Abstract
No systemic therapy is effective against pancreatic cancer (PC). Pancreatic cancer stem cells (PCSC) are hypothesized to account for therapeutic resistance. Several PCSC subpopulations were reported, each characterized by different markers. To be able to target PCSC, we sought to better define this putative heterogeneity. Therefore, we tested most of the known putative PCSC markers in established and fresh tumor cell lines. CD20, CD24, CD44, CD133, CD184 (CXCR4), CD326 (EpCam, ESA), Sox-2, OCT 3/4, and the side-population (SP) were tested in five PC cell lines, and the effects of confluency, hypoxia, radiation, and gemcitabine on the SP. The testing phase suggested several putative PCSC populations that were further tested and validated for their tumor-initiating capacity against known PCSC in 3 established and 1 fresh PC cell lines. Cell surface and intracellular markers showed significant variability among cell lines. SP was the only common marker in all cell lines and consistently less than 1%. SP response to confluence, hypoxia, radiation, and gemcitabine was inconsistent between cell lines. The initial testing phase suggested that SP/CD44-CD24-CD326+ cells might be a novel PCSC subpopulation. Tumor initiation capacity tests in nude mice confirmed their increased tumorigenicity over previously reported PCSC. Our data better define the heterogeneity of reported PCSC in cell lines tested in this study. We propose that prior to targeting PC via PCSC, one will need to gain more insight into this heterogeneity. Finally, we show that SP/CD44− CD24-CD326+ cells are a novel subpopulation of pancreatic cancer tumor initiating cells. Further mechanistic studies may lead to better targeting of PC via targeting this novel PCSC.
Keywords: pancreatic cancer, stem cell biology, side population, C44, CD24
Introduction
Pancreatic cancer is the fourth-leading cause of cancer deaths in both men and women in the United States. While the average cancer patient can expect a 69% 5-year survival, a patient with pancreatic cancer has a dismal 5% 5-year survival (Horner MJ, 2009). Despite advances in other cancer treatments, pancreatic cancer survival has not significantly improved over the last several decades. As a result, new pancreatic cancer therapies could be of significant value. One such therapy could target pancreatic cancer stem cells (PCSC).
The cancer stem cell hypothesis suggests that current forms of chemotherapy and radiation may not target cancer stem cells, thus allowing for recurrence (Reya et al., 2001). Cancer stem cells, originally described in leukemia (Bonnet and Dick, 1997), have recently been evaluated in many solid organ cancers (Rosen and Jordan, 2009; Visvader and Lindeman, 2008). One of several problems with the cancer stem cells hypothesis is the significant heterogeneity reported among cancer stem cells; different markers are reported to identify cancer stem cells in the same histology. In pancreatic cancer alone, CD24, CD44, ESA (Epithelial surface antigen), CD133, CXCR4, and the side population (SP) have all been reported by different laboratories to designate populations of PCSC (Hermann et al., 2007; Li et al., 2007; Sergeant et al., 2009). In particular, one seminal study has suggested that CD44+/CD24+/ESA+ cells have a 100-fold increased tumorigenic potential (Li et al., 2007). Additionally, other groups have independently suggested CD44+/CD24− and CD44+/CD133+ as possible tumor-initiating cells in 4 established pancreatic cancer cell lines (Kallifatidis et al., 2009). Although not necessarily mutually exclusive, these studies lead us to question the identity of an optimal PCSC in terms of potential therapeutic targeting. The ideal PCSC should be found in as many pancreatic cancer cell lines and fresh tumors should as possible.
Tumorigenic potential and resistance to therapy has been evaluated by growth in immunosuppressed mice as well as resistance to therapy assays (Reya et al., 2001). The SP and CD44+CD24− cells in pancreatic cancer have been reported to be resistant to gemcitabine, the standard chemotherapy agent for pancreatic cancer (Kallifatidis et al., 2009; Zhou et al., 2008). Yet, other laboratories suggested that cancer stem cells are radiation and hypoxia resistant (Tavaluc et al., 2007; Vlashi et al., 2009). We reasoned that to effectively target pancreatic cancer via targeting cancer stem cells, it is necessary to identify an optimal population of cancer stem cells that can be found in all or the majority of pancreatic cells tested and that possess greater tumor-initiating capacity. Therefore, we designed the present study to comprehensively investigate previously reported populations of PCSC, within one laboratory and in common pancreatic cancer cell lines and fresh tumors.
Several pancreatic cancer cell lines and human tissue samples were surveyed for cell surface, intracellular, and functional markers. CD20, CD24, CD44, CD133, CD184 (CXCR4), CD326 (EpCam), Sox-2, and OCT3/4 were examined in established pancreatic cancer cell lines. The functional marker, i.e., SP, was also determined in these cell lines. Side population was a term originally used to define a group of hematopeotic stem cells that had the ability to efflux the fluorescent vital dye Hoechst 33342 (Goodell et al., 1996). These cells have been shown to be long-term repopulating cells found in both normal tissues and cancer cells. However, when compare to non-SP, the SP has higher expression of genes linked to regulation of stem cell function and has been described in a variety of cancers (Wu and Alman, 2008). Since previous reports also suggest that the SP is influenced by cell culture density, we examined SP under 2 different density conditions (Tavaluc et al., 2007). The cardinal aim was to define a marker or a combination of markers that were common to all cancers tested, and to test its therapeutic resistance and tumor-initiating capacity. Various logistic combinations of surface markers and SP were examined; the combination of SP plus surface markers was found to be a potentially better indicator for PCSC in terms of tumor-initiating capacity. Using these techniques, we prospectively identified a population of novel PCSC in the testing phase. In the validating phase, these cells were subjected to a xenotransplantation assay and found to have superior tumorigenicity compared to controls. Our findings potentially indicate that PCSC are a very heterogeneous population of cells in terms of markers, the response to chemotherapy, radiation, and hypoxia. Importantly, we show a novel class of putative PCSC or pancreatic cancer cells with greater tumor-initiating capacity that has not previously been described.
Results
Expression of individual cell surface and intracellular markers is heterogeneous among various pancreatic cancer cells
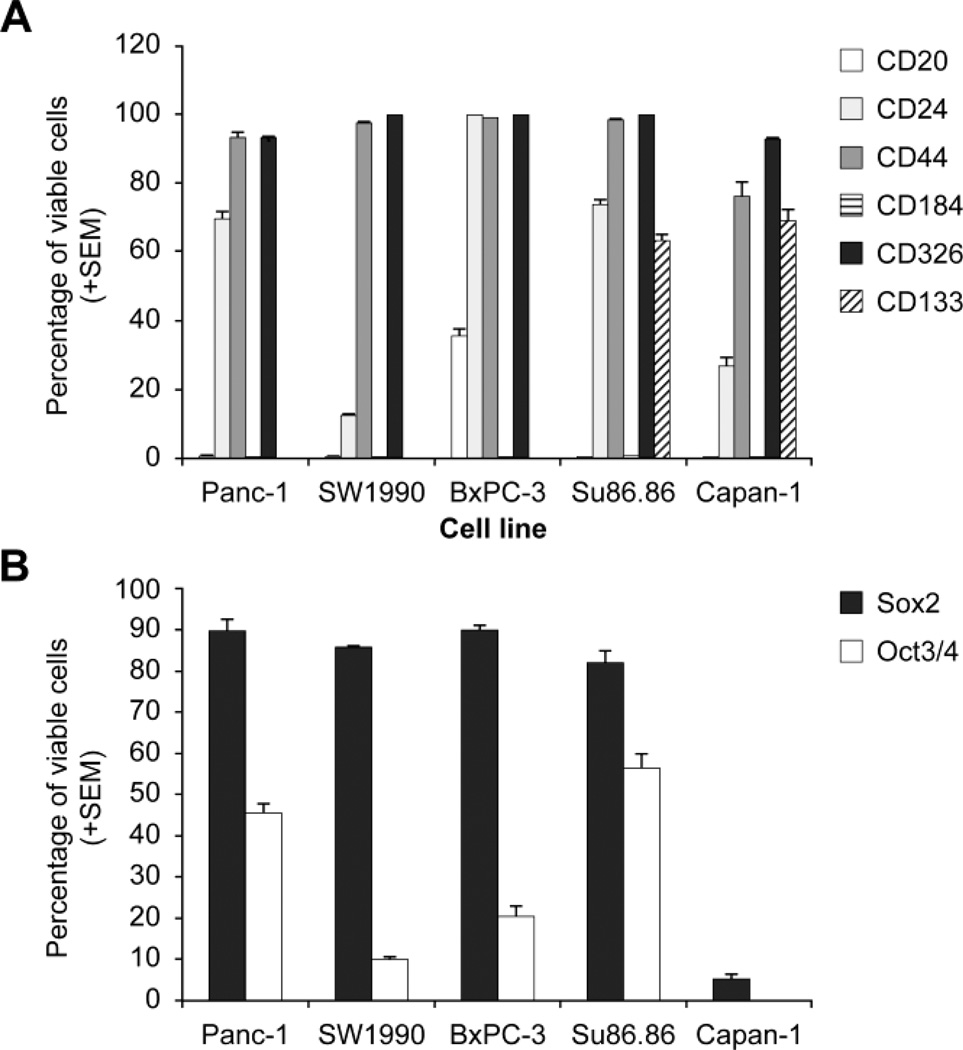
CD20, CD24, CD44, CD184, CD326, CD133, Sox-2, and OCT 3/4 were examined by flow cytometry in 5 pancreatic cancer cell lines (Fig. 1A, 1B). CD20 is expressed by less than 0.5% of the cells in four cell lines, and by 35% of BxPC-3 cells. CD24 is expressed by 12.4–99.6% of cells. CD44 is expressed by 75% of cells in all cell lines tested. CD184 is expressed by 0.21% of Su86.86 cells and is virtually undetectable in other cell lines. CD326 is ubiquitous in greater than 93% of cells in all cell lines tested. CD133 is only detectable in Su86.86 and Capan-1, comprising 63–69% of total cells. Interestingly, Su86.86 and Capan-1 are the only cell lines derived from pancreatic cancer liver metastasis, suggesting a potential role of CD133 in metastases. Intracellular markers, Sox-2 and OCT 3/4 are present in 5–90% and 0.2–57% of total cells, respectively.
