Abstract
Background
To investigate the mechanism underlying the anxiolytic properties of riluzole, a glutamate-modulating agent, we previously studied the effect of this drug on hippocampal N-Acetylaspartate (NAA) and volume in patients with Generalized Anxiety Disorder (GAD). In the same cohort, we now extend our investigation to the occipital cortex, a brain region that was recently implicated in the antidepressant effect of riluzole.
Methods
Fourteen medication-free adult patients with GAD received 8-week of open-label riluzole. Ten healthy subjects served as a comparison group. The healthy group did not receive riluzole treatment. Both groups underwent magnetic resonance imaging and spectroscopy at baseline and at the end of Week 8. Hamilton Anxiety Rating Scale (HAM-A) and Penn State Worry Questionnaire (PSWQ) were used as the primary and secondary outcome measures, respectively.
Results
At baseline, we found clusters of increased cortical thickness in the occipital region in GAD compared to healthy subjects. In the right hemisphere, eight weeks of treatment reduced occipital cortical thickness in the GAD group (t = 3.67, p = 0.004). In addition, the improvement in HAM-A scores was negatively correlated with post-treatment right occipital NAA (r = − 0.68, p = 0.008), and with changes in NAA levels (r = − 0.53, p = 0.051). In the left hemisphere, we found positive associations between changes in occipital cortical thickness and improvement in HAM-A (r = 0.60, p = 0.04) and PSWQ (r = 0.62, p = 0.03).
Conclusion
These pilot findings implicate the occipital cortex as a brain region associated with pathology and clinical improvement in GAD. In addition, the region specific effect of riluzole implies a distinct pathophysiology in the occipital cortex – compared to other, previously studied, frontolimbic brain structures.
Keywords: Riluzole, generalized anxiety disorder, biomarkers, glutamate, N-Acetylaspartate, occipital cortex, magnetic resonance spectroscopy, structural MRI
Introduction
Anxiety and mood disorders are among the leading causes of disability worldwide [10]. Yet, treatment options have been largely restricted to monoamine-based antidepressants with limited efficacy [25, 31]. More recently, converging evidence strongly implicated the glutamatergic system in the pathophysiology and treatment of mood and anxiety disorders. These early findings generated excitement in the field to develop a novel class of drug which directly targets the glutamatergic system [28]. One such medication is riluzole, an agent possessing neuroprotective properties approved by the US Food and Drug Administration for treatment of Amyotrophic Lateral Sclerosis. Riluzole is believed to exert its pharmacological effects primarily by reducing pre-synaptic glutamate release and potentiating glutamate reuptake [23, 34]. In pilot open-label studies, riluzole showed a potent anxiolytic and antidepressant effect (for review see [23, 34]). Hence, unraveling the neural effects of riluzole could have a major impact on drug development, biomarker identification, and our understanding of anxiety and mood disorders. In the current pilot study, we employed a multimodal pharmaco-neuroimaging paradigm to investigate the effect of riluzole in patients with generalized anxiety disorder (GAD).
Using proton magnetic resonance spectroscopy (1H-MRS), we previously found a positive correlation between an increase in hippocampal N-Acetylaspartate (NAA) – a marker of neuronal integrity and metabolism – and improvement in anxiety scores in patients with GAD treated with 8 weeks of riluzole [16]. Moreover, we demonstrated a positive correlation between increased hippocampal volume, and anxiety symptom improvement [1]. Taken together, these findings support a positive association between markers of neuroplasticity (i.e. volume and NAA) and clinical improvement following riluzole treatment. Intriguingly, in patients with bipolar depression treated with riluzole, Brennan et al. (2010) reported a similar positive correlation between prefrontal NAA levels and clinical improvement. However, an unexpected negative association was found between NAA changes in the occipital cortex and improvement in depressive severity [7]. These occipital findings are particularly important given that the occipital cortex has been previously associated with mood and anxiety disorders [27, 32]. Therefore, we extended our previous analysis to examine the effect of riluzole on the occipital cortex in GAD patients. The involvement of the occipital lobe in the pathophysiology of GAD has long been hypothesized. This was primarily based on the presence of high concentration of benzodiazepine receptors in this brain region [6, 21] and the reduction of occipital cortex metabolism and activity following treatment with benzodiazepines in GAD patients [8, 9]. In fact, an early PET study showed increased metabolism in the occipital lobe of GAD patients compared to healthy controls [32].
