Abstract
The irreversible receptor antagonist N-ethoxycarbonyl-2-ethoxy-1,2-dihydroquinoline (EEDQ) has been used to study the ontogeny of dopamine (DA) receptor functioning in the young and adult rat. Most notably, systemic administration of EEDQ blocks the DA agonist-induced behaviors of adult rats, while leaving the behavior of preweanling rats unaffected. The purpose of the present study was to: (a) determine whether the age-dependent actions of EEDQ involve receptors located in the dorsal caudate-putamen (CPu) and (b) confirm that EEDQ's behavioral effects result from the inactivation of DA receptors rather than some other receptor type. In Experiment 1, EEDQ or DMSO were bilaterally infused into the CPu on PD 17 or PD 84. After 24 h, rats were given bilateral microinjections of the full DA agonist R(–)-propylnorapomorphine (NPA) or vehicle into the dorsal CPu and behavior was assessed for 40 min. In Experiment 2, preweanling rats were treated as just described, except that DA receptors were protected from EEDQ-induced alkylation by administering systemic injections of D1 (SCH23390) and D2 (sulpiride) receptor antagonists. As predicted, microinjecting EEDQ into the dorsal CPu attenuated the NPA-induced locomotor activity and stereotypy of adult rats. In contrast, rats given bilateral EEDQ infusions on PD 17 exhibited a potentiated locomotor response when treated with NPA. Experiment 2 showed that DA receptor inactivation was responsible for NPA's actions. A likely explanation for these results is that EEDQ inactivates a sizable percentage of DA receptors on PD 17, but leaves the remaining receptors in a supersensitive state. This receptor supersensitivity, which probably involves alterations in G protein coupling, could account for NPA-induced locomotor potentiation. Either adult rats do not show a similar EEDQ-induced change in receptor dynamics or DA receptor inactivation was more complete in older animals and effectively eliminated the expression of DA agonist-induced behaviors.
Keywords: caudate-putamen; R(−)-propylnorapomorphine (NPA); N-ethoxycarbonyl-2-ethoxy-1,2-dihydroquinoline (EEDQ); behavior; ontogeny
INTRODUCTION
There is a growing understanding that dopamine (DA) agonists and antagonists affect the developing and mature brain differently (Andersen, 2005). Support for this idea is abundant and includes data showing that dopaminergic drugs frequently induce quantitatively different behavioral effects across ontogeny (for a review, see Spear, 1979). In some cases, the potency of DA-acting drugs changes linearly with age, but often the relationship between age and drug responsiveness is nonmonotonic (Spear and Brick, 1979; Campbell and Baldessarini, 1981). For example, Lepekhina and Tsitsurina (2007) reported that apomorphine-induced stereotyped sniffing peaked at postnatal day (PD) 14 and subsequently declined, whereas stereotyped licking increased progressively until 12 months of age. DA agonists occasionally induce qualitatively different behavioral effects depending on age, however these ontogenetic differences usually involve the emergence of age-specific responses (Moody and Spear, 1992).
Most studies examining ontogenetic differences in drug responsiveness rely on systemic administration procedures, however microinjection studies have provided important information about age-dependent changes in the localization of DA-mediated brain function. In most cases, infusing nonselective and selective DA agonists into the caudate-putamen (CPu) affects preweanling and adult rats in a generally similar manner. For example, microinjecting a selective D1 agonist into the CPu increases the locomotor activity of preweanling and adult rats (Kreipke and Walker, 2004; Krolewski et al., 2005; Charntikov et al., 2011). Both age groups also show an intensification of stereotypy when a D1 or D2 agonist is infused into the CPu (Bordi and Meller, 1989; Canales and Iversen, 1998; Waszczak et al., 2002; Kreipke and Walker, 2004; Krolewski et al., 2005; Charntikov et al., 2011). Some interesting age-dependent differences are apparent, however, because selectively stimulating D2 receptors in the dorsal CPu triggers a pronounced locomotor response in preweanling rats (Charntikov et al., 2011), while causing either a reduction (Bordi and Meller, 1989; Canales and Iversen, 1998) or a subtle, biphasic increase in the locomotor activity of adult rats (Van Hartesveldt et al., 1992). Perhaps most importantly, co-activation of D1 and D2 receptors in the CPu of adult rats causes both an intensification of stereotypy and a pronounced reduction in locomotor activity (Bordi and Meller, 1989; Bordi et al., 1989; Waszczak et al., 2002); whereas, preweanling rats continue to exhibit a robust locomotor response and show only a modest intensification of stereotyped behaviors (Charntikov et al., 2008, 2011).