Figure 1.
Cancer Stem Cells Markers Expression. Comparative expression of extracellular (A) and intracellular (B) cancer stem cells markers in 5 pancreatic cancer cell lines.
The premise of this study was that in order to target PC that is derived from a very heterogenic population of patients via targeting PCSC, one would need to identify a population of putative PCSC that exists in as many pancreatic cancer cells as possible. CD184 and CD133 are not expressed by all pancreatic cell lines and thus, individually, would not be good candidate markers in PC cells tested. Based on other biological systems generated from stem cells excluding embryological systems, stem cells comprise a rare population of cells. CD24, CD44, CD136, Sox2, and OCT3/4 are expressed by > 50% of the PC cells and as such are unlikely to be individually good candidates to designate PCSC markers. None of these markers appear to be present at a consistent level throughout all cell lines tested. This wide range of expression of both extracellular and intracellular markers suggests that no individual marker alone is sufficient to identify a putative cancer stem cell.
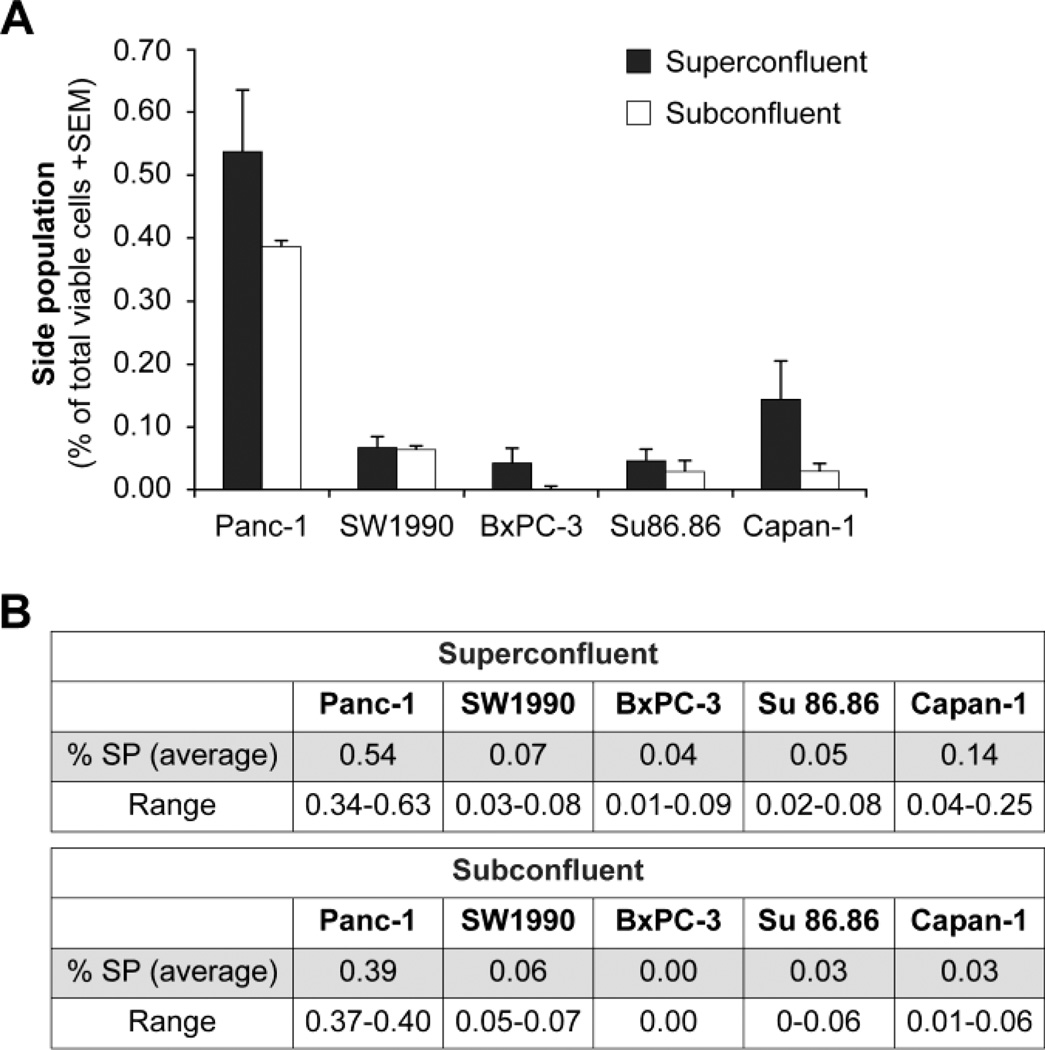
Side population is consistently rare in all cell lines
In panc-1, SW1990, Su86.86, BxPC-3, and Capan-1, the SP comprises less than 1% of total cells when defined by the inhibitor verapamil (Fig. 2). The SP was examined in cells that were 1 day beyond complete confluency (super-confluent) and in cells that were approximately 70–80% confluent (sub-confluent). Previous studies had suggested that lower confluency increases the SP (Tavaluc et al., 2007). However, our results cannot confirm those findings. In contrast, our results show that there is no statistical difference in the SP detected under super-confluent or sub-confluent conditions. Given these findings, for subsequent SP studies, we elected to use subconfluent cell culture conditions. Based on the fact that the SP is consistently rare and exists in all cell lines, it appears to be a better candidate for a ubiquitous marker of PCSC than the individual cell surface markers tested. Based on these findings and on the fact that the SP is a functional marker, we reasoned that the SP could potentially be a common ubiquitous marker of PCSC, and that other previously reported surface markers potentially better define various subpopulations within the SP. Additionally, since the SP was the only consistent marker, we further tested their response to chemotherapy, radiation, and hypoxia.
Figure 2.
Side Population. The side population is rare and exists in all pancreatic cancer cell lines tested. Comparisons between cells grown under super-confluent and sub-confluent conditions show no statistical difference in the side population for any of the cell lines, with p > 0.05 in all cases and using a 2-sided t-test.