The current study aims were -1- to investigate the relationship between occipital NAA levels and clinical improvement in GAD patients receiving 8 weeks of riluzole. -2- To examine the association between morphological changes in occipital cortex and clinical improvement following riluzole treatment. -3- To conduct a exploratory analysis of whole brain cortical thickness in GAD patients and healthy volunteers.
Material and Methods
Subjects
Sample characteristics were previously described [16]. Fourteen GAD patients (8 women, mean (±SEM) age 33.9 ± 2.7 years) and 10 healthy subjects (6 women, mean (±SEM) age 30.3 ± 2.4 years) successfully completed baseline high-resolution MRI scans. Thirteen GAD and 8 healthy subjects completed an additional MRI at Week 8. Fourteen GAD patients had viable 1H-MRS data at both assessments. Subjects flow and attrition were reported elsewhere [1, 16]; all available data were used in the statistical analyses. GAD patients were medication-free for at least 2 weeks prior to the initial scan. Following baseline scans, GAD patients, but not the healthy group, received open-label riluzole (50 mg twice per day) monotherapy for 8 weeks. All patients met DSM-IV-TR criteria for GAD according to the Structured Clinical Interview for DSM-IV (SCID) [12]. Healthy subjects had no lifetime history of Axis I psychiatric disorders, as established by the SCID-NP [30] (see supplements). An Institutional Review Board approved the study. All participants provided written informed consent prior to research procedures.
MR acquisition and processing
For detailed description of MR imaging and spectroscopy methods see previous reports [1, 16]. In summary, MRI and MRS acquisition were performed using a 1.5T GE Genesis Horizon 5.x Signa MR system. The 1H MRS imaging (MRSI) scans were performed using the method of Duyn et al. [11]. The raw MRSI data were processed offline and analyzed voxel by voxel. Voxels that best covered the right and left occipital cortex in each subject were selected on the basis of their location on the matching high-resolution MR images. Peak areas derived from spectral fitting were converted to “absolute” (i.e., molar) metabolite concentrations using phantom replacement methodology (see Supplements). Cortical surface reconstruction was performed with the fully automated Freesurfer image analysis package [13]. FreeSurfer post-processing quality checking routines were followed; no manual corrections were necessary. For the region of interest (ROI) analyses, we used the lateral occipital segmentation (the largest occipital structure) generated by FreeSurfer based on the Desikan-Killiany Atlas (for further details see Supplements).
Statistical analyses
Hamilton Anxiety Rating Scale (HAM-A) and Penn State Worry Questionnaire (PSWQ), a self-reported anxiety rating scale, were used as the primary and secondary clinical outcome measures, respectively. To examine the effect of riluzole on occipital NAA and thickness, a general linear model (GLM) with repeated measures (mixed effects) was constructed, with time of scan (baseline & Week 8) as the within-subject factor (repeated measure) and group (GAD vs. healthy) as the between-subjects factor. To reduce outliers’ effect and because few variables were not normally distributed, Spearman’s Rank Order was used for correlational analysis. Pearson’s correlations are provided in Supplements. The correlational analysis was limited to the GAD group. All tests were two-tailed, with significance level set at p ≤ 0.05.
Cortical Thickness Analysis
We used the QDEC module in FreeSurfer to estimate a GLM at each vertex across the cortical surface, with cortical thickness as dependent variable, group (GAD vs. healthy) as a discrete factor, and age as a nuisance factor. This procedure generated statistical maps at a threshold of p ≤ 0.01, uncorrected. Given the small sample size, correction for multiple comparisons was not permitted. Hence, caution is warranted in interpreting this exploratory analysis, as some of the observed difference could be a chance correlation – particularly in the case of small clusters.
