswender and Conn, 2010 for review). By inhibiting GABA release from terminals of the overactive striato-GPe pathway, mGluR4 activation may indeed have antiparkinsonian effects, allowing GPe neurons to regain their inhibitory control over STN activity (Hopkins et al., 2009). However, the concomitant expression of mGluR4 in putative glutamatergic synapses in the GPe, and in striatal GABAergic projections to the SNr and GPi would have the opposite effect. Amalric and colleagues have, indeed, shown that infusion of group III agonists into the GP improves, while infusion into the SNr worsens motor deficits in parkinsonian rats (Lopez et al., 2007). Furthermore, as discussed in more detail below, group III mGluR activation is also likely to affect glutamatergic and GABAergic transmission in the striatum. In light of these findings, it appears that the antiparkinsonian effects of group III mGluR agonists (or mGluR4 allosteric potentiators) are probably not simply mediated by modulation of GABA release from the overactive striatopallidal pathway, but may involve more complex regulation of GABAergic and glutamatergic synaptic mechanisms at various levels of the basal ganglia circuitry. It is possible that some of these mechanisms are adaptive in character, and would only be seen with chronic application of the group III mGluR agonists, an issue that was not addressed in the present study.
4.2 MGluR4 in the striatum: A target for the regulation of extrinsic glutamatergic and intrinsic GABAergic circuitry
The striatum is another target through which mGluR4 activation could influence the activity of basal ganglia neurons, and elicit antiparkinsonian effects. We found that about half of mGluR4-immunoreactive terminals in the monkey striatum formed asymmetric synapses, the large majority of which contacted spines, suggesting that the main source of mGluR4-containing glutamatergic terminals in the primate striatum is the cerebral cortex (although some may also originate from the thalamus, see Smith et al., 2004). Taking into consideration evidence for increased corticostriatal glutamatergic transmission in parkinsonism (Calabresi et al., 1993; Calabresi et al., 1996; Galarraga et al., 1987; Gubellini et al., 2002), increased activation of mGluR4 in striatal glutamatergic afferents by agonist application may help tone down this abnormal corticostriatal overactivity. Cuomo and colleagues have, in fact, shown that cortically-evoked striatal excitatory postsynaptic potentials are attenuated by activation of group III mGluRs, and that intrastriatal injection of group III agonist improves akinesia in parkinsonian rats (Cuomo et al., 2009). Although drugs used in these studies could not discriminate between the different subtypes of group III mGluRs, there is strong evidence that mGluR4 is the most likely receptor involved in these effects (see below).
In addition to the modulation of striatal glutamatergic transmission, activation of group III mGluRs also attenuates inhibitory postsynaptic potentials induced by intrastriatal stimulation in rat brain slices (Cuomo et al., 2009). In light of our anatomical data showing that about half of mGluR4-containing terminals in the monkey striatum form symmetric synapses with the ultrastructural features of GABAergic terminals, combined with the fact that the most common response of striatal neurons to group III activation was an increase in firing rate, the inhibition of intrastriatal GABAergic transmission may be an important mechanism by which group III mGluRs mediate their functional effects upon the basal ganglia circuitry in normal and parkinsonian states.
4.3 MGluR4 regulation of striatal and pallidal firing rates
Local infusion of L-AP4 increased the firing rate of about one third of GPe cells and half of striatal neurons in parkinsonian monkeys. These effects were likely due to mGluR4-mediated inhibition of overactive GABAergic striatopallidal terminals in the GPe, and blockade of intrastriatal GABAergic inhibition in the striatum, respectively. It is important to note that while many striatal and pallidal neurons increased their firing rates in response to L-AP4, half of striatal neurons and two thirds of pallidal neurons either showed an opposite response, or did not display any significant effect in response to L-AP4. Such diverse physiological effects, combined with the fact that presynaptic mGluR4 is also heavily expressed in GPi and SNr (Corti et al., 2002), highlight the possibility that the mechanisms by which systemically administered mGluR4-related compounds mediate their potential behavioral and therapeutic effects in normal and diseased states may be more complex than suggested by the rodent studies mentioned above (East and Gerlach, 2010; Niswender and Conn, 2010; Niswender et al., 2008).