Side population is not consistently affected by exposure to gemcitabine
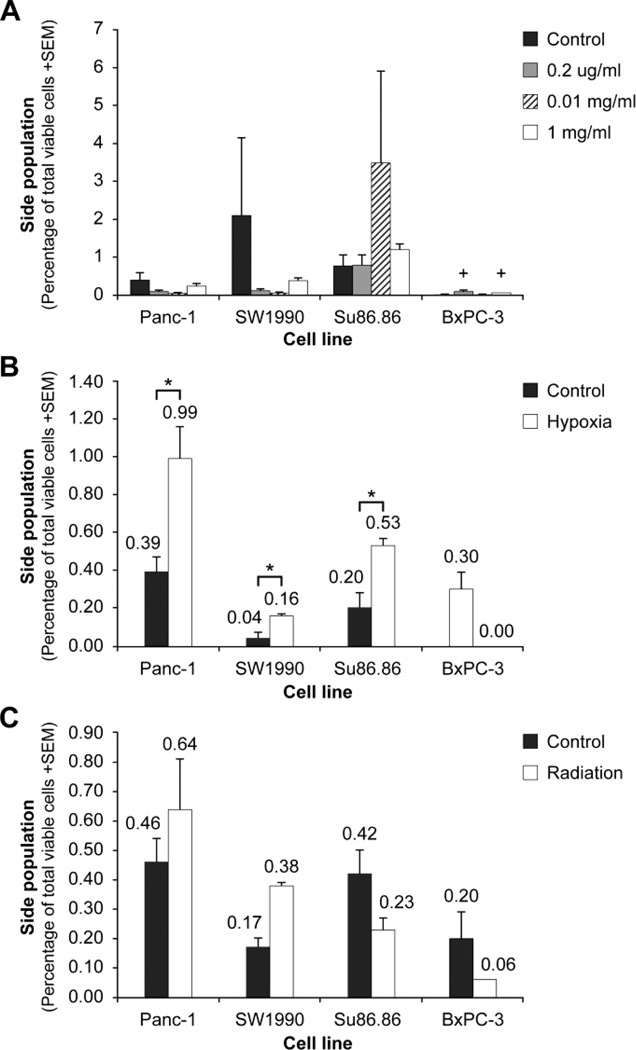
Gemcitabine is a commonly used chemotherapy in patients with pancreatic cancers. Previous in vitro studies have suggested that panc-1 cells exposed to 5µg/ml gemcitabine have a 10.7% increase in the SP (Zhou et al., 2008). We hypothesized that gemcitabine would increase the relative size of the SP, suggesting that SP cells may be relatively resistant to gemcitabine. Cells were exposed to clinically relevant increasing doses of gemcitabine: 0.02µg/ml, 0.01mg/ml, and 1mg/ml. The clinically relevant dose is approximately 0.01mg/ml since clinical peak serum concentration is 0.01mg/ml to 0.04mg/ml (Noble and Goa, 1997). Cells were exposed for 48 hours or 1 cell cycle. Our results demonstrate no statistically significant change in the SP with gemcitabine exposure (Fig. 3A). Given these findings, we concluded that the SP might not be affected by chemotherapy in terms of relative proportion in all pancreatic cancer cells tested with the exception of BxPC-3 (0.01 < p < 0.05). To further test the SP as a putative pancreatic cancer stem cell, we next examined its response to hypoxia.
Figure 3.
Side population response to chemotherapy, hypoxia, and radiation. (A) Cells were exposed to clinically relevant increasing doses of gemcitabine: 0.2µg/ml; 0.01mg/ml; and 1mg/ml. There is no statistical significance in the relative proportion of the SP in 4 of 5 cell lines tested. Only in BxPC-3 does there appear to be a statistical trend (+, 0.01 < p < 0.05, adjusted to multiple comparisons) where 0.01mg/ml and 1mg/ml may increase the relative proportion of the side population relative to control cell. (Significance evaluated by Dunnett’s test, n = 3). (B) Cells exposed to 1% oxygen were compared with cells under normal culture conditions in terms of side population. There is a statistically significant increase in side population under hypoxic conditions in panc-1 and Su86.86 cells. (*, p = 0.009 and p = 0.004, respectively). In SW1990 cells, there is a statistical trend (+, p = 0.015) toward increased side population with hypoxic exposure (adjusted to multiple comparisons). Significance was evaluated by a 2-sample t-test. (C) Cells exposed to 4 Gy radiation were compared with cells under normal culture conditions. Although in 2 of 4 cell lines tested the SP increased after radiation, there is no statistically significant difference between radiated cells and control cells. Significance was evaluated by a 2- sample t-test. Results are an average of 3 repetitions.
Side population increases with hypoxic exposure
Tumors, as they enlarge, tend to have some degree of hypoxia, particularly at central portions of the tumor. In neuroblastoma, hypoxia increases the SP and results in a highly invasive migratory group of cells that show increased tumorigenicity (Das et al., 2008). In colon cancer, a 3.2-fold increase in SP was found with exposure to 0.5% oxygen (Tavaluc et al., 2007). However, there are no studies that have reported the effect of hypoxia on SP in pancreatic cancer. We exposed cells to 1% oxygen, 5% carbon dioxide, and 94% nitrogen. Our results show that in 3 of 4 cell lines tested, hypoxia increases the SP (Fig. 3B). Panc-1 and Su86.86 show a statistically significant increase of about 2.5-fold with hypoxia. SW1990 cells have a 4-fold increase in the SP with hypoxia with a statistical trend (p = 0.015 adjusted to multiple comparisons). Our results demonstrate that although the SP might not be resistant to gemcitabine, it may well be resistant to hypoxia.
Side population is not consistently affected by radiation exposure
Radiation is often used to treat the local tumor bed after surgery or to decrease the size of locally advanced tumors before surgery. However many tumors re-grow in the irradiated field, suggesting the possible radiation resistance of PCSC. Radiation resistance of the SP has been suggested in studies of bladder cancer and breast cancer cell lines (Han and Crowe, 2009; Ning et al., 2009). However, there are no studies of the SP and radiation in pancreatic cancer. After exposure to 4Gy radiation, pancreatic cell lines appear to have variable responses to radiation (Fig. 3C). Our results demonstrate that 2 of 4 cell lines show an increase in SP, while other cell lines show a decrease. However, none of the comparisons are statistically significant.
Overall, our survey of pancreatic cancer SP response to therapy shows significant variability and inconsistency. Evaluation of 4 pancreatic cancer cell lines shows that the SP is not gemcitabine resistant in 3 out of 4 cell lines, or radiation resistant in 2 out of 4 cell lines. In contrast, hypoxia appears to increase the SP in the majority of cell lines tested. This variability in response suggests that the SP alone may not be an adequate cancer stem cell marker.