N-ethoxycarbonyl-2-ethoxy-1,2-dihydroquinoline (EEDQ), an alkylating agent that inactivates DA receptors, has been used to study both the time-course of receptor recovery and the behavioral relevance of D1 and D2 receptor stimulation (Hamblin and Creese, 1983; Arnt et al., 1988; Cameron and Crocker, 1988; Meller et al., 1989). When assessed 24 hr after systemic EEDQ (7.5 mg/kg, IP) administration, adult and preweanling rats show an approximately 61- 86% reduction in D1 and D2 receptor densities in the CPu, with the percent decline being somewhat greater in adult rats (Crawford et al., 1992; Gnanalingham et al., 1994). Despite a substantial decline in DA receptors, systemically administered EEDQ affects the behaviors of adult and preweanling rats differently. In adult rats, the behavior-inducing properties of DA agonists are fully attenuated when measured 24 h after EEDQ treatment (Arnt et al., 1988; Cameron and Crocker, 1988; Meller et al., 1989; McDougall et al., 1992); whereas, DA receptor inactivation leaves the behavior of preweanling rats unaffected. More specifically, preweanling rats show normal levels of NPA-, quinpirole-, and SKF38393-induced locomotor activity, grooming, and head-down sniffing when tested 24 h after systemic administration of EEDQ (McDougall et al., 1992, 1993; Mestlin and McDougall, 1993). The reason for this age-dependent difference has not been determined.
In adult rats, EEDQ has been microinjected into various brain regions in order to examine whether D1 and/or D2 receptor stimulation contributes to the mediation of muscle tone, various unlearned behaviors, ipsilateral circling, and fixed-interval operant responding (Hamblin and Creese, 1983; Bordi et al., 1989; Cameron and Crocker, 1989; Giorgi and Biggio, 1990a, b; Lee et al., 1995; Neisewander et al., 1995; Hemsley et al., 2002; Cory-Slechta et al., 2002). For example, Neisewander et al. (1995) have shown that infusing EEDQ into the lateral CPu attenuates the SKF38393-induced grooming and oral movements of adult rats. To determine the receptor type responsible for mediating a particular behavior, DA receptors can be selectively protected from EEDQ-induced inactivation by pretreating rats with D1 (e.g., SCH23390) or D2 (e.g., sulpiride or raclopride) reversible antagonists (Meller et al., 1985). Using this technique, Cameron and Crocker (1989) reported that D2 receptors in the ventrolateral CPu are necessary for apomorphine-induced head-down sniffing. Likewise, quinpirole stimulates ipsilateral circling in EEDQ-treated adult rats, but not when D2 receptors were protected by a pretreatment injection of raclopride (Giorgi and Biggio, 1990a). In sum, administering EEDQ either systemically or via intracerebral infusion produces the same general pattern of effects in adult rats; namely, nonselective and selective DA agonists have minimal behavioral impact if DA receptors are inactivated. It is not known whether intrastriatal infusions of EEDQ affect the behavior of preweanling rats in an adult-like manner.
When considered together, previous studies typically report that reversible DA agonists and antagonists either affect preweanling and adult rats in a similar manner or they induce quantitative differences in behavioral responsiveness (i.e., a particular drug causes more intense behavioral effects at one age vs. another). One goal of the present study was to determine whether microinjecting the irreversible DA receptor antagonist EEDQ into the dorsal CPu would produce qualitatively different behavioral effects in preweanling and adult rats. Qualitative differences are of particular interest because they may result from significant ontogenetic changes in the neural mechanisms underlying behavior. To examine this issue, EEDQ or vehicle were bilaterally infused into the dorsal CPu on PD 17 or PD 84 and the full DA agonist NPA (0–20 μg) was microinjected into the same structure 24 h later. To ensure that NPA (0.5 μl per side) was not stimulating DA receptors outside the area of EEDQ-induced alkylation, a relatively greater volume of EEDQ (0.75 μl per side) was infused into the dorsal CPu of both age groups. A second purpose of this study was to determine whether EEDQ's behavioral effects result from the inactivation of DA receptors or some other receptor type. To accomplish this goal, separate groups of preweanling rats were pretreated with SCH23390 and sulpiride in order to protect D1 and D2 receptors, respectively, from EEDQ-induced alkylation. Homogenate ligand binding assays were used to meaure D1 and D2 receptor concentrations in the CPu, while autoradiography was used to examine the dispersal pattern of EEDQ after microinjection.