4.4 Effects of group III mGluRs agonist vs. mGluR4 PAM on striatal and pallidal activity
Two drugs were used in our study to activate group III mGluRs in the monkey striatopallidal complex: L-AP4, a classical group III mGluR orthosteric agonist with strong affinity for mGluR4 and mGluR8 but much less for mGluR7, and VU0155041, a highly selective mGluR4 positive allosteric modulator (Niswender et al., 2008). Because of the striking differences in the EC50 of L-AP4 for mGluR4/8 versus mGluR7, the lack of significant mGluR8-mediated physiological and behavioral effects in the striatum and GPe (Cuomo et al., 2009; Lopez et al., 2007; Valenti et al., 2003), the lack of L-AP4 effects in mGluR4 knockout mice (Cuomo et al., 2009; Valenti et al., 2003), and the restricted expression of mGluR6 in the retina (Nakajima et al., 1993), it is reasonable to suggest that the main L-AP4-mediated physiological effects described in the present study were induced by mGluR4 activation, although minor contributions of mGluR7 and mGluR8 cannot be completely ruled out.
In contrast to L-AP4, VU0155041 was much less effective at changing the firing rates of striatal and pallidal neurons in parkinsonian monkeys. One reason for this somewhat surprising result could be that the two drugs have different mechanisms of action. L-AP4 directly activates mGluR4 via the glutamate binding site, while VU0155041 is a mixed allosteric agonist/positive allosteric modulator which binds mGluR4s at a site distinct from the glutamate binding site, necessitating activation of the receptor by endogenous glutamate for optimal effects (Niswender and Conn, 2010; Niswender et al., 2008). In future experiments, improved efficacy may be achieved by administering a combination of mGluR4 orthosteric agonist and positive allosteric modulator, in order to enhance the activity of mGluR4s, in addition to directly activating them. We also cannot rule out the possibility that the concentration of VU0155041 used in our study (4 mM) was too low to produce electrophysiological effects in the majority of recorded GPe neurons. Although this remains to be assessed using compounds that display better solubility properties, it is worth noting that this concentration of VU0155041 induced significant changes in the firing pattern of some striatal neurons (Fig. 7B), thereby suggesting differential properties between GPe and striatal neurons in their responses to mGluR4 allosteric potentiators. Finally, the possibility of a species difference between striatopallidal neurons in rodents and primates in their responses to mGluR4 allosteric potentiators cannot be ruled out.
Several individual striatal neurons, indeed, showed significant increases in CV when treated with L-AP4 or VU0155041, suggesting that activation of mGluR4 increases firing irregularity in a subpopulation of striatal neurons. Most GPe neurons did not display CV changes in response to drug microinjections, but in many cells the incidence of pauses in discharge was greatly reduced in response to L-AP4 treatment (Table 1). Based on the observation that, at baseline, GPe neurons in MPTP-treated monkeys showed a significant reduction in indices of pausing (Table 1), this effect of L-AP4 might be considered pro-parkinsonian. To our knowledge there are no previous reports of decreased pausing in GPe neurons in parkinsonism, but an increase in pausing in the GPe has sometimes been associated with hyperkinetic disorders (Bronfeld et al., 2010; Matsumura et al., 1995).
4.5 Concluding Remarks
Our results demonstrate that mGluR4 receptors are widely distributed throughout the primate basal ganglia circuitry in both normal and parkinsonian conditions, and suggest that the potential antiparkinsonian effects of mGluR4-related compounds in primates is not simply explained through an inhibition of GABA release from striatopallidal terminals, but may depend on a more complex regulation of glutamatergic and GABAergic transmission at various key synapses within the basal ganglia and, possibly, also in other brain regions. Such effects need to be taken into account in future studies evaluating the efficacy of mGluR4-mediated antiparkinsonian therapy in MPTP-treated monkeys.
Research Highlights.
Basal ganglia mGluR4 in primates is concentrated in GP, SNr and striatum.
mGluR4 is found in glutamatergic and GABAergic terminals in the GPe and striatum.