Various combinations of side population and cell surface markers may identify a better population of pancreatic cancer stem cells
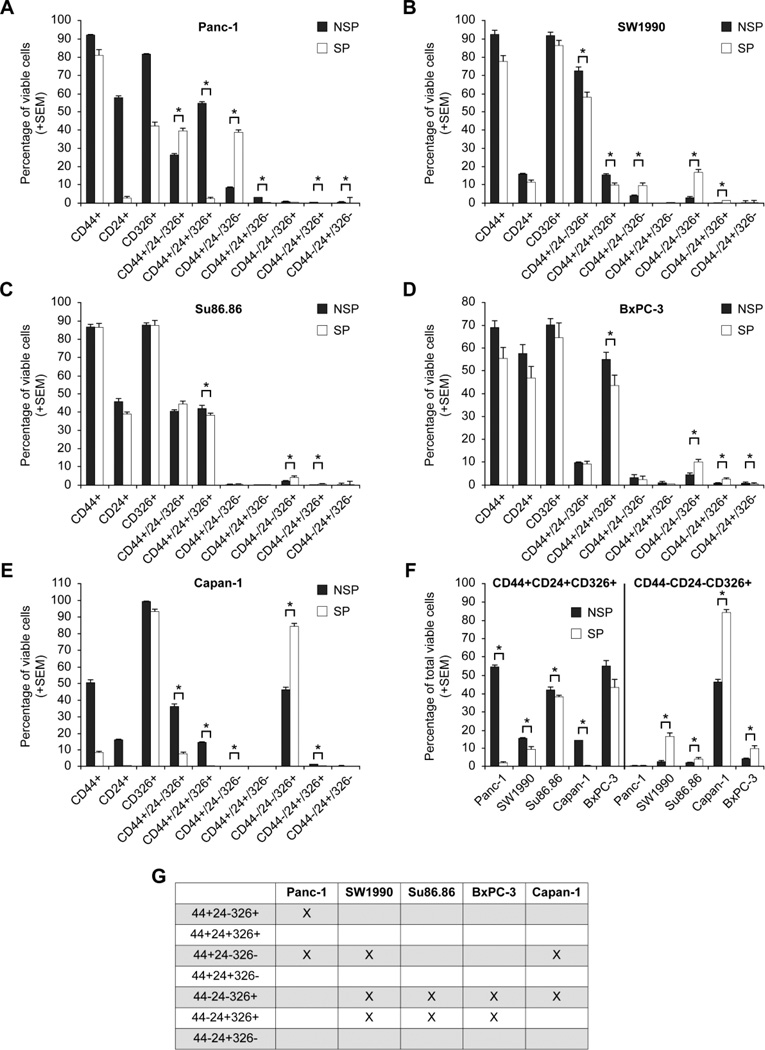
Since neither surface markers nor the functional marker of SP alone appear to be optimal cancer stem cell markers, we subsequently examined them in combinations. Previous studies in pancreatic cancer have shown the CD44+ CD24+ CD 326+ cells have 100-fold increased tumorigenicity (Li et al., 2007). Additionally, a recent study examined the surface markers within the SP of a single cell line, SW 1990. This study suggests that SP cells have more CD44+CD24+ cells than NSP cells (Yao et al., 2010). Therefore, we hypothesized that a surface marker combination that is present in relatively higher quantities in the SP compared to the non- SP could be a better marker combination for a putative cancer stem cell. Our in vitro studies of SP and CD44, CD 24, CD 326 demonstrated significant variability between cell lines (Fig. 4AE). Focusing on combinations that are statistically significantly higher in the SP than the NSP, we discovered a pattern between cell lines (Fig. 4G). CD44-CD24-CD326+ cells are higher in the SP than NSP in 4 out of 5 cell lines. No other combinations are consistently higher in the SP than NSP. When comparing this combination to the previously described CD44+CD24+CD236+ cells, our results show that these “triple positive” cells are lower in the SP than in the NSP in all cell lines (Fig. 4F). Given these in vitro results, we further hypothesized that SP/CD44-CD24-CD326+ may be better candidates for a putative cancer stem cells than the triple positive cells. We further tested this hypothesis with xenotransplantation.
Figure 4.
Pancreatic cancer stem cells surface markers expression in the side population (SP) versus the non-side population (NSP). CD44, CD24, CD326 positive and negative combinations are shown within the non-side population (NSP) and side population (SP) in various cell lines. Here we show that various combinations are statistically significantly different from each other (*, p < 0.01) in different cell lines (A–E). Comparing the previously described CD44+CD24+CD326+ cells with CD44-CD24-CD326+ cells shows that the latter is present in relatively higher amounts in the SP than in the NSP in 4 of 5 cell lines (F). The triple positive cells are lower in the SP than NSP in all cell lines. Cell surface marker combinations that are statistically higher in the SP than in the NSP are shown: CD44+/CD24−/CD 326+ is only significant in panc-1 cells; CD 44+CD24-CD326- and CD44-CD24+CD326+ are significant in 3 of 5 cell lines. CD 44-CD24-CD326+ is significant in 4 out of 5 cell lines(G).
Xenotransplantation demonstrates that CD44-CD24-CD326+ has greater tumor initiating capacity
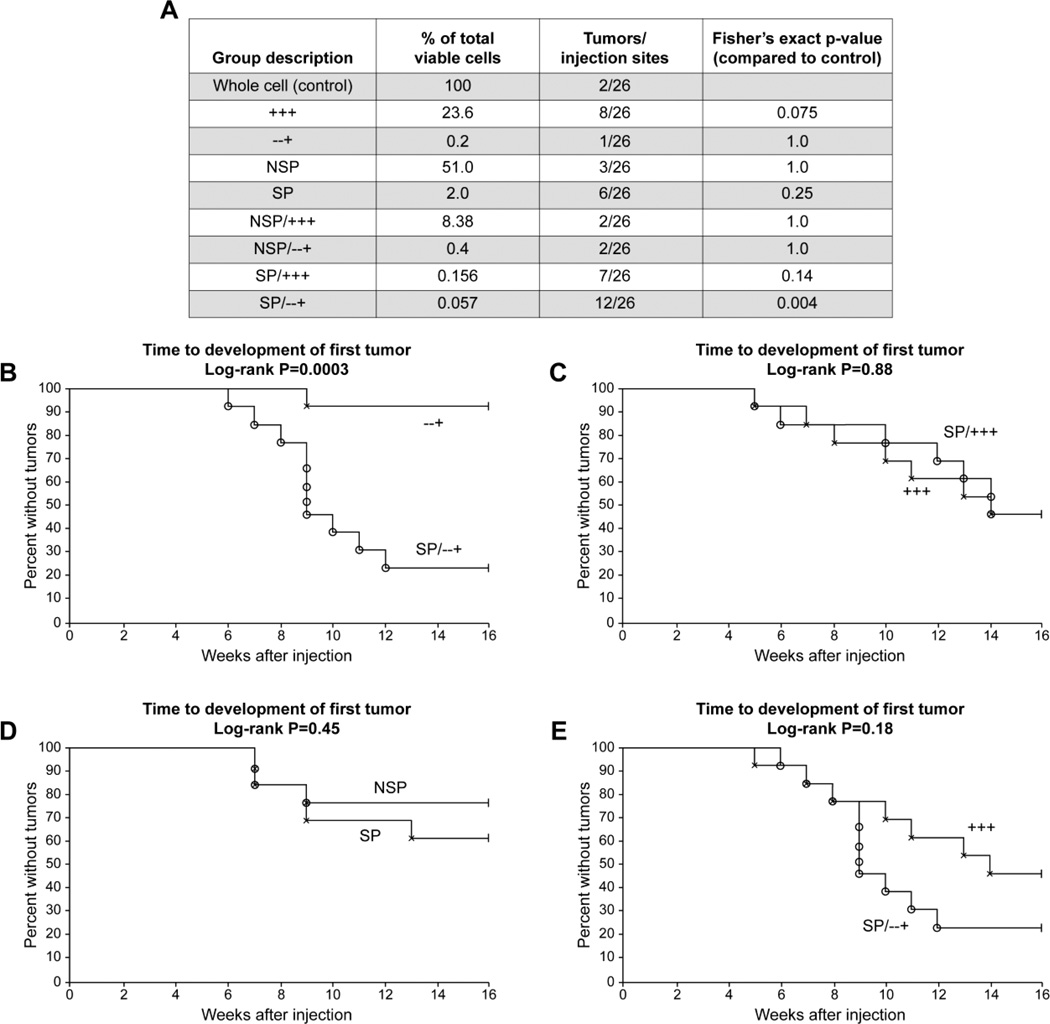
Since transplantation of all combinations and permutations of CD44, CD24, CD326, NSP, and SP is costly prohibitive (testing 5 markers will require 5! permutaional combinations, i.e., 250 different combinations). Thus, we selected 9 groups of particular interest and appropriate controls. Based on the surface marker distribution reported above (Fig. 4G), a pilot testing experiment was conducted using 1 cell line, SW1990; we transplanted the following groups: whole cell line; SP; NSP; CD44+CD24+CD326+; CD44-CD24-CD326+; SP/CD44+CD 24+CD326+; NSP/CD44+CD24+CD326+; SP/CD44-CD24-CD326+; and NSP/CD44-CD24-CD326+. We transplanted these 9 different groups, each with 100 cells per injection, into aythmic nude mice. Two front limb flank injections were used per animal, and 13 animals per group, resulting in 26 injections per group. We chose this number of animals based on prospective statistical analysis to have an 80% power to detect a difference between groups in which groups with tumor development were estimated to have 62% of animals with tumor. Analysis was done with a 2-tailed Fisher’s exact test with significance determined by a very strict p < 0.005 because of proper adjustment for multiple comparisons. Statistical evaluation showed that the presence of tumor on one flank of a single animal did not influence the growth of a second tumor on the adjacent flank of the same animal. Therefore, we counted each injection as a separate and independent event, giving a total of 26 evaluable sites for each group tested. Actual FACS sort data are shown in Supplementary Fig. S1. Tumor growth results demonstrate that the SP/CD44-CD 24-CD326+ cells grew in 12 of 26 sites, which is the only group that is statistically significantly different than control that is sorted whole cells (Fig. 5A). Of note, the CD44+CD24+CD326+ cells had the second-largest number of tumors, 8 of 26; however, this was not statistically different than control. These results suggest SP/CD44-CD 24-CD326+ are more tumorigenic than controls. Interestingly, whole cells grew in 2 of 26 injections. The reasons for this are unclear; it is the first reported tumor growth of a whole cell line, when only 100 cells have been injected.
