Abstract
Structure-based protein design has enabled the engineering of insulin analogs with improved pharmacokinetic and pharmacodynamic properties. Exploiting classical structures of zinc insulin hexamers, the first insulin analog products focused on destabilization of subunit interfaces to obtain rapid-acting (prandial) formulations. Complementary efforts sought to stabilize the insulin hexamer or promote higher-order self-assembly within the subcutaneous depot toward the goal of enhanced basal glycemic control with reduced risk of hypoglycemia. Current products either operate through isoelectric precipitation (insulin glargine, the active component of Lantus®; Sanofi-Aventis) or employ an albumin-binding acyl tether (insulin detemir, the active component of Levemir®; Novo-Nordisk). In the past year second-generation basal insulin analogs have entered clinical trials in an effort to obtain ideal flat 24-hour pharmacodynamic profiles. The strategies employ non-standard protein modifications. One candidate (insulin degludec; Novo-Nordisk a/s) undergoes extensive subcutaneous supramolecular assembly coupled to a large-scale allosteric reorganization of the insulin hexamer (the TR transition). Another candidate (LY2605541; Eli Lilly and Co.) utilizes coupling to polyethylene glycol to delay absorption and clearance. On the other end of the spectrum, advances in delivery technologies (such as microneedles and micropatches) and excipients (such as the citrate/zinc-ion chelator combination employed by Biodel, Inc.) suggest strategies to accelerate PK/PD toward ultra-rapid-acting insulin formulations. Next-generation insulin analogs may also address the feasibility of hepatoselective signaling. Although not in clinical trials, early-stage technologies provide a long-range vision of “smart insulins” and glucose-responsive polymers for regulated hormone release.
Keywords: glycemic control, protein engineering, polyethylene glycol, basal, prandial, insulin analog, diabetes mellitus
Introduction
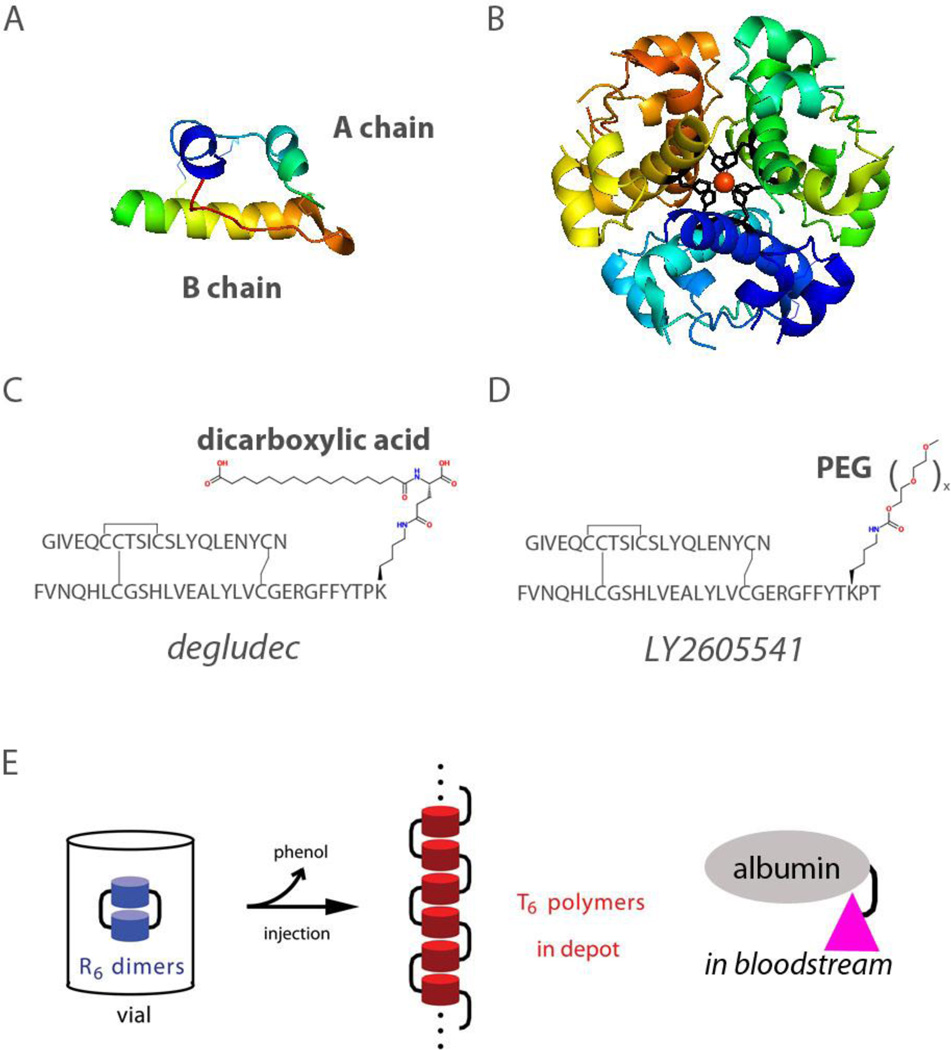
The crystal structure of the zinc insulin hexamer, first elucidated by Hodgkin and colleagues in 1969 [1, 2], defines a landmark in the history of structural biology (Fig. 1A and 1B). Providing the first depiction of a protein homo-oligomer, this and related crystal structures continue to provide a foundation for the design of insulin analogs and their stable pharmaceutical formulation [3, 4]. Analysis of the structure of an insulin hexamer at atomic-resolution [5] enabled design of rapid-acting (prandial) insulin analogs by targeted destabilization of self-association surfaces (Table 1A); their design and clinical use represent a triumph of rational protein design [6, 7]. Complementary efforts have led to the introduction of basal analog formulations superior to traditional NPH formulations and intended for once-a-day injection (Table 1B). The overarching therapeutic objective of insulin analog products, singly or in combination, is to recapitulate the physiologic pattern of insulin secretion by pancreatic β-cells in the course of metabolic homeostasis. Advances in the past year have focused on second-generation basal insulin analogs. In this mini-review we seek to relate therapeutic objectives to the utility of non-standard protein modifications.