Results
Clinical outcomes were previously reported [15]. Briefly, GAD patients had a chronic course of illness (mean (±SEM) 14.7 ± 2.5 years) with moderate severity (HAM-A, mean (±SEM) 20.0 ± 0.9). Eight weeks of riluzole treatment exerted a significant reduction in anxiety scores, with 64% decline in HAM-A scores over the treatment period (see [15]). Age, gender, body mass index (BMI), IQ, and education level did not differ between the GAD and healthy groups (p > 0.4).
-1- What is the effect of riluzole on occipital NAA in GAD patients? What is the correlation between occipital NAA and clinical improvement?
Chronic riluzole administration had no significant effect on NAA levels in right or left occipital cortex, with no group, time, or group-by-time effects (p > 0.4; Table S1). In the GAD group, there was a strong negative correlation between Week 8 right occipital NAA and improvement in HAM-A (r = − 0.68, p = 0.008; Fig. 1). A similar inverse relationship was found with PSWQ at a trend level of significance (r = − 0.50, p = 0.07). In the left occipital cortex, NAA levels negatively correlated at a trend level with improvement in HAM-A (r = − 0.52, p = 0.06), but not with PSWQ (r = − 0.40, p = 0.2).
Figure 1.
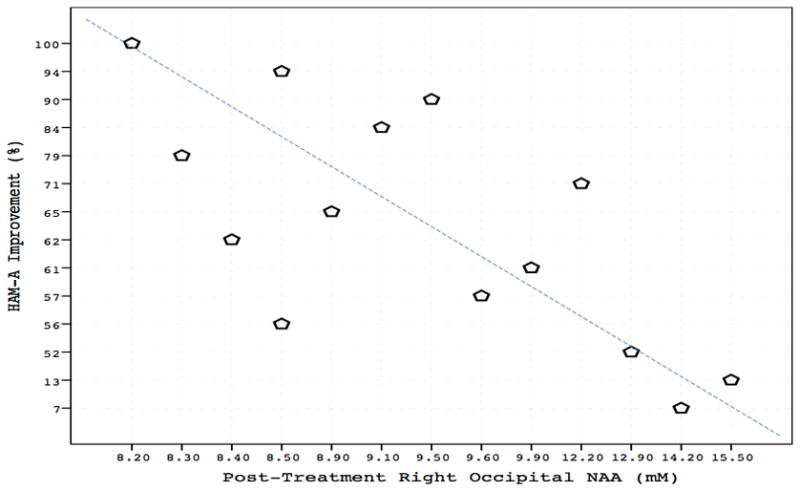
In GAD patients, there was a strong negative correlation between right occipital NAA concentration and improvement in HAM-A scores (r = − 0.68, p = 0.008) following 8 weeks of riluzole treatment.
Then, we examined the association between changes in occipital NAA (Week 8 minus baseline) and clinical improvement in the GAD group. There was a trend for a negative correlation between reduction in right occipital NAA and HAM-A improvement (r = − 0.53, p = 0.051), but no other correlations (p > 0.1; Table S2 & S3).
-2- What is the effect of riluzole on occipital cortical thickness? What is the relationship between occipital cortical thickness and clinical improvement?
For right occipital cortical thickness, there was significant group-by-time interaction (F(1,18) = 8.20, p = 0.01; Fig. S1), but no main effect of group or time (Table S1). A post-hoc paired t-test within the GAD group showed a significant reduction in right occipital cortical thickness following treatment with riluzole (t = 3.67, p = 0.004). Riluzole had no significant effect on left occipital cortical thickness; no group, time, or group-by-time effects (p > 0.4; Table S1).
Left occipital cortical thickness changes (Week 8 minus baseline) positively correlated with improvement in HAM-A (r = 0.60, p = 0.04) and PSWQ (r = 0.62, p = 0.03; Fig. S2). No other significant correlations were found (p > 0.05; Table S2 & S3).
-3- How does the baseline cortical thickness of the GAD group compare to the healthy group?