Figure 5.
Tumor-initiating capacity testing experiment. Nine different subpopulation of cells derived from SW1990 cells were xenotrasplanted. Each group was compared to a whole cell control with p-values listed. The side population CD44- CD 24-CD326+ (SP/−−+) is the only group that is statistically significantly more tumorigenic than controls (A). Further testing was done using Kaplan-Meier analysis to test whether the time to development of first tumor was different between the various groups (B–E). In comparing the following couplets: SP/−−+ vs −−+; SP vs. NSP (p = 0.45); SP/+++ vs. +++ (p = 0.88); and SP/−−+ vs. +++ (p = 0.18), only SP/−−+ vs. −−+ showed a statistically significant difference (p = 0.0003) (B).
Several comparisons were made between the groups using Kaplan-Meier analysis to test whether the time to development of first tumors was different between the various groups (Fig. 5B–D). In comparing the contribution of SP to surface markers, Fig. 5B demonstrates that the SP enhances tumorigenicity of CD44-CD 24-CD326+ cells (p = 0.0003). However, SP does not appear to change the tumorigenicity of CD44+CD24+CD326+ cells (Fig. 5C). Interestingly, SP alone does not provide the same tumorigenicity, suggesting that SP alone or surface markers alone have less tumorigenicity than the combination (Fig. 5D, p = 0.45). Comparing our putative cancer stem cell population of SP/CD44-CD24-CD326+ to the triple positive cells, we find that the former has better tumor formation (12/26 versus 8/26), but the difference is not statistically significant based on the p-value set at 0.005 to adjust for multiple comparisons. Similarly, the time to development of first tumor between CD44+CD24+CD326+ and SP/CD44-CD 24-CD326+ is not statistically significant (p = 0.18).
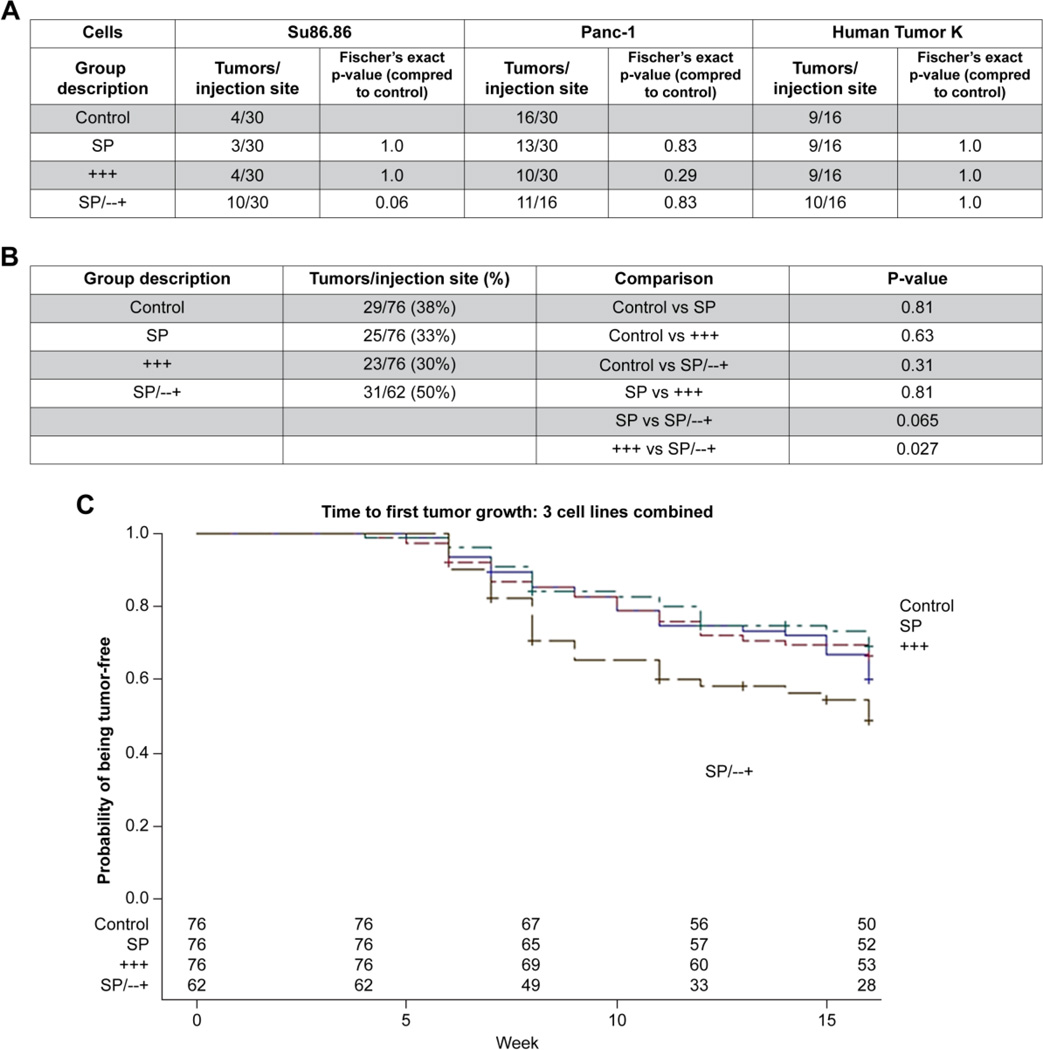
To further verify these results, we conducted a validating experiment. We examined these selected populations in several additional cell lines. Xenotransplantation of 100 cells of SP, CD44+CD24+CD326+, SP/ CD44-CD 24-CD326+, and control cells was done using Su86.86 and Panc-1 cell lines. One additional human fresh pancreatic ductal adenocarcinoma cell line, designated human tumor K, was also tested. Fig. 6 shows the number of tumors that grew in these xenotransplantation experiments. Up to 15 mice per group had 100 cells of the sorted populations injected into their flanks bilaterally (Fig. 6). When looking at each cell line, the comparisons to control do not show statistically different numbers of tumors in any of the 3 sorted groups. Additionally, comparisons between groups within a cell line show no difference (data not shown). However, when the groups are pooled across the 3 cell lines to approximate a population of various patients, the SP/ CD44-CD 24-CD326+ have absolute 20% higher growth rate than the CD44+CD24+CD326+ cells (Fig. 6B, p = 0.027). Furthermore, examined in another way, the time to first tumor development also shows advantage to the SP/ CD44-CD24-CD326+ subpopulation. In each individual cell line, none of the groups were statistically different from each other in terms of which group developed tumors first (data not shown). However, when the 3 cell lines were examined together (Fig. 6C), the SP/CD44-CD 24-CD326+ developed tumors more quickly than the CD44+CD24+CD326+ (p = 0.037).
Figure 6.
Tumor-initiating capacity validating experiemnt. Xenotransplantation using 100 cells in Su86.86, Panc-1, and Human tumor K. Table A shows the number of tumors that grew per total number of sites injected. Each group was compared to the whole cell control with p-values listed. Table B shows combined analysis pooling numbers of tumors from all 3 cell lines and the comparisons between groups. Graph C shows the time to the development of a tumor combining all 3 cell lines together. SP/CD44-CD 24-CD326+ developed tumors more quickly than the CD44+CD24+CD326+ (p = 0.037).