Figure 1.
(A) Ribbon model of wild-type insulin (B) Structure of insulin hexamer. Two axial zinc ions (red; overlaid at center) are coordinated by six histidine side chains (residue B10; black). The structure shown is the R6 hexamer form characteristic of a pharmaceutical formulation [83]; coordinates were obtained from Protein Databank entry 1ZNJ. (C) Structure of insulin degludec showing the dicarboxylic acid attached to the ε-amino group of LysB29. (D) Structure of LY2605541 showing PEG attached to the ε-amino group of LysB28. (E) Exploiting the TR transition in supramolecular protein engineering: schematic representation of the mechanism of insulin degludec. Left, Insulin degludec is formulated at neutral pH as dimers of phenol- (or meta-cresol) stabilized R6 zinc insulin hexamers (blue). The acyl modification of LysB29 is shown in schematic form as a black bar (in principle 6 per hexamer); for simplicity only two are shown. Center, On subcutaneous injection, diffusion of the phenolic ligand into cellular membranes triggers the R → T transition, leading in turn to linear polymerization of T6 zinc hexamers (red). Classical hexamer reorganization is thus coupled to a change in mode of hexamer-hexamer assembly mediated in part by the B29-linked acyl group. Right, Upon entering the bloodstream as monomers (pink) insulin degludec binds to circulating albumin forming another depot that further protracts its action.
Table 1.
Current Insulin Analogs and Modes of Actiona
| Analog | Modification | Mechanism |
|---|---|---|
| A | ||
| Lispro (Humalog®) Eli Lilly and Co. |
ProB28 → Lys LysB29 → Pro |
IGF-I-related motif impairs dimerization |
| Aspart (NovoLog®) Novo-Nordisk |
ProB28 → Asp | Charge repulsion at dimer interface |
| Glulisine (Apidra®) Sanofi-Aventis |
AsnB3 → Lys LysB29 → Glu |
Decreased zinc-free self-association |
| B | ||
| Glargine (Lantus®) Sanofi-Aventis |
ArgB31-ArgB32 tag AspA21 → Gly |
Shift in pI to pH 7 leads to isoelectric precipitation on injection |
| Detemir (Levemir®) Novo-Nordisk |
Modification of LysB29 by a tethered fatty acid | Stabilization of hexamer and binding to serum albumin |
| C | ||
| Insulin degludec Novo-Nordisk | Modification of LysB29 by a dicarboxylic acid | Allosteric assembly of a linear T6 polymer and albumin binding |
| LY2605541 Eli Lilly and Co. |
PEGylation of LysB28 in insulin lispro | Increased hydrodynamic radius |
Panel A lists rapid-acting analogs employed in prandial regimens and in insulin pumps. Panel B describes basal insulin analogs with protracted action. Panel C lists novel basal insulin analogs undergoing Phase 3 clinical trials; PEG, polyethylene glycol.
Prandial Insulin Analogs
The design of rapid-acting insulin analogs provided an initial paradigm for the development and application of general principles of protein folding and assembly. Working toward the goal of accelerating hexamer disassembly [8, 9], these efforts posited that more rapid disassembly in the subcutaneous depot would facilitate capillary absorption of zinc-free insulin monomers and dimers [6, 10]. Mutations at subunit interfaces were found to weakened or prevent formation of dimers and hexamers with retention of native biological activity. Three such rapid-acting analogs are in current use (Table 1A): insulin lispro (the active component of Humalog®; Eli Lilly) [11], insulin aspart (Novolog®; Novo-Nordisk) [12, 13], and insulin glulisine (Apidra®; Sanofi-Aventis) [14, 15]. These products have been proven safe and effective in multi-injection regimens [7, 16] and for use in continuous subcutaneous infusion devices (insulin pumps) [17]. That distinct molecular strategies may equally well confer more rapid insulin absorption (e.g., introduction of a negative charge near the C-terminus of the B-chain in insulin apart (AspB28) or glulisine (GluB29) versus rearrangement of a positive charge adjoining a proline in insulin lispro (LysB28-ProB29)) reflects the multiple ways that the wild-type insulin hexamer may be rendered less stable with maintenance of biological activity.
Rapid-acting insulin analog formulations have proven to be safe and effective for use in insulin pumps [17]. Increasing interest in closed-loop systems (in which control of the pump is controlled by an algorithm based on feedback from a continuous glucose monitor [18, 19]) has highlighted the need for insulin analogs even faster than current products [20–23]. Although early-stage protein engineering efforts have been undertaken to address this need [24], in recent years progress has been obtained in small clinical trials and pre-clinical trials done in animals through ancillary technologies. These include use of heating pads at the site of injection to increase local blood flow [25, 26], co-injection of the enzyme hyaluronidase to break down connective tissue at the site of injection [27], trans-dermal micro-needle patches (with active infusion [28]), intradermal microneedles administration [29], needle-free jet injection [30], and excipients that accelerate insulin hexamer disassembly [31, 32]. Enthusiasm for approaches unrelated to molecular features of insulin itself in part reflects concern that any further pharmacokinetic advantage of accelerated protein disassembly in a subcutaneous depot (beyond that achieved by current products) may be incremental. Any further destabilization of the insulin hexamer may also incur a tradeoff between rapid action and the stability requirements of a pharmaceutical formulation. It is possible that extension of protein engineering to include use of unnatural amino acids (as can now be incorporated in microbial expression systems by expanded genetic-code technology [33]) may encourage efforts to combine novel molecular design with innovative delivery systems.