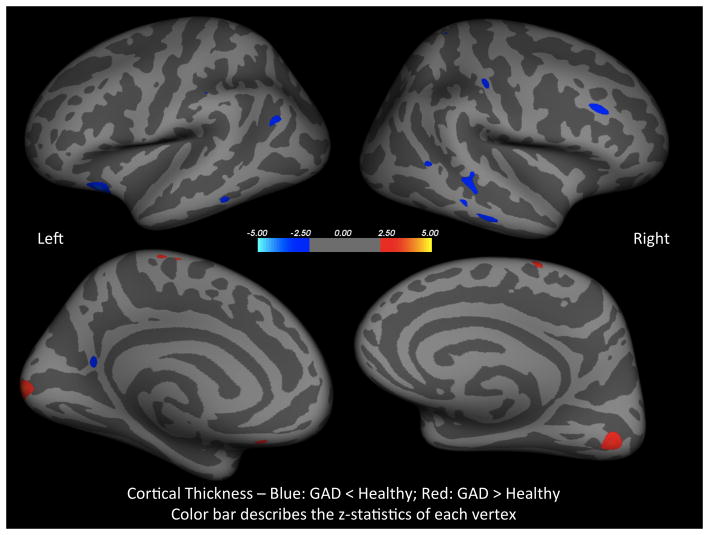
Controlling for age, baseline cortical thickness maps showed few clusters of reduced cortical thickness in the GAD group, compared to the healthy group, in the right and left middle temporal, right rostral middle frontal, right postcentral, left inferior parietal, and left lateral orbitofrontal (Fig. 2). Also, there were clusters of increased cortical thickness in the GAD group in the left lateral occipital, right lingual, right and left paracentral, and a small cluster in the left medial orbitofrontal (Fig. 2).
Figure 2.
Controlling for age, these statistical maps show clusters of reduced (blue) and increased (red) cortical thickness in the GAD group, as compared to healthy control (z-statistics of each vertex are color coded).
Discussion
In this brief report, we investigated the relationship between occipital measures (structural and metabolic) and clinical outcomes in GAD patients treated for 8 weeks with the glutamate modulating agent riluzole. First, we observed a pattern of negative correlation between post-treatment occipital NAA and clinical improvement (Fig. 1). In the right occipital cortex, this pattern translated in a trend toward negative association between changes in NAA following riluzole treatment and the clinician-rated anxiety improvement. Second, the exploratory analysis showed bilateral clusters of increased baseline cortical thickness in the occipital brain of GAD group (Fig. 2). Moreover, the pharmacologic treatment was associated with a reduction of cortical thickness in the right occipital cortex (Fig. S1). In contrast, we observed a significant positive correlation between changes in the left occipital cortical thickness and improvement in clinician and self-rated anxiety scores (Fig. S2). Finally, we observed several differences in baseline cortical thickness between groups, as shown in Fig. 2.
A main finding of the current study is the region specific effect of riluzole treatment (i.e. occipital vs. hippocampus/anterior cingulate). As mentioned earlier, we previously found, in the same cohort, a significant positive correlation between clinical improvement and hippocampal NAA and volume [1, 16]. Here, we see a reduction in the right occipital cortical thickness following treatment, a positive association between improvement and left occipital thickness, and a pattern of negative correlations between occipital NAA and clinical improvement. These latter findings are consistent with Brennan et al. report [10], which showed similar negative correlations of occipital NAA and clinical improvement in patients with bipolar depression treated with riluzole. In their study, positive correlation was seen between NAA levels in anterior cingulate cortex and clinical improvement [7]. Together these pilot studies suggest that the occipital cortex may play a distinctive role in influencing expression of anxiety and response to anxiolytic medication, in comparison to other rostral frontolimbic brain structures, such as hippocampus and anterior cingulate.