Discussion
Despite significant research, the identity of pancreatic cancer stem cells remains controversial (Hermann et al., 2009). While different groups have identified CD44+CD24+CD326+ cells as cancer stem cells, others suggest that CD44+CD 24- and CD 44+CD133+ are also cancer stem cells (Kallifatidis et al., 2009; Li et al., 2007). Although the identity remains questionable, detailed proteomic and therapeutic studies have already been conducted on different putative cancer stem cells (Dai et al., 2010; Mueller et al., 2009; Rausch et al., 2010). The value of these types of studies would be improved if an agreement could be established on the identity of pancreatic cancer stem cells.
This study was designed to examine previously described cancer stem cell assays in pancreatic cancer. Specifically, we examined surface markers, intracellular markers, SP, and effects of radiation, hypoxia, and gemcitabine. In studying the combinations of surface markers and SP, we have identified a novel combination that may be more tumorigenic than previously described CD44+CD24+CD326+ cells. By xenotransplantation, we find that SP/CD44-CD24- CD326+ cells are highly tumorigenic in pancreatic cancers.
In this study of PCSC, we prospectively defined the characteristics of the population we thought would be the best pancreatic cancer stem cells. Most researchers in this field would agree that cancer stem cells are a small subset of cancer cells that have the ability to self-renew and maintain a tumor (Clarke et al., 2006). In accordance with the cancer stem cells hypothesis, we searched for a rare population of cells, since cancer stem cells are thought to be a very small fraction of the bulk of the tumor. We also searched for a population that would be present in the majority of cell lines, i.e., consistency between cell lines. A putative cancer stem cell would also likely be resistant to treatments such as chemotherapy and radiation. Finally, a cancer stem cell also must demonstrate self-renewal by growth in an immunocompromised mouse model.
Our survey of the effects of radiation, hypoxia, and gemcitabine on the SP shows that there may not be a consistent effect on this sub-population. This suggests that the SP alone may not be the best method to isolate pancreatic cancer stem cells. Other studies have found that CD24 and ALDH are enriched after gemcitabine exposure and are strongly associated with subsequent tumor growth (Jimeno et al., 2009). Although we did not examine the effects of therapy on the SP/ CD44-CD24-CD326+ cells in this survey study, that may be a worthy topic of future studies as we begin to elucidate the mechanisms of increased tumorigenicity of this population.
The combination in vitro results suggested that CD44-CD24-CD326+ cells are present in greater quantities in the SP, while CD 44+CD 24+CD326+ cells are higher in the NSP. These results directly conflict with previous reports which suggested that SP contains more CD44+CD24+ than NSP (Yao et al., 2010). However, since our findings are present in 4 of 5 cell lines, compared to a single cell line in previous studies, we believe that our results may be more relevant. Of note, CD44-CD24-CD326+ cells were also detected in fresh tumor cells (Figure 6). Also, these same studies suggest that the SW1990 cell line contains a significant amount of CD133+ cells, particularly in SP cells. However, our results suggest that only Su86.86 and Capan-1 cell lines have significant amounts of CD133+ cells.
Xenotransplantation assays were performed in athymic mice. However, xenotransplantation assay to examine stem cell tumorigenicity has become more controversial. The exact immunocompromised nature of the model may significantly influence results. Commonly athymic or NOD/SCID mice are used. However, in melanoma, 27% of unselected melanoma single cells were reported to give rise to tumor in a model that used highly immunocompromised NOD/SCID interleukin-2 receptor gamma chain null (Il2rg−/−) mice (Quintana et al., 2008). These authors suggest that using NOD/SCID mice may underestimate the population of possible cancer stem cells. However, we speculate that perhaps using a highly immunocompromised mouse such as Il2rg−/− may overestimate putative cancer stem cell. In all cancer studies, models such as xenotransplantation are used to model the actual human condition as closely as possible. Using a highly immunocompromised mouse is possibly further from the state of a human being with cancer than using simply athymic mice. Tumors that grow in less immunocompromised models may be a more rigorous test of tumorigenicity. To address this controversy, some experts have suggested the use of genetically engineered mouse models that may allow examination of the cancer stem cell hypothesis in more syngeneic, immunocompetent setting (Lonardo et al., 2010).
Additionally, the exact method of xenotransplantation may be important. As in previous studies, we used a 25% matrigel cell suspension for injection. Although commonly used in maintenance of human embryonic stem cells, its variability has led researchers to investigate alternate methods (Nagaoka et al., 2010). Several studies have suggested increased tumor formation from tumor cell lines when injected in matrigel (Kleinman and Martin, 2005). The properties of matrigel may explain why 2 of 26 tumors grew in mice injected with 100 whole, unsorted cells. However, previous studies that identified CD44+CD24+CD326+ cells as cancer stem cells also used matrigel (Li et al., 2007). Regardless, when compared to this control group that grew 2 tumors, the SP/CD44-CD24-CD326+ cells showed statistically significant increased tumorigenicity in SW1990. The previously described CD44+CD24+CD326+ cells did not show statistically significant increased tumorigenicity over control. One explanation for this finding is that the previously described triple positive cells were studied exclusively in fresh human pancreatic cancer tissues. The different results between previously reported PCSC from fresh tumors and our results may be another source of heterogeneity. Additionally, the present study uses rigorous statistical planning to pre-determine the number of animals needed to show a difference in growth. Most studies use between 3 and 18 injections for analysis, while we have used between 16 and 30 for each group. While the increased tumorigenicity of SP/CD44-CD24- CD326+ cells is most statistically significant in our SW1990 results, it is also present in the pooled confirmatory studies done in Su86.86, panc-1, and human tumor K. However, it cannot be ignored that each individual cell line has variable results. Individually, the increased tumorigenicity of SP/ CD44-CD24-CD326+ is most apparent for SW1990 and Su86.86 cells; for panc-1 and human tumor K, this increased tumorigenicity seems to be abrogated. One technical explanation for this could be poor FACS sorting, which could also possibly explain the growth of numerous (16 of 30) tumors in the whole-cell population of panc-1 cells. Another possibility is that different cell lines may have different tumor-initiating cells. Based on other biological systems where stem cells were described, stem cell markers were not expressed by the majority of the cells within a system. Any stem-cell marker should be rare enough to denote a unique population of cells with stem cells properties. The only biological system where stem cell markers are expressed by the majority of cells is in embryonic systems. However, it is possible that in cancer, this rule does not apply. Moreover, in this study, using the cell lines we used, and the fresh tumor we used, PCSC seem to be heterogeneous. Potentially, these findings should make future studies aware of heterogeneity and should be explored and reported as such in future studies on the subject.
In terms of PCSC function, and based on this study alone, we can discuss only one functional aspect of PCSC: Tumor initiating capacity. Cancer stem cells are hypothesized to have play roles in asymmetric division, metastasis, and resistance to therapeutic agents. However, the current manuscript only examines the methods by which we can identify the putative PCSC. The SP+/ CD44- CD24-CD326+ cells were found to be more tumorigenic, suggesting that they may play a role in tumor initiation and propagation. There can be several sources of functional heterogeneity in PCSC. Different origins i.e. established cell lines vs. fresh surgical specimen, and cell lines originated from primary tumors vs. metastases can potentially results in different ability to generate tumors ro other functions. We propose that when denoting a PCSC-marker one should always define it by these four parameters for better accuracy. Although we examined the ability of some PCSC to resist radiation, chemotherapy and hypoxia in this study, we have not examined the exact role these cells might play in metastasis or direct resistance to chemotherapy. Clearly, not all subpopulations of putative PCSC have similar ability to negotiate chemotherapy, radiation or hypoxia and thus might not be ideal tools to identify putative PCSC.
In summary, the present study offers an extensive survey of pancreatic cancer stem cells. It challenges previous studies that suggest that surface markers or SP alone are sufficient to define the cancer stem cell compartment. Instead, we find that the combination of these markers may be better putative cancer stem cells than either population alone.
Materials and Methods
Cell culture
All cells were maintained in 5%CO2, 37.5 °C incubators. Panc-1, SW-1990, Su86.86, BxPC-3, were obtained from ATCC and grown in RPMI 1640 with 10% fetal bovine serum and 1% antibiotic/antimycotic (Invitrogen). Capan-1 cells were also obtained from ATCC and grown in IMDM (Invitrogen). Originally, panc-1 (ATCC, CRL-1469) and BxPC-3 (ATCC, CRL-1687) were obtained from primary pancreatic adenocarcinoma, while SW-1990 (ATCC, CRL-2172) was from a spleen metastasis and Su86.86 (ATCC, CRL-1837) and Capan-1 (ATCC, HTB-79) were from liver metastasis.