Basal Insulin Analogs
A cornerstone in the non-pump-based treatment of diabetes mellitus is provided by basal insulin analog formulations [34]. Whereas fast-acting analogs are essential for the management of Type 1 diabetes mellitus, basal insulin analogs enhance glycemic control in multi-injection regimens [35] and offer superior pharmacokinetic and pharmacodynamic profiles with fewer hypoglycemic events as compared to older basal insulin products which also benefits patients with Type 2 diabetes mellitus [36]. The current and predicted global need for such products exceeds that of rapid-acting formulations due to the increasing prevalence of the metabolic syndrome and Type 2 diabetes mellitus. In such patients controllable by either prandial or basal analogs alone, basal regimens are preferred to their simplicity and reduced risk of weight gain (as exclusive meal-time injections enhance insulin’s anabolic effects) [37].
Targeted stabilization of the insulin hexamer has posed a more subtle challenge to the protein engineer than its targeted destabilization [4, 38]. Evolutionary optimization of the insulin hexamer and the molecular elegance of its conserved self-assembly surfaces [5] limit possible structural routes to further improvement. Although pioneering efforts in this direction were proposed by Dodson and colleagues [4, 5], current products (Table 1B) have employed non-peptidic modifications to circumvent the need for detailed molecular analysis. Insulin glargine (the active component of Lantus®; Sanofi-Aventis) thus exploits isoelectric precipitation, a reversible transition to insolubility that classically occurs between pH 5 and 6 (under which conditions wild-type insulin exhibits little or no net charge) [39, 40]. This strategy is robust to the details of molecular structure. Insulin glargine contains a two-residue basic extension of the B-chain (ArgB31 and ArgB32) whose positive charges result in a shift in the isoelectric point to neutrality. Injection of an unbuffered pH 4 formulation results in precipitation within the subcutaneous depot with protracted absorption for 16–24 hours [41]. The di-arginyl extension is disordered and largely removed by endogenous exopeptidases. Lantus® is the most widely-used long-acting insulin currently on the market [42]. Related analogs containing additional basic residues N-terminal to GlyA1 have also been described but are not in clinical use [43]. Insulin detemir (the active component of Levemir®; Novo-Nordisk) by contrast contains a prosthetic fatty acyl group on LysB29, intended to mediate binding to serum albumin and hence provide a circulating depot [44]. Although this mechanism is active, the tethered moiety serendipitously also enhances the stability of hexamers of the modified insulin [45]. Levemir® is often administered twice a day as its duration of action is less prolonged than that of Lantus®. Thus, non-standard modification of the insulin molecule has enabled the exploitation of chemical tactics beyond those made possible by the 20 naturally occurring amino acids – and so complement classical structural relationships implicitly optimized by evolution.
Next-Generation Insulin Analogs
Two candidate basal insulin analogs are under investigation (Table 1C). These employ different structural and biophysical principles as described in turn below.
Allosteric Assembly of a Subcutaneous Insulin Depot
A next-generation basal insulin analog developed by Novo-Nordisk, designated insulin degludec, is under clinical investigation by a network of academic collaborators [46–49]. Formulated at neutral pH, the protein solution contains a dimer of zinc hexamers linked by a novel acyl modification (Fig. 1C). Remarkably, the analog undergoes multi-hexamer assembly in the subcutaneous depot and thereby achieves protracted action. The designation “degludec” reflects three chemical features: de, the absence of ThrB30 (i.e., des-B30), glu, side-chain addition of a glutamic acid residue via a non-standard peptide bond between its δ-carboxylate function and the ε-amino group of LysB29, and dec, referring to a dicarboxylic acid (HOOC-(CH2)n-COOH) in turn linked to the α-amino group of attached Glu. Optimization of this class of modifications by Novo-Nordisk led to the choice of n=14, corresponding to an ester of thapsic acid. Whereas insulin glargine contains two additional positive charges at neutral pH (ArgB31 and ArgB32; see above) and is insoluble, the net formal charge of insulin degludec differs from wild-type insulin by -2 (loss of the positive charge of LysB29 and gain of one negative charge from the peptide-linked thapsic acid), thereby permitting formulation as a clear solution at pH 7.4.
Results of two Phase 3 clinical trials of insulin degludec in the respective treatment of patients with type 1 and type 2 diabetes mellitus have been released [50, 51]. These open-label treat-to-target trials were designed to demonstrate non-inferiority to insulin glargine in a basal-bolus algorithm (the bolus component was in each case provided by insulin aspart). The primary endpoint was change in HbA1c over the course of 1 year. The trials showed that the efficacy of insulin degludec was similar to that of insulin glargine, confirming the findings of smaller previous studies [48, 49]. Notably, the Phase 3 studies documented lower rates of nocturnal hypoglycemia in both type 1 and type 2 patients; fewer overall hypoglycemic events were reported in the study involving type 2 patients. These differences were significant. Reduced risk of hypoglycemic events was hypothesized to be due to the flatter and more extended pharmacokinetic and pharmacodynamic profile of insulin degludec. These favorable features are presumably due to the prolonged duration of the subcutaneous insulin depot and subsequent binding of the circulating degludec monomer to albumin. Reduced risk of nocturnal and overall hypoglycemia may encourage more aggressive efforts by patients and providers to achieve individualized glycemic targets.