To elucidate this hypothesis, we briefly review existing supportive evidence. Previous studies showed increased metabolism in the occipital lobe, but reduced metabolic rate in the basal ganglia, of GAD patients [32]. Moreover, benzodiazepine treatment of patients with GAD resulted in a reduction in glucose metabolic rate of the occipital cortex, but a relative increase in the thalamus and basal ganglia metabolism [9]. Since glucose brain consumption has been shown to largely reflect glutamatergic activity [29], the elevated metabolism evident in the occipital cortex implies increased pretreatment glutamatergic neurotransmission in this brain area. Additional evidence suggestive of region specific glutamatergic alteration, distinguishing between the occipital and frontolimbic structures, comes from 1H-MRS studies in depressed patients diagnosed with major depressive disorder (MDD). These studies showed increased glutamate levels in the occipital brain [3, 27]. In contrast, there is a reduction of glutamate concentration in the hippocampus [5], amygdala [19], prefrontal cortex [14, 18], and anterior cingulate [2, 22] of depressed patients with MDD; although these glutamatergic abnormalities were not evident in all MDD patients [4, 20, 24] (for recent review see [33]). Together these data provide evidence for region specific alterations in the glutamatergic system, showing increased occipital, but decreased frontolimbic, activity and total glutamate levels as suggested by PET studies in GAD and 1H-MRS in MDD, respectively. Hence, we speculate that the glutamate modulating agent riluzole may have differential impact on the occipital brain as compared the other frontolimbic structures.
An important question concerns how NAA levels are affected by these glutamatergic alterations. In summary, in addition to the role of NAA as a marker of neuronal density and integrity, recent accumulating evidence supports the role of NAA as a subacute index of neuronal metabolism and overall glutamatergic activity (see Supplements). Moreover, a direct positive correlation between NAA and glutamate concentration in the occipital brain has been previously reported [27]. Thus, it is possible that riluzole effect on the glutamatergic system, subsequently affected total NAA levels. However, further investigations are needed to explore this possibility. Future studies may use ultra-high-field (7T) 1H-MRS protocols with short echo time optimized to resolve glutamine/glutamate signals [17], or 13C-MRS to directly measure glutamine/glutamate cycling [26] at baseline and following treatment with glutamate modulating agents, such as riluzole. Further, multimodal approaches combining spectroscopy with structural measures may provide additional insight into underlying neuronal mechanisms.
Strength and Limitations
The strengths of the current study include a well-matched healthy control group that received repeated scans, a medication-free patient cohort, and a multimodal neuroimaging approach. In addition, we utilized well-validated methods -1- of spectroscopy quantification to measure the absolute concentration of NAA, and -2- of surface-based morphometric analysis to accurately measure cortical thickness. Yet, it is important to be aware of the limitations of this study, particularly the sample size and the lack of direct measure of glutamate levels – an amino acid neurotransmitter targeted by riluzole. In addition, the study design cannot distinguish between primary and secondary consequences of riluzole intake on brain metabolites and structure.
Conclusions
This pilot study provided preliminary evidence detailing the effect of riluzole, a glutamate-based drug with anxiolytic activity in GAD patients, on occipital cortical thickness and NAA levels. In addition, the regionally specific effect of riluzole may suggest a distinct pathophysiology in the occipital cortex compared to other rostral frontolimbic brain structures. Future studies are needed to confirm and extend these findings and to directly probe the glutamatergic system to better understand the neurobiological mechanisms of therapeutics for this disorder.
Supplementary Material
Highlights.
The GAD group had clusters of increased cortical thickness in the occipital brain.
Riluzole treatment reduced right occipital cortical thickness in the GAD group.
Occipital NAA was negatively associated with clinical improvement.
Overall the findings suggested a distinct occipital pathophysiology in GAD.
Acknowledgments
Funding of this study was provided by Brain and Behavior Research Foundation, Sackler Institute of Columbia University, National Institute of Mental Health (K23-MH-069656), and National Institute on Drug Abuse (T32-DA-022975). The funding sources have no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Footnotes
Contributors
C.G.A. conceptualized the MRI study, managed the literature searches, undertook the statistical analysis, contributed to the interpretation of the data and wrote the manuscript. J.D.C. conceptualized the MRI study and contributed to the interpretation of the data. A.J. and J.R.S. performed the MRI image processing, segmentations, quality control and analyses. D.C.S. and X.M. performed the MRS and MRI studies, and MRS image processing and analysis. S.J.M. conceived the study, wrote the protocol, performed the study, and contributed to the interpretation of the data. All authors contributed to and have approved the final manuscript.
Disclaimer: The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the United States government.