Local group III mGluRs activation increases pallidal activity in monkeys.
Group III mGluRs activation increases and decreases striatal activity in monkeys.
Group III mGluRs activation increases firing irregularity in the monkey striatum.
Acknowledgments
This work was supported by grants from the RJG Foundation, the Michael J Fox Foundation, the Udall Center for Parkinson’s Disease Research (NIH P50 NS071669), and the Yerkes National Primate Research Center base grant (NIH P51 RR-000165). Many thanks are due to Jeff Conn, Colleen Niswender, Corey Hopkins and other members of the Vanderbilt Center for Neuroscience Drug Discovery for providing VU0155041, and to Jeff Paré and Susan Jenkins for technical support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference list
- Aosaki T, Tsubokawa H, Ishida A, Watanabe K, Graybiel AM, Kimura M. Responses of tonically active neurons in the primate’s striatum undergo systematic changes during behavioral sensorimotor conditioning. J Neurosci. 1994;14:3969–3984. doi: 10.1523/JNEUROSCI.14-06-03969.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blazquez PM, Fujii N, Kojima J, Graybiel AM. A network representation of response probability in the striatum. Neuron. 2002;33:973–982. doi: 10.1016/s0896-6273(02)00627-x. [DOI] [PubMed] [Google Scholar]
- Beurrier C, Lopez S, Revy D, Selvam C, Goudet C, Lherondel M, Gubellini P, Kerkerian-LeGoff L, Acher F, Pin JP, Amalric M. Electrophysiological and behavioral evidence that modulation of metabotropic glutamate receptor 4 with a new agonist reverses experimental parkinsonism. FASEB J. 2009;23:3619–3628. doi: 10.1096/fj.09-131789. [DOI] [PubMed] [Google Scholar]
- Blandini F, Greenamyre JT. Prospects of glutamate antagonists in the therapy of Parkinson’s disease. Fundam Clin Pharmacol. 1998;12:4–12. doi: 10.1111/j.1472-8206.1998.tb00918.x. [DOI] [PubMed] [Google Scholar]
- Blandini F, Greenamyre JT, Nappi G. The role of glutamate in the pathophysiology of Parkinson’s disease. Funct Neurol. 1996;11:3–15. [PubMed] [Google Scholar]
- Bogenpohl JW, Galvan A, Hu X, Pare JF, Wichmann T, Smith Y. 7th FENS Forum of European Neuroscience. Amsterdam, Netherlands: 2010. Electrophysiological and behavioral effects of activation of group III metabotropic glutamate receptors in the striatopallidal complex of parkinsonian monkeys. [Google Scholar]
- Bogenpohl JW, Galvan A, Hu X, Pare JF, Wichmann T, Smith Y. Subcellular localization and electrophysiological effects of metabotropic glutamate receptor 4 activation in the striatopallidal complex of parkinsonian monkeys; Society for Neuroscience 41st Annual Meeting; Washington D.C. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley SR, Standaert DG, Rhodes KJ, Rees HD, Testa CM, Levey AI, Conn PJ. Immunohistochemical localization of subtype 4a metabotropic glutamate receptors in the rat and mouse basal ganglia. J Comp Neurol. 1999;407:33–46. [PubMed] [Google Scholar]
- Breysse N, Amalric M, Salin P. Metabotropic glutamate 5 receptor blockade alleviates akinesia by normalizing activity of selective basal-ganglia structures in parkinsonian rats. J Neurosci. 2003;23:8302–8309. doi: 10.1523/JNEUROSCI.23-23-08302.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronfeld M, Belelovsky K, Erez Y, Bugaysen J, Korngreen A, Bar-Gad I. Bicuculline-induced chorea manifests in focal rather than globalized abnormalities in the activation of the external and internal globus pallidus. J Neurophysiol. 2010;104:3261–3275. doi: 10.1152/jn.00093.2010. [DOI] [PubMed] [Google Scholar]
- Calabresi P, Mercuri NB, Sancesario G, Bernardi G. Electrophysiology of dopamine-denervated striatal neurons. Implications for Parkinson’s disease. Brain. 1993;116 (Pt 2):433–452. [PubMed] [Google Scholar]