The mechanism of protracted action by insulin degludec exploits the classical structural reorganization of zinc insulin hexamers (designated the T → R transition) [52–54]. Higher-order assembly of insulin in the subcutaneous depot is associated with a change in hexamer conformation triggered by release of the phenolic preservative from its R-state binding sites. Thus, whereas the vial or pen formulations contain dimers of stable R-type hexamers (advantageous for stability and resistance to degradation), the depot contains supramolecular acyl-bridged stacks of T-type hexamers, yielding linear polymers that only slowly disassemble (Fig. 1E). Once in the bloodstream, the acyl modification also permits albumin binding as achieved by insulin detemir. Because the pharmacokinetic profile of insulin degludec exceeds 24 hours, once-a-day dosing promises to provide a flatter pharmacodynamic profile and reduced risk of hypoglycemia relative to first-generation products [47–49]. Acylated insulin analogs conferring protracted action have also been disclosed in patents awarded to the Lilly Research Laboratories [43, 55–58]. Although the physical state of proteins in a subcutaneous depot is not amenable to atomic-level characterization, many of these unanticipated structural features lead to the favorable pharmacokinetic properties of this next-generation analog and represent a combination of rational design and serendipity. The mechanism of action of insulin degludec highlights the structure and organization of a subcutaneous insulin depot as a biomaterial in its own right [59]. An independent approach to the supramolecular “depot engineering” in the absence of unnatural modifications exploits the introduction of novel zinc-ion binding sites on the surface of the insulin hexamer to enable its pH-dependent supramolecular assembly in the subcutaneous depot [60].
Development of a PEGylated Basal Insulin Analog
A derivative of insulin lispro containing a single polyethylene-glycol (PEG) moiety is under clinical investigation as a basal insulin analog formulation. Developed at the Lilly Research Laboratories and designated LY2605541, the analog contains a 20-kDa PEG unit attached to LysB28 via its ε-amino group by means of a urethane bond (Fig. 1D). The modified insulin analog exhibits a duration of action that is more protracted than that of Lantus and offers the potential promise of reduced intra-patient variability [61]. Although no formal reports have been published, presentations and posters at the recent 2012 American Diabetes Association's 72nd Scientific Sessions have provided evidence that LY2605541 may be associated with reduced risk of nocturnal hypoglycemia relative to Lantus [62]. The Lilly investigators hypothesize that the increased hydrodynamic radius of the analog (a fourfold increase afforded by the attached PEG moiety; see below) retards both absorption from the subcutaneous depot and renal clearance [63]. Preliminary data suggest that treatment with LY2605541 is associated with modest weight loss rather than the weight gain often seen on treatment with Lantus. Studies of the analog in dogs further suggest that the PEGylated analog may have hepatoselective action to blunt hepatic glucose output [64]. Although no molecular mechanism has been established for the partial hepatoselective effect exhibited by this analog, we speculate that it may be a consequence of reduced receptor-mediated clearance and reduced uptake by hepatic Kupffer cells due to the bulk of the PEG moiety, leading in turn to longer residence times in the microcirculation of the liver [65].
PEGylation, although novel in the context of insulin, provides a well-established strategy to enhance the therapeutic properties of proteins [66]. Pioneered by F. Davis (Rutgers University) more than 40 years ago, PEGylation leads to increased solubility (due to its hydrophilicity), decreased degradation (due to decreased access of proteases to the protein itself), and augmented hydrodynamic radius, markedly decreasing renal clearance [67]. Molecular diversity can be explored as PEG polymers can vary by size (5–40 kDa), degree of branching, and site (or sites) of attachment to a protein. To date, several PEGylated proteins have been approved for US Food & Drug Administration for human use, including PEGylated adenosine deaminase (for treatment for severe combined immunodeficiency syndrome) and PEGylated interferon alpha (for treatment of treatment for Hepatitis C).
Conclusions
The discovery of insulin in 1922 was a transformational event in molecular medicine and elicited broad public support for the development of innovative therapeutic products [68]. Characterization of the atomic-level structure of insulin and its conformational repertoire has extended over eight decades and involved international networks of laboratories, with current focus on the relationship between classical structures [5] and the mechanism of binding to (and triggering) the insulin receptor [69]. It is remarkable that this small globular protein continues to inspire molecular innovation, motivated by unmet clinical needs. Short-term risks of insulin replacement therapy reflect daily steering between treatment-related hypoglycemia (with its neurocognitive and adrenergic symptoms), on the one hand, and hyperglycemic excursions on the other. In addition to acute metabolic decompensation, hyperglycemic excursions are associated with omnipresent long-term risks of microvascular, macrovascular, and neurologic complications.
Considerable attention has been paid in the past year to potential public-health concerns regarding a possible link between basal insulin analogs and cancer. These concerns were stimulated by a study in 2008 suggesting that use of Lantus at high doses lead to an increased risk of several common cancers. Such concerns were exacerbated by reports that the analog exhibits a sixfold-increased affinity relative to human insulin for the mitogenic IGF-1R and by in vitro studies suggesting an almost eightfold increase in mitogenic potential in cancer cell lines [70–72]. The claimed association between insulin glargine and risk of cancer is controversial and is not supported by recent studies [73–75]. Additional population-based studies of cancer prevalence in long-term users of insulin products (ideally prospective and randomized) will nevertheless be required to address the issue of carcinogenicity and its potential relationship to in vitro properties of insulin analogs.
Next-generation insulin analogs in affluent societies seek to enhance the convenience and safety by which patients can achieve metabolic control. Ultra-rapid delivery technologies and ultra-flat basal insulin analog formulations promise to enable patients to recapitulate with greater precision endogenous mechanisms of hormonal regulation. The cost-effectiveness of such technologies, an of increasing societal concern in relation to aggregate health-care expenditures [76–78], will require population-based analysis of relative impact on rates of complications leading to expensive interventions and long-term disability [79]. The majority of patients in the coming decades will be living in underprivileged regions of the developing world. In such regions intertwined scientific, technical, and societal challenges are posed by the cold chain of insulin delivery in the absence of refrigeration [80–82]. Given this humanitarian need and its growing scale, we anticipate that third-generation insulin analogs must combine ultra-stability with optimized pharmacokinetic properties. Such efforts define a new frontier of translational research with application to global health.
Acknowledgments
This work was supported by grants from the National Institutes of Health and American Diabetes Association to one of the authors (M.A.W.). V.P. is a predoctoral fellow of the NIH Medical Scientist Training Program at the CWRU School of Medicine and is supported by NIH Fellowship F30DK094685-02.