EXPERIMENTAL PROCEDURES
Animals
A total of 273 male and female Sprague-Dawley rats were used. Adult male rats (N = 65) were purchased from Charles River (Hollister, CA, USA) and began testing on PD 83; whereas, 208 male and female rats were born and bred at California State University, San Bernardino (CSUSB) and underwent experimental procedures beginning on PD 16. Litters were culled to 10 pups on PD 3. Rat pups were kept with the dam and littermates in large polycarbonate maternity cages (56 × 34 × 22 cm) with wire lids, while adult rats were group housed in maternity cages until time of surgery. Food and water were freely available. The colony room was maintained at 22−24°C and kept under a 12-h light/dark cycle. Testing was done in a separate experimental room and was conducted during the light phase of the cycle. Subjects were cared for in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80–23) under a research protocol approved by the Institutional Animal Care and Use Committee of CSUSB.
Apparatus
Behavioral testing was done in commercially available (Coulbourn Instruments, Allentown, PA, USA) activity monitoring chambers, consisting of acrylic walls, a plastic floor, and an open top. Each chamber included an X–Y photobeam array, with 16 photocells and detectors, that was used to determine distance traveled (locomotor activity). The position of each rat was determined every 100 msec. To somewhat control for differences in body size, preweanling rats were tested in smaller sized chambers (26 × 26 × 41 cm) than adult rats (41 × 41 × 41 cm). In all other regards, the different-sized chambers were identical to each other.
Drugs
R(+)-SCH 23390 hydrochloride, (+)-butaclamol hydrochloride, and mianserin hydrochloride were dissolved in saline. (−)-Sulpiride was dissolved in a minimal amount of glacial acetic acid and diluted with saline; whereas, R(−)-propylnorapomorphine hydrochloride (NPA) was dissolved in distilled water containing 0.1% metabisulfite (an antioxidant). N-ethoxycarbonyl-2-ethoxy-1,2-dihydroquinoline (EEDQ) was dissolved in dimethyl sulfoxide (DMSO). Systemically administered drugs were injected intraperitoneally (IP) at a volume of 5 ml/kg for preweanling rats and 1 ml/kg for adult rats. EEDQ was microinjected at a volume of 0.75 μl per side, while NPA was infused at a volume of 0.5 μl per side. Nonlabeled ligands were purchased from Sigma (St. Louis, MO, USA), whereas [3H]-SCH 23390 (84.9 Ci/mmol) and [3H]-spiperone (83.9 Ci/mmol) were purchased from PerkinElmer (Boston, MA, USA).
Surgery
On PD 16 and PD 83, anesthesia was induced by isoflurane (2.5–5%) mixed with oxygen. A topical lidocaine solution (1%) was applied to the scalp and ibuprofen (2 mg/kg IP) was administered. A standard Kopf stereotaxic apparatus was used, with preweanling rats requiring a Cunningham Neonatal Rat Adapter for proper positioning. For the behavioral experiments, two craniotomies were performed and a stainless steel double guide cannula (22 gauge; Plastics One, Roanoke, VA, USA) was implanted in the dorsal CPu of preweanling rats (A/P +6.5, M/L ±2.4, D/V −5.6 mm from the interaural line; Sherwood and Timiras, 1970), whereas two single guide cannulae (22 gauge; Plastics One) were implanted in the dorsal CPu of adult rats (A/P −0.26, M/L ±3.0, D/V −5.8 mm from bregma; Paxinos and Watson, 1998). At both ages, guide cannulae were implanted 1 mm above the target location and were fixed in place using cyanoacrylate gel followed by dental cement and two stainless steel screws (adults only). Stainless steel stylets (Plastics One) were used to seal guide cannulae until time of testing. For the homogenate ligand binding experiments, two craniotomies were performed and needles attached to Hamilton microsyringes (2 μl) were bilaterally lowered into the dorsal striatum. EEDQ (100 μg) or DMSO were infused (0.75 μl per side) over a 2 min period. Needles were left in place for an additional 2 min before being retracted. After surgery, rats were allowed to recover in a temperature-controlled chamber (30°C). When fully responsive, preweanling rats were returned to the home cage with the dam and littermates, while adult rats were housed singly. The differential housing procedures were a consequence of preweanling rats needing to suckle for nourishment. Although the possibility cannot be excluded, we do not believe that the age-specific housing arrangements were responsible for ontogenetic differences in drug responsiveness.