- Calabresi P, Pisani A, Mercuri NB, Bernardi G. The corticostriatal projection: from synaptic plasticity to dysfunctions of the basal ganglia. Trends Neurosci. 1996;19:19–24. doi: 10.1016/0166-2236(96)81862-5. [DOI] [PubMed] [Google Scholar]
- Chase TN, Bibbiani F, Oh JD. Striatal glutamatergic mechanisms and extrapyramidal movement disorders. Neurotox Res. 2003;5:139–146. doi: 10.1007/BF03033378. [DOI] [PubMed] [Google Scholar]
- Corti C, Aldegheri L, Somogyi P, Ferraguti F. Distribution and synaptic localisation of the metabotropic glutamate receptor 4 (mGluR4) in the rodent CNS. Neuroscience. 2002;110:403–420. doi: 10.1016/s0306-4522(01)00591-7. [DOI] [PubMed] [Google Scholar]
- Cuomo D, Martella G, Barabino E, Platania P, Vita D, Madeo G, Selvam C, Goudet C, Oueslati N, Pin JP, Acher F, Pisani A, Beurrier C, Melon C, Kerkerian-Le Goff L, Gubellini P. Metabotropic glutamate receptor subtype 4 selectively modulates both glutamate and GABA transmission in the striatum: implications for Parkinson’s disease treatment. J Neurochem. 2009;109:1096–1105. doi: 10.1111/j.1471-4159.2009.06036.x. [DOI] [PubMed] [Google Scholar]
- DeLong MR. Activity of pallidal neurons during movement. J Neurophysiol. 1971;34:414–427. doi: 10.1152/jn.1971.34.3.414. [DOI] [PubMed] [Google Scholar]
- Deltheil T, Turle-Lorenzo N, Amalric M. Orthosteric group III mGlu receptor agonists for Parkinson’s disease treatment: symptomatic and neurochemical action on pallidal synaptic transmission. 7th International Meeting on Metabotropic Glutamate Receptors; Taormina, Italy. 2011. [Google Scholar]
- East SP, Gerlach K. mGluR4 positive allosteric modulators with potential for the treatment of Parkinson’s disease: WO09010455. Expert Opin Ther Pat. 2010;20:441–445. doi: 10.1517/13543770903551295. [DOI] [PubMed] [Google Scholar]
- Elias S, Joshua M, Goldberg JA, Heimer G, Arkadir D, Morris G, Bergman H. Statistical properties of pauses of the high-frequency discharge neurons in the external segment of the globus pallidus. J Neurosci. 2007;27:2525–2538. doi: 10.1523/JNEUROSCI.4156-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engers DW, Field JR, Le U, Zhou Y, Bolinger JD, Zamorano R, Blobaum AL, Jones CK, Jadhav S, Weaver CD, Conn PJ, Lindsley CW, Niswender CM, Hopkins CR. Discovery, synthesis, and structure-activity relationship development of a series of N-(4-acetamido)phenylpicolinamides as positive allosteric modulators of metabotropic glutamate receptor 4 (mGlu(4)) with CNS exposure in rats. J Med Chem. 2011;54:1106–1110. doi: 10.1021/jm101271s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engers DW, Gentry PR, Williams R, Bolinger JD, Weaver CD, Menon UN, Conn PJ, Lindsley CW, Niswender CM, Hopkins CR. Synthesis and SAR of novel, 4-(phenylsulfamoyl)phenylacetamide mGlu4 positive allosteric modulators (PAMs) identified by functional high-throughput screening (HTS) Bioorg Med Chem Lett. 2010;20:5175–5178. doi: 10.1016/j.bmcl.2010.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engers DW, Niswender CM, Weaver CD, Jadhav S, Menon UN, Zamorano R, Conn PJ, Lindsley CW, Hopkins CR. Synthesis and evaluation of a series of heterobiarylamides that are centrally penetrant metabotropic glutamate receptor 4 (mGluR4) positive allosteric modulators (PAMs) J Med Chem. 2009;52:4115–4118. doi: 10.1021/jm9005065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galarraga E, Bargas J, Martinez-Fong D, Aceves J. Spontaneous synaptic potentials in dopamine-denervated neostriatal neurons. Neurosci Lett. 1987;81:351–355. doi: 10.1016/0304-3940(87)90409-5. [DOI] [PubMed] [Google Scholar]