Footnotes
Disclosure
Conflicts of Interest: The intellectual property pertaining to zinc-stapled human insulin analogs and its long-acting formulations are owned by Case Western Reserve University and licensed to Thermalin Diabetes, LLC. M.A. Weiss: holds shares in and is Chief Scientific Officer of Thermalin Diabetes, LLC.; he has also been a consultant to Merck, Inc. and the DEKA Research and Development Corp.; V. Pandyarajan: none. The authors otherwise declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Adams MJ, Blundell TL, Dodson EJ, et al. Structure of rhombohedral 2 zinc insulin crystals. Nature. 1969;224:491–495. [Google Scholar]
- 2.Blundell TL, Cutfield JF, Cutfield SM, et al. Atomic positions in rhombohedral 2-zinc insulin crystals. Nature. 1971;231:506–511. doi: 10.1038/231506a0. [DOI] [PubMed] [Google Scholar]
- 3.Brange J, Skelbaek-Pedersen B, Langkjaer L, et al. Galenics of insulin: the physico-chemical and pharmaceutical aspects of insulin and insulin preparations. Berlin: Springer-Verlag; 1987. [Google Scholar]
- 4.Dodson GG, Dodson EJ, Turkenburg JP, Bing X. Molecular recognition in insulin assembly. Biochem Soc Trans. 1993;21:609–614. doi: 10.1042/bst0210609. [DOI] [PubMed] [Google Scholar]
- 5.Baker EN, Blundell TL, Cutfield JF, et al. The structure of 2Zn pig insulin crystals at 1.5 Å resolution. Philos Trans R Soc Lond B Biol Sci. 1988;319:369–456. doi: 10.1098/rstb.1988.0058. [DOI] [PubMed] [Google Scholar]
- 6.Brange J. The new era of biotech insulin analogues. Diabetologia. 1997;40:S48–S53. doi: 10.1007/s001250051400. [DOI] [PubMed] [Google Scholar]
- 7.Freeman JS. Insulin analog therapy: Improving the match with physiologic insulin secretion. J Am Osteo Assoc. 2009;109:26–36. [PubMed] [Google Scholar]
- 8.Brange J, Hansen JF, Havelund S, Melberg SG. Studies of insulin fibrillation process. In: Brunetti P, Waldhäusl WK, editors. Advanced models for the therapy of insulin-dependent diabeses. New York: Raven Press; 1987. pp. 85–90. [Google Scholar]
- 9.Brems DN, Alter LA, Beckage MJ, et al. Altering the association properties of insulin by amino acid replacement. Protein Eng. 1992;5:527–533. doi: 10.1093/protein/5.6.527. [DOI] [PubMed] [Google Scholar]
- 10.DeFelippis MR, Chance RE, Frank BH. Insulin self-association and the relationship to pharmacokinetics and pharmacodynamics. Crit Rev Ther Drug Carrier Syst. 2001;18:201–264. [PubMed] [Google Scholar]
- 11.DiMarchi RD, Chance RE, Long HB, et al. Preparation of an insulin with improved pharmacokinetics relative to human insulin through consideration of structural homology with insulin-like growth factor. I. Horm Res. 1994;41(Suppl. 2):93–96. doi: 10.1159/000183967. [DOI] [PubMed] [Google Scholar]
- 12.Heinemann L, Heise T, Jorgensen LN, Starke AA. Action profile of the rapid acting insulin analogue: human insulin B28Asp. Diabet Med. 1993;10:535–539. doi: 10.1111/j.1464-5491.1993.tb00116.x. [DOI] [PubMed] [Google Scholar]
- 13.Owens D, Vora J. Insulin aspart: a review. Expert Opin Drub Metab Toxicol. 2006;2:793–804. doi: 10.1517/17425255.2.5.793. [DOI] [PubMed] [Google Scholar]
- 14.Barlocco D. Insulin glulisine. Aventis Pharma. Curr Opin Investig Drugs. 2003;4:1240–1244. [PubMed] [Google Scholar]
- 15.Helms KL, Kelley KW. Insulin glulisine: an evaluation of its pharmacodynamic properties and clinical application. Ann Pharmacother. 2009;43:658–668. doi: 10.1345/aph.1E662. [DOI] [PubMed] [Google Scholar]
- 16.Garg S, Ampudia-Blasco FJ, Pfohl M. Rapid-acting insulin analogues in Basal-bolus regimens in type 1 diabetes mellitus. Endocr Pract. 2010;16:486–505. doi: 10.4158/EP09294.RA. [DOI] [PubMed] [Google Scholar]
- 17.Colquitt J, Royle P, Waugh N. Are analogue insulins better than soluble in continuous subcutaneous insulin infusion? Results of a meta-analysis. Diab Med. 2003;20:863–866. doi: 10.1046/j.1464-5491.2003.01018.x. [DOI] [PubMed] [Google Scholar]
- 18.Elleri D, Dunger DB, Hovorka R. Closed-loop insulin delivery for treatment of type 1 diabetes. BMC Med. 2011;9:120. doi: 10.1186/1741-7015-9-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schaepelynck P, Darmon P, Molines L, et al. Advances in pump technology: insulin patch pumps, combined pumps and glucose sensors, and implanted pumps. Diabetes Metab. 2011;37(Suppl 4):S85–93. doi: 10.1016/S1262-3636(11)70972-7. [DOI] [PubMed] [Google Scholar]
- 20.Brown L, Edelman ER. Optimal control of blood glucose: the diabetic patient or the machine? Sci Transl Med. 2010;2:27ps18. doi: 10.1126/scitranslmed.3001083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.NIDDK . In: Advances and emerging opportunities in diabetes research: a Strategic Planning report of the DMICC. Committee DMIC, editor. Washington, DC: 2011. [Google Scholar]
- 22.ADA. American Diabetes Association Scientific Sessions 2011 San Diego, California 2011 June 24 – 28. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scutcher M, Vasilakis-Scaramozza C, Sullivan K. Vital Biopharmaceutical Insights and Analytics for Experts from Experts. Burlington, Mass.: Decision Resources Group Co.; 2011. Diabetic Complications. [Google Scholar]