Conflict of interest
JDC received grant support from NIMH, NYSTEM, GlaxoSmithKline, Pfizer, and Alexza Pharmaceuticals. He is on the Pfizer advisory board and gives talks for BMS, AstraZeneca, GSK, and Pfizer. SJM: received research funding or salary support over the last three years from the Banner Family Fund, Brain and Behavior Fund (NARSAD), The Brown Foundation, Inc., Bristol-Myers Squibb, Department of Veterans Affairs, Evotec, Johnson & Johnson, and the National Institute of Mental Health. He has received consulting fees or honoraria from Allergan, AstraZeneca, Cephalon, Corcept, Noven, Roche, and Takeda. He has received medication (Rilutek) from Sanofi-Aventis for a NIMH sponsored study. Dr. Mathew has been named as an inventor on a use-patent of ketamine for the treatment of depression. Dr. Mathew has relinquished his claim to any royalties and will not benefit financially if ketamine were approved for this use. No biomedical financial interests or potential conflicts of interest are reported for CGA, AJ, JRS, XM, and DCS.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Abdallah CG, Coplan JD, Jackowski A, Sato JR, Mao X, Shungu DC, Mathew SJ. A pilot study of hippocampal volume and N-acetylaspartate (NAA) as response biomarkers in riluzole-treated patients with GAD. Eur Neuropsychopharmacol. 2012 doi: 10.1016/j.euroneuro.2012.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Auer DP, Putz B, Kraft E, Lipinski B, Schill J, Holsboer F. Reduced glutamate in the anterior cingulate cortex in depression: an in vivo proton magnetic resonance spectroscopy study. Biol Psychiatry. 2000;47:305–313. doi: 10.1016/s0006-3223(99)00159-6. [DOI] [PubMed] [Google Scholar]
- 3.Bhagwagar Z, Wylezinska M, Jezzard P, Evans J, Ashworth F, Sule A, Matthews PM, Cowen PJ. Reduction in occipital cortex gamma-aminobutyric acid concentrations in medication-free recovered unipolar depressed and bipolar subjects. Biol Psychiatry. 2007;61:806–812. doi: 10.1016/j.biopsych.2006.08.048. [DOI] [PubMed] [Google Scholar]
- 4.Binesh N, Kumar A, Hwang S, Mintz J, Thomas MA. Neurochemistry of late-life major depression: a pilot two-dimensional MR spectroscopic study. J Magn Reson Imaging. 2004;20:1039–1045. doi: 10.1002/jmri.20214. [DOI] [PubMed] [Google Scholar]
- 5.Block W, Traber F, von Widdern O, Metten M, Schild H, Maier W, Zobel A, Jessen F. Proton MR spectroscopy of the hippocampus at 3 T in patients with unipolar major depressive disorder: correlates and predictors of treatment response. Int J Neuropsychopharmacol. 2009;12:415–422. doi: 10.1017/S1461145708009516. [DOI] [PubMed] [Google Scholar]
- 6.Braestrup C, Albrechtsen R, Squires RF. High densities of benzodiazepine receptors in human cortical areas. Nature. 1977;269:702–704. doi: 10.1038/269702a0. [DOI] [PubMed] [Google Scholar]
- 7.Brennan BP, Hudson JI, Jensen JE, McCarthy J, Roberts JL, Prescot AP, Cohen BM, Pope HG, Jr, Renshaw PF, Ongur D. Rapid enhancement of glutamatergic neurotransmission in bipolar depression following treatment with riluzole. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2010;35:834–846. doi: 10.1038/npp.2009.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buchsbaum MS, Hazlett E, Sicotte N, Stein M, Wu J, Zetin M. Topographic EEG changes with benzodiazepine administration in generalized anxiety disorder. Biol Psychiatry. 1985;20:832–842. doi: 10.1016/0006-3223(85)90208-2. [DOI] [PubMed] [Google Scholar]