- Galvan A, Hu X, Smith Y, Wichmann T. Localization and function of GABA transporters in the globus pallidus of parkinsonian monkeys. Exp Neurol. 2010;223:505–515. doi: 10.1016/j.expneurol.2010.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvan A, Hu X, Smith Y, Wichmann T. Localization and pharmacological modulation of GABA-B receptors in the globus pallidus of parkinsonian monkeys. Exp Neurol. 2011;229:429–439. doi: 10.1016/j.expneurol.2011.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvan A, Villalba RM, West SM, Maidment NT, Ackerson LC, Smith Y, Wichmann T. GABAergic modulation of the activity of globus pallidus neurons in primates: in vivo analysis of the functions of GABA receptors and GABA transporters. J Neurophysiol. 2005;94:990–1000. doi: 10.1152/jn.00068.2005. [DOI] [PubMed] [Google Scholar]
- Greenamyre JT. Glutamatergic influences on the basal ganglia. Clin Neuropharmacol. 2001;24:65–70. doi: 10.1097/00002826-200103000-00001. [DOI] [PubMed] [Google Scholar]
- Gubellini P, Picconi B, Bari M, Battista N, Calabresi P, Centonze D, Bernardi G, Finazzi-Agro A, Maccarrone M. Experimental parkinsonism alters endocannabinoid degradation: implications for striatal glutamatergic transmission. J Neurosci. 2002;22:6900–6907. doi: 10.1523/JNEUROSCI.22-16-06900.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliday DM, Rosenberg JR, Amjad AM, Breeze P, Conway BA, Farmer SF. A framework for the analysis of mixed time series/point process data--theory and application to the study of physiological tremor, single motor unit discharges and electromyograms. Prog Biophys Mol Biol. 1995;64:237–278. doi: 10.1016/s0079-6107(96)00009-0. [DOI] [PubMed] [Google Scholar]
- Hopkins CR, Lindsley CW, Niswender CM. mGluR4-positive allosteric modulation as potential treatment for Parkinson’s disease. Future Med Chem. 2009;1:501–513. doi: 10.4155/fmc.09.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes AJ. Drug treatment of Parkinson’s disease in the 1990s. Achievements and future possibilities. Drugs. 1997;53:195–205. doi: 10.2165/00003495-199753020-00002. [DOI] [PubMed] [Google Scholar]
- Johnson KA, Conn PJ, Niswender CM. Glutamate receptors as therapeutic targets for Parkinson’s disease. CNS Neurol Disord Drug Targets. 2009;8:475–491. doi: 10.2174/187152709789824606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerner JA, Standaert DG, Penney JB, Jr, Young AB, Landwehrmeyer GB. Expression of Group II and III mGluR mRNAs in identified striatal and cortical neurons. Society for Neuroscience Annual Meeting; New Orleans, LA. 1997. [Google Scholar]
- Kimura M. The role of primate putamen neurons in the association of sensory stimuli with movement. Neurosci Res. 1986;3:436–443. doi: 10.1016/0168-0102(86)90035-0. [DOI] [PubMed] [Google Scholar]
- Kliem MA, Pare JF, Khan ZU, Wichmann T, Smith Y. Ultrastructural localization and function of dopamine D1-like receptors in the substantia nigra pars reticulata and the internal segment of the globus pallidus of parkinsonian monkeys. Eur J Neurosci. 2010;31:836–851. doi: 10.1111/j.1460-9568.2010.07109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliem MA, Wichmann T. A method to record changes in local neuronal discharge in response to infusion of small drug quantities in awake monkeys. J Neurosci Methods. 2004;138:45–49. doi: 10.1016/j.jneumeth.2004.03.015. [DOI] [PubMed] [Google Scholar]