- 24.Phillips NB, Whittaker J, Ismail-Beigi F, Weiss MA. Insulin fibrillation and protein design: topological resistance of single-chain analogs to thermal degradation with application to a pump reservoir. J Diabetes Sci Technol. 2012;6:277–288. doi: 10.1177/193229681200600210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Raz I, Weiss R, Yegorchikov Y, et al. Effect of a local heating device on insulin and glucose pharmacokinetic profiles in an open-label, randomized, two-period, one-way crossover study in patients with type 1 diabetes using continuous subcutaneous insulin infusion. Clin Ther. 2009;31:980–987. doi: 10.1016/j.clinthera.2009.05.010. [DOI] [PubMed] [Google Scholar]
- 26.Jakobsen LA, Jensen A, Larsen LE, et al. Effect of cutaneous blood flow on absorption of insulin: a methodological study in healthy male volunteers. Int J Physiol Pathophysiol Pharmacol. 2011;3:257–265. [PMC free article] [PubMed] [Google Scholar]
- 27.Vaughn DE, Muchmore DB. Use of recombinant human hyaluronidase to accelerate rapid insulin analogue absorption: experience with subcutaneous injection and continuous infusion. Endocr Pract. 2011;17:914–921. doi: 10.4158/EP11297.RA. [DOI] [PubMed] [Google Scholar]
- 28.Nordquist L, Roxhed N, Griss P, Stemme G. Novel microneedle patches for active insulin delivery are efficient in maintaining glycaemic control: an initial comparison with subcutaneous administration. Pharm Res. 2007;24:1381–1388. doi: 10.1007/s11095-007-9256-x. [DOI] [PubMed] [Google Scholar]
- 29.Pettis RJ, Ginsberg B, Hirsch L, et al. Intradermal microneedle delivery of insulin lispro achieves faster insulin absorption and insulin action than subcutaneous injection. Diabetes Technol Ther. 2011;13:435–442. doi: 10.1089/dia.2010.0184. [DOI] [PubMed] [Google Scholar]
- 30.Engwerda EE, Abbink EJ, Tack CJ, de Galan BE. Improved pharmacokinetic and pharmacodynamic profile of rapid-acting insulin using needle-free jet injection technology. Diabetes Care. 2011;34:1804–1808. doi: 10.2337/dc11-0182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hompesch M, McManus L, Pohl R, et al. Intra-individual variability of the metabolic effect of a novel rapid-acting insulin (VIAject) in comparison to regular human insulin. J Diabetes Sci Technol. 2008;2:568–571. doi: 10.1177/193229680800200406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heinemann L, Nosek L, Flacke F, et al. U-100, pH-Neutral formulation of VIAject((R)) : faster onset of action than insulin lispro in patients with type 1 diabetes. Diabetes Obes Metab. 2011 doi: 10.1111/j.1463-1326.2011.01516.x. [DOI] [PubMed] [Google Scholar]
- 33.Young TS, Schultz PG. Beyond the canonical 20 amino acids: expanding the genetic lexicon. J Biol Chem. 2010;285:11039–11044. doi: 10.1074/jbc.R109.091306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pollock RF, Erny-Albrecht KM, Kalsekar A, et al. Long-acting insulin analogs: a review of "real-world" effectiveness in patients with type 2 diabetes. Curr Diabetes Rev. 2011;7:61–64. doi: 10.2174/157339911794273892. [DOI] [PubMed] [Google Scholar]
- 35.King AB, Armstrong DU. Basal bolus dosing: a clinical experience. Curr Diabetes Rev. 2005;1:215–220. doi: 10.2174/1573399054022794. [DOI] [PubMed] [Google Scholar]
- 36.Baxter MA. The role of new basal insulin analogues in the initiation and optimization of insulin therapy in type 2 diabetes. Acta Diabetol. 2008;45:253–268. doi: 10.1007/s00592-008-0052-9. [DOI] [PubMed] [Google Scholar]
- 37.Pontiroli AE, Miele L, Morabito A. Increase of body weight during the first year of intensive insulin treatment in type 2 diabetes: systematic review and meta-analysis. Diabetes Obes Metab. 2011;13:1008–1019. doi: 10.1111/j.1463-1326.2011.01433.x. [DOI] [PubMed] [Google Scholar]
- 38.Markussen J, Diers I, Hougaard P, et al. Soluble, prolonged-acting insulin derivatives. III. Degree of protraction, crystallizability and chemical stability of insulins substituted in positions A21, B13, B23, B27 and B30. Protein Eng. 1988;2:157–166. doi: 10.1093/protein/2.2.157. [DOI] [PubMed] [Google Scholar]
- 39.Owens DR, Griffiths S. Insulin glargine (Lantus) Int J Pract. 2002;56:460–466. [PubMed] [Google Scholar]
- 40.Goykhman S, Drincic A, Desmangles JC, Rendell M. Insulin Glargine: a review 8 years after its introduction. Expert Opin Pharmacother. 2009;10:705–718. doi: 10.1517/14656560902775677. [DOI] [PubMed] [Google Scholar]
- 41.Heinemann L, Linkeschova R, Rave K, et al. Time-action profile of the long-acting insulin analog insulin glargine (HOE901) in comparison with those of NPH insulin and placebo. Diab Care. 2000;23:644–649. doi: 10.2337/diacare.23.5.644. [DOI] [PubMed] [Google Scholar]
- 42.Maggon K. Guild (KPG) Knol Publishing; 2011. Global Diabetes Market Review 2008 & World Top Ten Diabetes drugs:World Top Ten diabetic drug brands, market trends and top companies 2008. [Google Scholar]
- 43.Kohn WD, Micanovic R, Myers SL, et al. pI-shifted insulin analogs with extended in vivo time action and favorable receptor selectivity. Peptides (N Y) 2007;28:935–948. doi: 10.1016/j.peptides.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 44.Havelund S, Plum A, Ribel U, et al. The mechanism of protraction of insulin detemir, a long-acting, acylated analog of human insulin. Pharm Res. 2004;21:1498–1504. doi: 10.1023/b:pham.0000036926.54824.37. [DOI] [PubMed] [Google Scholar]
- 45.Whittingham JL, Jonassen I, Havelund S, et al. Crystallographic and solution studies of N-lithocholyl insulin: a new generation of prolonged-acting human insulins. Biochemistry. 2004;43:5987–5995. doi: 10.1021/bi036163s. [DOI] [PubMed] [Google Scholar]
- 46.Markussen J, Jonassen I, Havelund S, et al., inventors. Insulin derivatives. 6,620,780. U. S. A. patent. 2003 Sep 16; 2003.