- 9.Buchsbaum MS, Wu J, Haier R, Hazlett E, Ball R, Katz M, Sokolski K, Lagunas-Solar M, Langer D. Positron emission tomography assessment of effects of benzodiazepines on regional glucose metabolic rate in patients with anxiety disorder. Life Sci. 1987;40:2393–2400. doi: 10.1016/0024-3205(87)90753-3. [DOI] [PubMed] [Google Scholar]
- 10.Collins PY, Patel V, Joestl SS, March D, Insel TR, Daar AS, Anderson W, Dhansay MA, Phillips A, Shurin S, Walport M, Ewart W, Savill SJ, Bordin IA, Costello EJ, Durkin M, Fairburn C, Glass RI, Hall W, Huang Y, Hyman SE, Jamison K, Kaaya S, Kapur S, Kleinman A, Ogunniyi A, Otero-Ojeda A, Poo MM, Ravindranath V, Sahakian BJ, Saxena S, Singer PA, Stein DJ. Grand challenges in global mental health. Nature. 2011;475:27–30. doi: 10.1038/475027a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duyn JH, Gillen J, Sobering G, van Zijl PC, Moonen CT. Multisection proton MR spectroscopic imaging of the brain. Radiology. 1993;188:277–282. doi: 10.1148/radiology.188.1.8511313. [DOI] [PubMed] [Google Scholar]
- 12.First M, Spitzer R, Gibbon M, Williams J. Biometric Research. New York State Psychiatric Institute; New York: 1995. Structured Clinical Interview for DSM-IV Axis I Disorders: Patient Edition. SCIDI/P. Version 2.0. [Google Scholar]
- 13.Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:11050–11055. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hasler G, van der Veen JW, Tumonis T, Meyers N, Shen J, Drevets WC. Reduced prefrontal glutamate/glutamine and gamma-aminobutyric acid levels in major depression determined using proton magnetic resonance spectroscopy. Arch Gen Psychiatry. 2007;64:193–200. doi: 10.1001/archpsyc.64.2.193. [DOI] [PubMed] [Google Scholar]
- 15.Mathew SJ, Amiel JM, Coplan JD, Fitterling HA, Sackeim HA, Gorman JM. Open-label trial of riluzole in generalized anxiety disorder. The American journal of psychiatry. 2005;162:2379–2381. doi: 10.1176/appi.ajp.162.12.2379. [DOI] [PubMed] [Google Scholar]
- 16.Mathew SJ, Price RB, Mao X, Smith EL, Coplan JD, Charney DS, Shungu DC. Hippocampal N-acetylaspartate concentration and response to riluzole in generalized anxiety disorder. Biol Psychiatry. 2008;63:891–898. doi: 10.1016/j.biopsych.2007.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mekle R, Mlynarik V, Gambarota G, Hergt M, Krueger G, Gruetter R. MR spectroscopy of the human brain with enhanced signal intensity at ultrashort echo times on a clinical platform at 3T and 7T. Magn Reson Med. 2009;61:1279–1285. doi: 10.1002/mrm.21961. [DOI] [PubMed] [Google Scholar]
- 18.Michael N, Erfurth A, Ohrmann P, Arolt V, Heindel W, Pfleiderer B. Metabolic changes within the left dorsolateral prefrontal cortex occurring with electroconvulsive therapy in patients with treatment resistant unipolar depression. Psychol Med. 2003;33:1277–1284. doi: 10.1017/s0033291703007931. [DOI] [PubMed] [Google Scholar]
- 19.Michael N, Erfurth A, Ohrmann P, Arolt V, Heindel W, Pfleiderer B. Neurotrophic effects of electroconvulsive therapy: a proton magnetic resonance study of the left amygdalar region in patients with treatment-resistant depression. Neuropsychopharmacology. 2003;28:720–725. doi: 10.1038/sj.npp.1300085. [DOI] [PubMed] [Google Scholar]
- 20.Milne A, MacQueen GM, Yucel K, Soreni N, Hall GB. Hippocampal metabolic abnormalities at first onset and with recurrent episodes of a major depressive disorder: a proton magnetic resonance spectroscopy study. Neuroimage. 2009;47:36–41. doi: 10.1016/j.neuroimage.2009.03.031. [DOI] [PubMed] [Google Scholar]