Behavioral procedures
Experiment 1. Effects of EEDQ on the NPA-induced behaviors of adult and preweanling rats
On PD 17 or PD 84 (24 h after surgery), rats were taken to a separate room and the stainless steel stylets were replaced by infusion cannulae (Plastics One) which extended 1 mm below the guide cannulae. Hamilton microsyringes (10 μl) attached to a dual infusion pump were used to bilaterally microinject DMSO or EEDQ (100 μg) at a volume of 0.75 μl per side. Similar doses of EEDQ have been microinjected into various basal ganglia and limbic structures (Cameron and Crocker, 1989; Cory-Slechta et al., 1998, 2002; Hemsley and Crocker, 2001). Drugs were delivered at a constant rate over a 60 s period and the infusion cannulae were subsequently left in place for an additional 2 min.
On PD 18 or PD 85, preweanling (N = 48) and adult (N = 33) rats were habituated to the testing chambers for 20 min. Rats were then injected (IP) with saline and returned to the testing chambers for another 20 min habituation period [this procedure replicates the methods from previous studies (Charntikov et al., 2008, 2011)]. NPA (0 or 20 μg) was bilaterally infused into the dorsal CPu at a volume of 0.5 μl per side. After drug infusion, rats were returned to the testing chambers for 40 min. Distance traveled (a measure of horizontal locomotor activity) and repetitive motor movements (a measure of stereotypy) were assessed continuously across the 80-min session. Repetitive motor movements were defined as the total number of repetitive coordinate changes on the X–Y axes that occurred within 2 s (three back and forth movements were required before the behavior qualified as a repetitive motor movement). Rats were videotaped and various discrete behaviors (e.g., head-down sniffing and grooming) were quantified using the fixed interval momentary time sampling method described by Cameron et al. (1988). Using this technique, the presence or absence of a particular behavior was determined at 20 s intervals during each 5-min time block.
Experiment 2. Impact of dopamine receptor protection on the NPA-induced behaviors of preweanling rats
On PD 17 (24 h after surgery), rats (N = 96) were randomly divided into a nonprotected or D1/D2 protected condition. In the D1/D2 protected condition, preweanling rats were given an initial injection of sulpiride (100 mg/kg IP) followed, 30 min later, by an injection of SCH23390 (1 mg/kg IP). In the nonprotected condition, rats received two saline injections. Thirty min after the second injection, the stainless steel stylets were removed and DMSO or EEDQ (100 μg) were microinjected into the CPu at a volume of 0.75 μl per side.
On PD 18, rats were placed in the testing chambers and habituated as described in Experiment 1. After 40 min, rats were removed from the chambers and NPA (0, 10, or 20 μg) was bilaterally infused into the dorsal CPu at a volume of 0.5 μl per side. Rats were immediately returned to the testing chambers where distance traveled, repetitive motor movements, and head-down sniffing were recorded for 40 min.
D1 and D2 homogenate ligand binding assays
On PD 18 or PD 85 (48 h after surgery and 24 h after EEDQ or DMSO infusions), rats (N = 32 adults; N = 64 preweanling rats) were rapidly decapitated and CPu sections were dissected bilaterally and stored at −80°C. On the day of assay, tissue was thawed on ice and crude membrane homogenates were made using the following protocol. Tissue was homogenized in 100 volumes of 50 mM Tris-HCl buffer (pH 7.4) for approximately 20 s using a Brinkmann Polytron. Homogenates were then centrifuged at 20,000 × g for 30 min. The pellet was resuspended in 100 volumes of the same buffer and centrifuged again at 20,000 × g for 30 min. The final pellet was suspended in approximately 30 volumes of buffer (pH 7.4). Procedures for the two age groups were identical, with the exception that homogenates from PD 18 rats consisted of two pooled tissue samples. Protein concentrations for the final pellet were determined using the Bio-Rad Protein Assay.
For both the D1 and D2 receptor binding assays, tissue suspensions (50–100 μg/protein) were added to duplicate tubes containing 50 mM Tris, 2 mM NaCl2, 5 mM KCl, 1 mM MgSO4, and 2 mM CaCl2 (pH 7.4) at a final volume of 1 ml. For the D1 assay, tubes included [3H]-SCH23390 in concentrations ranging from 0.1 to 5 nM. Nonspecific binding was determined in the presence of 10 μM (+)-butaclamol. To prevent binding of [3H]-SCH23390 to serotonin receptors 100 nM mianserin was added to all tubes. For the D2 assay, tubes included [3H]-spiperone in concentrations ranging from 0.05 to 0.8 nM. Nonspecific binding was determined in the presence of 10 μM (−)-sulpiride. Due to the specificity of sulpiride, mianserin was not used in the D2 assay (Boyson et al., 1986). Incubation time for both assays was 30 min at 37°C. Incubation was terminated by vacuum filtration over glass fiber filters (Whatman GF/B, pretreated with 0.1% polyethylenimine). Filters were washed twice with ice-cold Tris-HCl buffer and radioactivity was measured by liquid scintillation spectrometry.