- 47.Zinman B, Fulcher G, Rao PV, et al. Insulin degludec, an ultra-long-acting basal insulin, once a day or three times a week versus insulin glargine once a day in patients with type 2 diabetes: a 16-week, randomised, open-label, phase 2 trial. Lancet. 2011;377:924–931. doi: 10.1016/S0140-6736(10)62305-7. [DOI] [PubMed] [Google Scholar]
- 48.Birkeland KI, Home PD, Wendisch U, et al. Insulin degludec in type 1 diabetes: a randomized controlled trial of a new-generation ultra-long-acting insulin compared with insulin glargine. Diabetes Care. 2011;34:661–665. doi: 10.2337/dc10-1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Heise T, Tack CJ, Cuddihy R, et al. A new-generation ultra-long-acting basal insulin with a bolus boost compared with insulin glargine in insulin-naive people with type 2 diabetes: a randomized, controlled trial. Diabetes Care. 2011;34:669–674. doi: 10.2337/dc10-1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Heller S, Buse J, Fisher M, et al. Insulin degludec, an ultra-longacting basal insulin, versus insulin glargine in basal-bolus treatment with mealtime insulin aspart in type 1 diabetes (BEGIN Basal-Bolus Type 1): a phase 3, randomised, open-label, treat-to-target non-inferiority trial. Lancet. 2012;379:1489–1497. doi: 10.1016/S0140-6736(12)60204-9. Phase 3 open label treat-to-target clinical trial data for insulin degludec done in patients with type 1 diabetes.
- 51. Garber AJ, King AB, Del Prato S, et al. Insulin degludec, an ultra-longacting basal insulin, versus insulin glargine in basal-bolus treatment with mealtime insulin aspart in type 2 diabetes (BEGIN Basal-Bolus Type 2): a phase 3, randomised, open-label, treatto- target non-inferiority trial. Lancet. 2012;379:1498–1507. doi: 10.1016/S0140-6736(12)60205-0. Phase 3 open label treat-to-target clinical trial data for insulin degludec done in patients with type 2 diabetes.
- 52.Bentley G, Dodson E, Dodson G, et al. Structure of insulin in 4-zinc insulin. Nature. 1976;261:166–168. doi: 10.1038/261166a0. [DOI] [PubMed] [Google Scholar]
- 53.Brader ML, Dunn MF. Insulin hexamers: new conformations and applications. Trends Biochem Sci. 1991;16:341–345. doi: 10.1016/0968-0004(91)90140-q. [DOI] [PubMed] [Google Scholar]
- 54.Derewenda U, Derewenda Z, Dodson EJ, et al. Phenol stabilizes more helix in a new symmetrical zinc insulin hexamer. Nature. 1989;338:594–596. doi: 10.1038/338594a0. [DOI] [PubMed] [Google Scholar]
- 55.Flora DB, inventor. Fatty acid-acylated insulin analogs. 6,444,641. U. S. A. patent. 2002 Sep 3; 2002.
- 56.Baker JC, Hanquier JM, inventors. Acylated insulin analogs. 5,922,675. U. S. A. patent. 1999 Jul 13; 1999.
- 57.Beals JM, DeFelippis MR, DiMarchi RD, et al., inventors. Eli Lilly and Company, assignee. Insulin molecule having protracted time action. 2005/0014679. U. S. A. patent. 2005 Jan 20; 2005.
- 58.Kohn WD, Zhang L, DiMarchi RD, inventors. Insulin analogs having protracted time action. 2006/0217290. U. S. A. patent. 2006 Sep 28; 2006.
- 59. Jonassen I, Havelund S, Hoeg-Jensen T, et al. Design of the novel protraction mechanism of insulin degludec, an ultra-long-acting basal insulin. Pharm Res. 2012 doi: 10.1007/s11095-012-0739-z. The mechanism of the long-acting insulin degludec analog is clearly laid out here along with much of the relevant biophysical data.
- 60.Phillips NB, Wan ZL, Whittaker L, et al. Supramolecular protein engineering: design of zinc-stapled insulin hexamers as a long acting depot. J Biol Chem. 2010;285:11755–11759. doi: 10.1074/jbc.C110.105825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Sinha VP, Howey DC, Kwang D, et al. Poster presented at the American Diabetes Association's 72nd Scientific Sessions. Philadelphia, PA: Eli Lilly and Co.; 2012. Single-dose pharmacokinetics (PK) and glucodynamics (GD) of the novel, long-acting basal insulin LY2605541 in healthy subjects. Data that illustrates the extended effect of pegylated insulin lispro obtained in healthy human adults using a single dose.