- 21.Persson A, Ehrin E, Eriksson L, Farde L, Hedstrom CG, Litton JE, Mindus P, Sedvall G. Imaging of [11C]-labelled Ro 15-1788 binding to benzodiazepine receptors in the human brain by positron emission tomography. J Psychiatr Res. 1985;19:609–622. doi: 10.1016/0022-3956(85)90080-9. [DOI] [PubMed] [Google Scholar]
- 22.Pfleiderer B, Michael N, Erfurth A, Ohrmann P, Hohmann U, Wolgast M, Fiebich M, Arolt V, Heindel W. Effective electroconvulsive therapy reverses glutamate/glutamine deficit in the left anterior cingulum of unipolar depressed patients. Psychiatry Res. 2003;122:185–192. doi: 10.1016/s0925-4927(03)00003-9. [DOI] [PubMed] [Google Scholar]
- 23.Pittenger C, Coric V, Banasr M, Bloch M, Krystal JH, Sanacora G. Riluzole in the treatment of mood and anxiety disorders. CNS Drugs. 2008;22:761–786. doi: 10.2165/00023210-200822090-00004. [DOI] [PubMed] [Google Scholar]
- 24.Price RB, Shungu DC, Mao X, Nestadt P, Kelly C, Collins KA, Murrough JW, Charney DS, Mathew SJ. Amino acid neurotransmitters assessed by proton magnetic resonance spectroscopy: relationship to treatment resistance in major depressive disorder. Biol Psychiatry. 2009;65:792–800. doi: 10.1016/j.biopsych.2008.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ravindran LN, Stein MB. The pharmacologic treatment of anxiety disorders: a review of progress. J Clin Psychiatry. 2010;71:839–854. doi: 10.4088/JCP.10r06218blu. [DOI] [PubMed] [Google Scholar]
- 26.Rothman DL, De Feyter HM, de Graaf RA, Mason GF, Behar KL. 13C MRS studies of neuroenergetics and neurotransmitter cycling in humans. NMR Biomed. 2011;24:943–957. doi: 10.1002/nbm.1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sanacora G, Gueorguieva R, Epperson CN, Wu YT, Appel M, Rothman DL, Krystal JH, Mason GF. Subtype-specific alterations of gamma-aminobutyric acid and glutamate in patients with major depression. Arch Gen Psychiatry. 2004;61:705–713. doi: 10.1001/archpsyc.61.7.705. [DOI] [PubMed] [Google Scholar]
- 28.Sanacora G, Zarate CA, Krystal JH, Manji HK. Targeting the glutamatergic system to develop novel, improved therapeutics for mood disorders. Nat Rev Drug Discov. 2008;7:426–437. doi: 10.1038/nrd2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shulman RG, Rothman DL, Behar KL, Hyder F. Energetic basis of brain activity: implications for neuroimaging. Trends Neurosci. 2004;27:489–495. doi: 10.1016/j.tins.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 30.Spitzer R, Williams J, Gibbon M. Structured clinical interview for DSM-IV: non-patient edition (SCID-NP) New York: Biometrics Research Department, New York State Psychiatric Institute; 1996. [Google Scholar]
- 31.Trivedi MH, Rush AJ, Wisniewski SR, Nierenberg AA, Warden D, Ritz L, Norquist G, Howland RH, Lebowitz B, McGrath PJ, Shores-Wilson K, Biggs MM, Balasubramani GK, Fava M. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psychiatry. 2006;163:28–40. doi: 10.1176/appi.ajp.163.1.28. [DOI] [PubMed] [Google Scholar]
- 32.Wu JC, Buchsbaum MS, Hershey TG, Hazlett E, Sicotte N, Johnson JC. PET in generalized anxiety disorder. Biol Psychiatry. 1991;29:1181–1199. doi: 10.1016/0006-3223(91)90326-h. [DOI] [PubMed] [Google Scholar]
- 33.Yuksel C, Ongur D. Magnetic resonance spectroscopy studies of glutamate-related abnormalities in mood disorders. Biol Psychiatry. 2010;68:785–794. doi: 10.1016/j.biopsych.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zarate CA, Manji HK. Riluzole in psychiatry: a systematic review of the literature. Expert Opin Drug Metab Toxicol. 2008;4:1223–1234. doi: 10.1517/17425255.4.9.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.