Side population assay
Adherent cells were harvested with 0.5% trypsin. Aliquots of 106 cells were exposed to 10µg/ml Hoechst 33342 (Invitrogen) in a 37.5 °C incubator for 90 minutes with constant gentle agitation. For samples with inhibitor, cells were exposed first to either 100µM Verapamil (Sigma) or 10 µM Fumitremorgin C (Alexis Biochemicals) for 15 minutes before adding Hoechst. After Hoechst incubation, samples were rapidly cooled on ice for 5–10 minutes. Samples were centrifuged and supernatant removed. Pellets were resuspended in 2% FBS/ PBS solution at a concentration of 106 cells/ml. 2µg/ml of 7-aminoactinomycin D (Invitrogen) was added as a viability detector. Samples were evaluated for SP on a LSR II cell analyzer (BD Biosciences).
Extracellular and intracellular marker flow cytometry
Adherent cells were harvested, and aliquots of 106 cells/ml were placed in FACS tubes. Cells were washed twice with 2% FBS/ PBS solution. For extracellular antibody markers, cells were resuspended in 100µl of 2%FBS/PBS before incubating with antibody. Anti-CD20 (FITCconjugated, 5 µl), anti-CD133/1 (APC-conjugated, 5 µl), and anti-CD326 (APC-conjugated, 5 µl) were obtained from Miltenyi Biotec. Anti-CD24 (FITC-conjugated, 5 µl) was obtained from Invitrogen. Anti-CD44 (PE-conjugated, 10 µl), and anti-CD184 (PE-conjugated, 10 µl) were obtained from BD Biosciences. Appropriate isotype controls were also used. Antibodies were incubated for 30 minutes at 4°C. Cells were then washed twice with 2% FBS/ PBS solution, and resuspended in 500 µl of 2% FBS/ PBS prior to reading on a Canto II Flow cytometer (BD Biosciences). 10 µl of propidium iodine was added as a viability stain.
For intracellular markers, Sox-2 and OCT3/4, cells were aliquoted as above and then fixed with 500µl of 4% paraformaldehyde for 30 minutes, and cells permeablized with 500µl of 2% Triton-x for 10 minutes, prior to incubating with antibodies. Sox-2 (PE-conjugated, 10µl) and OCT ¾ (FITC- conjugated) were used.
Gemcitabine, hypoxia, and radiation exposure
Cells were plated in a sub-confluent fashion. The next day, they were exposed to normal growth media with various doses of gemcitabine (NCI veterinary pharmacy), 0.02µg/ml, 0.01mg/ml, and 1mg/ml. Control cells had a media change with normal growth media only. Cells were allowed to grow under these conditions for 48 hours (approximately 1 cell cycle), after which the cells were harvested and analyzed for SP. Similarly, for hypoxia evaluation, sub-confluent cells were exposed to 1% oxygen, 5% carbon dioxide, and 94% nitrogen in a hypoxia chamber at 37.5°C (Billups-Rothenberg, Inc.) for 48 hours. Control cells were placed in normal atmospheric conditions in a 37.5°C incubator. SP was analyzed as previously described. For experiments involving radiation exposure, sub-confluent cells were exposed to 4Gy and then replaced in the conventional incubator for 3 days and analyzed for SP.
Combined side population, and cell surface marker flow cytometry and sorting
Cells were grown in a sub-confluent fashion and harvested with 0.5% trypsin, washed. Aliquots of 3×106 cells/ml were made for analysis. Appropriate controls and isotypes were done to allow for setting of voltages and compensation. The SP assay was conducted as described above. After completing the 90-minute incubation, cells were placed on ice for 10 minutes in the dark. Cells were then centrifuged and washed with cold 2%FBS/PBS. Each FACS tube containing 3×106 cells was then resuspended in 100µl of 2% FBS/PBS. Anti-CD44 (PE-conjugated, 10 µl), anti- CD24 (FITC-conjugated, 5 µl), and anti-CD326 (APC-conjugated, 5 µl) were added and incubated for 30 minutes at 4°C, protected from light. Cells were then washed twice with 2%FBS/PBS, resuspended in 1ml. 2µg/ml of 7-aminoactinomycin D (Invitrogen) was added as a viability detector. Compensation beads (BD Biosciences, catalog No. 552843) were prepared using manufacturer instructions and used to set compensation for each experiment. Samples were run on the LSR II cell analyzer (BD Biosciences).
Xenotransplantation
Between 200 and 400 million cells were grown and harvested for sorting and xenotransplantation. Cells were harvested, counted, and aliquoted into 3×106 cells/ml. For sorting, 10ml of this cell concentration was placed in a 125-ml sterile Erlenmeyer flask before incubating with Hoechst (10µg/ml) with constant agitation at 37°C for 90 minutes. Cells were placed on ice for 10 minutes. Cells to be sorted were combined in 50ml conical tubes and washed twice with cold 2% FBS/PBS. Cell were resuspended with 100µl 2%FBS/PBS per 3× 106 cells. Antibodies were added: anti-CD44 (PE-conjugated, 10 µl per 3× 106); anti-CD24 (FITC-conjugated, 5 µl per 3× 106); and anti-CD326 (APC-conjugated, 5 µl per 3× 106). Cells were incubated for 30 minutes at 4°C, protected from light. Cells were then washed twice with 2% FBS/PBS and aliquoted into sorting tubes at 10×106 cells/ml. Appropriate samples were done to allow for compensation on a BD FACS Aria II (BD Biosciences, San Jose, CA). Isotype controls were used to set gates for each flourchrome. Nine populations were sorted: whole cell line; SP; non-side population (NSP); CD44+CD 24+CD326+; CD44-CD24-CD326+; SP/ CD44+CD 24+CD326+; NSP/ CD44+CD 24+CD326+; SP/ CD44-CD24-CD326+; and NSP/ CD44-CD24- CD326+ (NSP non SP). After sorting, cells were centrifuged, counted, and resuspended in 25% matrigel/staining media solution to allow for a concentration of 100 cells/100µl. Staining media was made in RPMI-1640 with 8% DEPC sterile water (Crystalgen), 1% antibiotic/antimycotic (Invitrogen), 1% 1M HEPES (Crystalgen). 100µl were used for each injection of aythmic nude mice (01B74 Athymic NCr-nu/nu) from NCI Frederick Mouse Repository (Frederick, MD). Several days prior to injection, mice had electronic IMI-1000 transponders (Bio Medic Data Systems) implanted in a dorsal-caudal subcutaneous location to aid in the blinding of the study. Injections were done on bilateral front limbs. Injection groups were blinded and mice were mixed between cages/groups to avoid measurement bias. Subsequent weekly measurements were blinded until the experiments were terminated at 16 weeks.
Human fresh pancreatic tumor
The “human tumor K” [male, age 48 at diagnosis, pancreatic tail tumor] cells were generated by mincing fresh human pancreatic adenocarcinoma tumor into < 1 mm pieces and transplanting them into the subcutaneous tissue of athymic nude mice. After 1 passage in mice, tumors were harvested and digested with 1 mg/ml of type IV collagenase (Sigma, USA) and plated onto tissue culture plates and maintained in DMEM/F12 with 10% FBS.
Supplementary Material
Highlights.
We tested all known putative pancreatic cancer stem cells (PCSC) markers.
Cell surface and intracellular markers showed significant variability among cell lines.
Side population response to confluence, hypoxia, radiation, and gemcitabine was inconsistent among cell lines.
A novel subpopulation of SP/CD44-CD24-CD326+ cells demonstrated increased tumorigenicity over previously reported PCSC.
Acknowledgments
Douglas Burka and Chenwi Ambe have contributed to this work. This study was supported by the intramural grant provided by the NIH/National Cancer Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Disclosure Statement
The authors indicate no potential conflicts of interest.