- Konieczny J, Wardas J, Kuter K, Pilc A, Ossowska K. The influence of group III metabotropic glutamate receptor stimulation by (1S,3R,4S)-1-aminocyclo-pentane-1,3,4-tricarboxylic acid on the parkinsonian-like akinesia and striatal proenkephalin and prodynorphin mRNA expression in rats. Neuroscience. 2007;145:611–620. doi: 10.1016/j.neuroscience.2006.12.006. [DOI] [PubMed] [Google Scholar]
- Legendy CR, Salcman M. Bursts and recurrences of bursts in the spike trains of spontaneously active striate cortex neurons. J Neurophysiol. 1985;53:926–939. doi: 10.1152/jn.1985.53.4.926. [DOI] [PubMed] [Google Scholar]
- Liang L, DeLong MR, Papa SM. Inversion of dopamine responses in striatal medium spiny neurons and involuntary movements. J Neurosci. 2008;28:7537–7547. doi: 10.1523/JNEUROSCI.1176-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez S, Turle-Lorenzo N, Acher F, De Leonibus E, Mele A, Amalric M. Targeting group III metabotropic glutamate receptors produces complex behavioral effects in rodent models of Parkinson’s disease. J Neurosci. 2007;27:6701–6711. doi: 10.1523/JNEUROSCI.0299-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez S, Turle-Lorenzo N, Johnston TH, Brotchie JM, Schann S, Neuville P, Amalric M. Functional interaction between adenosine A2A and group III metabotropic glutamate receptors to reduce parkinsonian symptoms in rats. Neuropharmacology. 2008;55:483–490. doi: 10.1016/j.neuropharm.2008.06.038. [DOI] [PubMed] [Google Scholar]
- MacInnes N, Messenger MJ, Duty S. Activation of group III metabotropic glutamate receptors in selected regions of the basal ganglia alleviates akinesia in the reserpine-treated rat. Br J Pharmacol. 2004;141:15–22. doi: 10.1038/sj.bjp.0705566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marino MJ, Conn PJ. Glutamate-based therapeutic approaches: allosteric modulators of metabotropic glutamate receptors. Curr Opin Pharmacol. 2006;6:98–102. doi: 10.1016/j.coph.2005.09.006. [DOI] [PubMed] [Google Scholar]
- Marino MJ, Hess JF, Liverton N. Targeting the metabotropic glutamate receptor mGluR4 for the treatment of diseases of the central nervous system. Curr Top Med Chem. 2005;5:885–895. doi: 10.2174/1568026054750263. [DOI] [PubMed] [Google Scholar]
- Marino MJ, Williams DL, Jr, O’Brien JA, Valenti O, McDonald TP, Clements MK, Wang R, DiLella AG, Hess JF, Kinney GG, Conn PJ. Allosteric modulation of group III metabotropic glutamate receptor 4: a potential approach to Parkinson’s disease treatment. Proc Natl Acad Sci U S A. 2003;100:13668–13673. doi: 10.1073/pnas.1835724100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui T, Kita H. Activation of group III metabotropic glutamate receptors presynaptically reduces both GABAergic and glutamatergic transmission in the rat globus pallidus. Neuroscience. 2003;122:727–737. doi: 10.1016/j.neuroscience.2003.08.032. [DOI] [PubMed] [Google Scholar]
- Matsumura M, Tremblay L, Richard H, Filion M. Activity of pallidal neurons in the monkey during dyskinesia induced by injection of bicuculline in the external pallidum. Neuroscience. 1995;65:59–70. doi: 10.1016/0306-4522(94)00484-m. [DOI] [PubMed] [Google Scholar]
- Nakajima Y, Iwakabe H, Akazawa C, Nawa H, Shigemoto R, Mizuno N, Nakanishi S. Molecular characterization of a novel retinal metabotropic glutamate receptor mGluR6 with a high agonist selectivity for L-2-amino-4-phosphonobutyrate. J Biol Chem. 1993;268:11868–11873. [PubMed] [Google Scholar]