- 62.Bergenstal RM, Rosenstock J, Arakaki RF, et al. Oral presentation at the American Diabetes Association's 72nd Scientific Sessions. Philadelphia, PA: Eli Lilly and Company; 2012. Reduced nocturnal hypoglycemia and weight loss with novel, long-acting basal insulin LY2605541 compared with insulin glargine in patients with type 2 diabetes. [Google Scholar]
- 63. Hansen RJ, Cutler JGB, Vick A, et al. Poster presented at the American Diabetes Association's 72nd Scientific Sessions. Philadelphia, PA: Lilly Laboratories; 2012. LY2605541: Leveraging hydrodynamic size to develop a novel basal insulin 896-P. Explains the rationale behind the design of pegylated insulin lispro and provides the supporting data done in rats.
- 64.Moore MC, Smith MS, Mace KF, et al. Poster presented at the American Diabetes Association's 72nd Scientific Sessions. Philadelphia, PA: Department of Molecular Physiology & Biophysics, Vanderbilt University School of Medicine; 2012. Novel PEGylated basal Insulin LY2605541 has a preferential hepatic effect on glucose metabolism. [Google Scholar]
- 65.Webster R, Didier E, Harris P, et al. PEGylated proteins: evaluation of their safety in the absence of definitive metabolism studies. Drug Metab Dispos. 2007;35:9–16. doi: 10.1124/dmd.106.012419. [DOI] [PubMed] [Google Scholar]
- 66.Davis FF. The origin of pegnology. Adv Drug Deliv Rev. 2002;54:457–458. doi: 10.1016/s0169-409x(02)00021-2. [DOI] [PubMed] [Google Scholar]
- 67.Veronese FM, Pasut G. PEGylation, successful approach to drug delivery. Drug Discov Today. 2005;10:1451–1458. doi: 10.1016/S1359-6446(05)03575-0. [DOI] [PubMed] [Google Scholar]
- 68.Bliss M. The discovery of insulin: twenty-fifth anniversary edition. Chicago: University of Chicago Press; 2007. [Google Scholar]
- 69.Whittaker J, Whittaker LJ, Roberts CT, Jr, et al. α-Helical element at the hormonebinding surface of the insulin receptor functions as a signaling element to activate its tyrosine kinase. Proc Natl Acad Sci U S A. 2012;109:11166–11171. doi: 10.1073/pnas.1205681109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zib I, Raskin P. Novel insulin analogues and its mitogenic potential. Diab Obes Metab. 2006;8:611–620. doi: 10.1111/j.1463-1326.2005.00567.x. [DOI] [PubMed] [Google Scholar]
- 71.Nagel JM, Staffa J, Renner-Muller I, et al. Insulin glargine and NPH insulin increase to a similar degree epithelial cell proliferation and aberrant crypt foci formation in colons of diabetic mice. Horm Cancer. 2010;1:320–330. doi: 10.1007/s12672-010-0020-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Teng JA, Hou RL, Li DL, et al. Glargine promotes proliferation of breast adenocarcinoma cell line MCF-7 via AKT activation. Horm Metab Res. 2011;43:519–523. doi: 10.1055/s-0031-1280780. [DOI] [PubMed] [Google Scholar]
- 73.Lind M, Fahlen M, Eliasson B, Oden A. The relationship between the exposure time of insulin glargine and risk of breast and prostate cancer: An observational study of the time-dependent effects of antidiabetic treatments in patients with diabetes. Prim Care Diabetes. 2011 doi: 10.1016/j.pcd.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 74.Blin P, Lassalle R, Dureau-Pournin C, et al. Insulin glargine and risk of cancer: a cohort study in the French National Healthcare Insurance Database. Diabetologia. 2012 doi: 10.1007/s00125-011-2429-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ruiter R, Visser LE, van Herk-Sukel MP, et al. Risk of cancer in patients on insulin glargine and other insulin analogues in comparison with those on human insulin: results from a large population-based follow-up study. Diabetologia. 2012;55:51–62. doi: 10.1007/s00125-011-2312-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Radermecker RP, Scheen AJ. Continuous subcutaneous insulin infusion with short-acting insulin analogues or human regular insulin: efficacy, safety, quality of life, and cost-effectiveness. Diabetes Metab Res Rev. 2004;20:178–188. doi: 10.1002/dmrr.447. [DOI] [PubMed] [Google Scholar]
- 77.Hirsch IB. Insulin analogues. N Engl J Med. 2005;352:174–183. doi: 10.1056/NEJMra040832. [DOI] [PubMed] [Google Scholar]
- 78.Leichter S. Is the use of insulin analogues cost-effective? Adv Ther. 2008;25:285–299. doi: 10.1007/s12325-008-0043-9. [DOI] [PubMed] [Google Scholar]
- 79.Cameron CG, Bennett HA. Cost-effectiveness of insulin analogues for diabetes mellitus. CMAJ. 2009;180:400–407. doi: 10.1503/cmaj.081180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Osei K. Global epidemic of type 2 diabetes: implications for developing countries. Ethn Dis. 2003;13:S102–S106. [PubMed] [Google Scholar]
- 81.Osei K, Schuster DP, Amoah AG, Owusu SK. Pathogenesis of type 1 and type 2 diabetes mellitus in sub-Saharan Africa: implication for transitional populations. J Cardiovasc Risk. 2003;10:85–96. doi: 10.1097/01.hjr.0000060841.48106.a3. [DOI] [PubMed] [Google Scholar]
- 82.Lefebvre P, Pierson A. The global challenge of diabetes. World Hosp Health Serv. 2004;40:37–40. 42. [PubMed] [Google Scholar]
- 83.Derewenda U, Derewenda Z, Dodson GG, et al. Molecular structure of insulin: the insulin monomer and its assembly. Br Med Bull. 1989;45:4–18. doi: 10.1093/oxfordjournals.bmb.a072320. [DOI] [PubMed] [Google Scholar]