References
- Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997;3:730–737. doi: 10.1038/nm0797-730. [DOI] [PubMed] [Google Scholar]
- Clarke MF, Dick JE, Dirks PB, Eaves CJ, Jamieson CH, Jones DL, Visvader J, Weissman IL, Wahl GM. Cancer stem cells--perspectives on current status and future directions: AACR Workshop on cancer stem cells. Cancer Res. 2006;66:9339–9344. doi: 10.1158/0008-5472.CAN-06-3126. [DOI] [PubMed] [Google Scholar]
- Dai L, Li C, Shedden KA, Lee CJ, Quoc H, Simeone DM, Lubman DM. Quantitative Proteomic Profiling Studies of Pancreatic Cancer Stem Cells. J Proteome Res. 2010 doi: 10.1021/pr100231m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das B, Tsuchida R, Malkin D, Koren G, Baruchel S, Yeger H. Hypoxia enhances tumor stemness by increasing the invasive and tumorigenic side population fraction. Stem Cells. 2008;26:1818–1830. doi: 10.1634/stemcells.2007-0724. [DOI] [PubMed] [Google Scholar]
- Goodell MA, Brose K, Paradis G, Conner AS, Mulligan RC. Isolation and functional properties of murine hematopoietic stem cells that are replicating in vivo. The Journal of experimental medicine. 1996;183:1797–1806. doi: 10.1084/jem.183.4.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han JS, Crowe DL. Tumor initiating cancer stem cells from human breast cancer cell lines. Int J Oncol. 2009;34:1449–1453. [PubMed] [Google Scholar]
- Hermann PC, Huber SL, Herrler T, Aicher A, Ellwart JW, Guba M, Bruns CJ, Heeschen C. Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell Stem Cell. 2007;1:313–323. doi: 10.1016/j.stem.2007.06.002. [DOI] [PubMed] [Google Scholar]
- Hermann PC, Mueller M-T, Heeschen C. Pancreatic cancer stem cells – insights and perspectives. Expert Opinion on Biological Therapy. 2009;9:1271–1278. doi: 10.1517/14712590903246362. [DOI] [PubMed] [Google Scholar]
- Horner MJ, R L, Krapcho M, Neyman N, Aminou R, Howlader N, Altekruse SF, Feuer EJ, Huang L, Mariotto A, Miller BA, Lewis DR, Eisner MP, Stinchcomb DG, Edwards BK. SEER Cancer Statistics Review, 1975–2006. Bethesda, MD: National Cancer Institute; 2009. pp. posted to the SEER web site, 2009. [Google Scholar]
- Jimeno A, Feldmann G, Suárez-Gauthier A, Rasheed Z, Solomon A, Zou G-M, Rubio-Viqueira B, García-García E, López-Ríos F, Matsui W, et al. A direct pancreatic cancer xenograft model as a platform for cancer stem cell therapeutic development. Molecular Cancer Therapeutics. 2009;8:310–314. doi: 10.1158/1535-7163.MCT-08-0924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallifatidis G, Rausch V, Baumann B, Apel A, Beckermann BM, Groth A, Mattern J, Li Z, Kolb A, Moldenhauer G, et al. Sulforaphane targets pancreatic tumour-initiating cells by NF-kappaB-induced antiapoptotic signalling. Gut. 2009;58:949–963. doi: 10.1136/gut.2008.149039. [DOI] [PubMed] [Google Scholar]
- Kleinman HK, Martin GR. Matrigel: Basement membrane matrix with biological activity. Seminars in Cancer Biology. 2005;15:378–386. doi: 10.1016/j.semcancer.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Li C, Heidt DG, Dalerba P, Burant CF, Zhang L, Adsay V, Wicha M, Clarke MF, Simeone DM. Identification of pancreatic cancer stem cells. Cancer Res. 2007;67:1030–1037. doi: 10.1158/0008-5472.CAN-06-2030. [DOI] [PubMed] [Google Scholar]
- Lonardo E, Hermann PC, Heeschen C. Pancreatic cancer stem cells - update and future perspectives. Molecular Oncology. 2010;4:431–442. doi: 10.1016/j.molonc.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller M-T, Hermann PC, Witthauer J, Rubio-Viqueira B, Leicht SF, Huber S, Ellwart JW, Mustafa M, Bartenstein P, D'Haese JG, et al. Combined Targeted Treatment to Eliminate Tumorigenic Cancer Stem Cells in Human Pancreatic Cancer. Gastroenterology. 2009;137:1102–1113. doi: 10.1053/j.gastro.2009.05.053. [DOI] [PubMed] [Google Scholar]
- Nagaoka M, Si-Tayeb K, Akaike T, Duncan S. Culture of human pluripotent stem cells using completely defined conditions on a recombinant E-cadherin substratum. BMC Developmental Biology. 2010;10:60. doi: 10.1186/1471-213X-10-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ning ZF, Huang YJ, Lin TX, Zhou YX, Jiang C, Xu KW, Huang H, Yin XB, Huang J. Subpopulations of Stem-like Cells in Side Population Cells from the Human Bladder Transitional Cell Cancer Cell Line T24. The Journal of International Medical Research. 2009;37:621–630. doi: 10.1177/147323000903700304. [DOI] [PubMed] [Google Scholar]
- Noble S, Goa KL. Gemcitabine. A review of its pharmacology and clinical potential in non-small cell lung cancer and pancreatic cancer. Drugs. 1997;54:447–472. doi: 10.2165/00003495-199754030-00009. [DOI] [PubMed] [Google Scholar]
- Quintana E, Shackleton M, Sabel MS, Fullen DR, Johnson TM, Morrison SJ. Efficient tumour formation by single human melanoma cells. Nature. 2008;456:593–598. doi: 10.1038/nature07567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rausch V, Liu L, Kallifatidis G, Baumann B, Mattern J, Gladkich J, Wirth T, Schemmer P, Buchler MW, Zoller M, et al. Synergistic Activity of Sorafenib and Sulforaphane Abolishes Pancreatic Cancer Stem Cell Characteristics. Cancer Res. 2010 doi: 10.1158/0008-5472.CAN-10-0066. [DOI] [PubMed] [Google Scholar]
- Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- Rosen JM, Jordan CT. The increasing complexity of the cancer stem cell paradigm. Science. 2009;324:1670–1673. doi: 10.1126/science.1171837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sergeant G, Vankelecom H, Gremeaux L, Topal B. Role of cancer stem cells in pancreatic ductal adenocarcinoma. Nat Rev Clin Oncol. 2009;6:580–586. doi: 10.1038/nrclinonc.2009.127. [DOI] [PubMed] [Google Scholar]
- Tavaluc RT, Hart LS, Dicker DT, El-Deiry WS. Effects of low confluency, serum starvation and hypoxia on the side population of cancer cell lines. Cell Cycle. 2007;6:2554–2562. doi: 10.4161/cc.6.20.4911. [DOI] [PubMed] [Google Scholar]
- Visvader JE, Lindeman GJ. Cancer stem cells in solid tumours: accumulating evidence and unresolved questions. Nat Rev Cancer. 2008;8:755–768. doi: 10.1038/nrc2499. [DOI] [PubMed] [Google Scholar]
- Vlashi E, McBride WH, Pajonk F. Radiation responses of cancer stem cells. J Cell Biochem. 2009;108:339–342. doi: 10.1002/jcb.22275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C, Alman BA. Side population cells in human cancers. Cancer letters. 2008;268:1–9. doi: 10.1016/j.canlet.2008.03.048. [DOI] [PubMed] [Google Scholar]
- Yao J, Cai HH, Wei JS, An Y, Ji ZL, Lu ZP, Wu JL, Chen P, Jiang KR, Dai CC, et al. Side population in the pancreatic cancer cell lines SW1990 and CFPAC-1 is enriched with cancer stem-like cells. Oncol Rep. 2010;23:1375–1382. doi: 10.3892/or_00000774. [DOI] [PubMed] [Google Scholar]
- Zhou J, Wang CY, Liu T, Wu B, Zhou F, Xiong JX, Wu HS, Tao J, Zhao G, Yang M, Gou SM. Persistence of side population cells with high drug efflux capacity in pancreatic cancer. World J Gastroenterol. 2008;14:925–930. doi: 10.3748/wjg.14.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.