- Nanda B, Galvan A, Smith Y, Wichmann T. Effects of stimulation of the centromedian nucleus of the thalamus on the activity of striatal cells in awake rhesus monkeys. Eur J Neurosci. 2009;29(3):588–98. doi: 10.1111/j.1460-9568.2008.06598.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen JB, Conway BA, Halliday DM, Perreault MC, Hultborn H. Organization of common synaptic drive to motoneurones during fictive locomotion in the spinal cat. J Physiol. 2005;569:291–304. doi: 10.1113/jphysiol.2005.091744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niswender CM, Conn PJ. Metabotropic glutamate receptors: physiology, pharmacology, and disease. Annu Rev Pharmacol Toxicol. 2010;50:295–322. doi: 10.1146/annurev.pharmtox.011008.145533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niswender CM, Johnson KA, Weaver CD, Jones CK, Xiang Z, Luo Q, Rodriguez AL, Marlo JE, de Paulis T, Thompson AD, Days EL, Nalywajko T, Austin CA, Williams MB, Ayala JE, Williams R, Lindsley CW, Conn PJ. Discovery, characterization, and antiparkinsonian effect of novel positive allosteric modulators of metabotropic glutamate receptor 4. Mol Pharmacol. 2008;74:1345–1358. doi: 10.1124/mol.108.049551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ossowska K, Konieczny J, Wardas J, Pietraszek M, Kuter K, Wolfarth S, Pilc A. An influence of ligands of metabotropic glutamate receptor subtypes on parkinsonian-like symptoms and the striatopallidal pathway in rats. Amino Acids. 2007;32:179–188. doi: 10.1007/s00726-006-0317-y. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Huang XF, Toga AW. The rhesus monkey brain in stereotaxic coordinates. San Diego, CA: Academic Press; 2000. [Google Scholar]
- Peters A, Palay SL, Webster Hd. The fine structure of the nervous system: neurons and their supporting cells. Oxford University Press; New York: 1991. [Google Scholar]
- Shink E, Smith Y. Differential synaptic innervation of neurons in the internal and external segments of the globus pallidus by the GABA- and glutamate-containing terminals in the squirrel monkey. J Comp Neurol. 1995;358:119–141. doi: 10.1002/cne.903580108. [DOI] [PubMed] [Google Scholar]
- Smith Y, Bevan MD, Shink E, Bolam JP. Microcircuitry of the direct and indirect pathways of the basal ganglia. Neuroscience. 1998;86:353–387. doi: 10.1016/s0306-4522(98)00004-9. [DOI] [PubMed] [Google Scholar]
- Smith Y, Raju DV, Pare JF, Sidibe M. The thalamostriatal system: a highly specific network of the basal ganglia circuitry. Trends Neurosci. 2004;27:520–527. doi: 10.1016/j.tins.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Smith Y, Wichmann T, Factor SA, Delong MR. Parkinson’s Disease Therapeutics: New Developments and Challenges Since the Introduction of Levodopa. Neuropsychopharmacology. 2012 doi: 10.1038/npp.2011.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares J, Kliem MA, Betarbet R, Greenamyre JT, Yamamoto B, Wichmann T. Role of external pallidal segment in primate parkinsonism: comparison of the effects of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced parkinsonism and lesions of the external pallidal segment. J Neurosci. 2004;24:6417–6426. doi: 10.1523/JNEUROSCI.0836-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenti O, Marino MJ, Wittmann M, Lis E, DiLella AG, Kinney GG, Conn PJ. Group III metabotropic glutamate receptor-mediated modulation of the striatopallidal synapse. J Neurosci. 2003;23:7218–7226. doi: 10.1523/JNEUROSCI.23-18-07218.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wichmann T, Kliem MA, DeLong MR. Antiparkinsonian and behavioral effects of inactivation of the substantia nigra pars reticulata in hemiparkinsonian primates. Exp Neurol. 2001;167:410–424. doi: 10.1006/exnr.2000.7572. [DOI] [PubMed] [Google Scholar]
- Wichmann T, Soares J. Neuronal firing before and after burst discharges in the monkey basal ganglia is predictably patterned in the normal state and altered in parkinsonism. J Neurophysiol. 2006;95:2120–2133. doi: 10.1152/jn.01013.2005. [DOI] [PubMed] [Google Scholar]