Histology
After behavioral testing, rats were given an overdose of sodium pentobarbital and brains were fixed in 4% paraformaldehyde. Brains were cryoprotected in a 20% sucrose solution, sectioned coronally (70 μm) using a cryostat, and then stained with thionin. Histological assessment of cannula placements was done by observers blind to drug treatment conditions. Overall, 92.9% of rats (210 out of 226) had proper cannula placements in the dorsal CPu. Data from animals with inappropriate guide cannula placements were not included in the statistical analyses. Replacement rats were added as needed, thus experiments had 8–9 subjects per group.
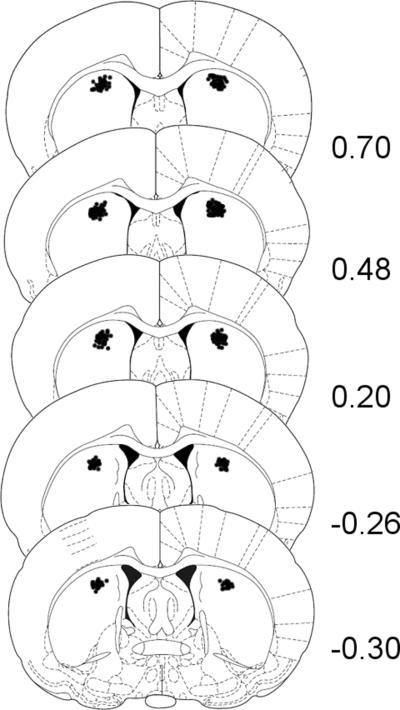
Individual preweanling and adult rats received bilateral microinjections of EEDQ or DMSO (0.75 μl per side) and D1 and D2 receptor autoradiography was used to determine dispersion. Briefly, 20 μM slices from DMSO- and EEDQ-treated rats were assayed for D1 and D2 binding sites with [3H]-SCH23390 and [3H]-spiperone, respectively, and apposed to x-ray film for 10 weeks (Crawford et al., 2011). EEDQ spread in a concentric pattern that encompassed the medial and most of the lateral portion of the dorsal CPu (Fig. 1). To determine dispersion of NPA, additional preweanling and adult rats received bilateral microinjections (0.5 μl per side) of crystal violet and brains were removed 5 or 30 min later. Examination of coronal sections indicated that dye was restricted within the dorsal CPu. Cannula placements of rats included in the statistical analyses are shown in Fig. 2.
Fig. 1.
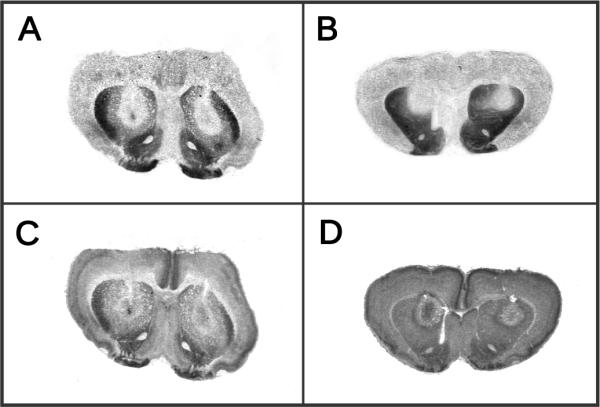
Representative autoradiograms of [3H]-SCH23390 and [3H]-spiperone binding after bilateral infusions of EEDQ into the dorsal CPu on PD 17 or PD 84. A, Adult–[3H]-SCH23390; B, Pup–[3H]-SCH23390; C, Adult–[3H]-spiperone; D, Pup–[3H]-spiperone.
Fig. 2.
Schematic representations of cannula placements in the dorsal CPu of preweanling and adult rats from Experiments 1 and 2. In all cases, numbers on the right indicate distance (mm) from Bregma using coordinates from the rat brain atlas of Paxinos and Watson (1998).
Data analysis
Repeated measures (5-min time blocks) analyses of variance (ANOVAs) were used for statistical analysis of distance traveled, repetitive motor movement, and head-down sniffing data. Because of ongoing experimental manipulations, separate ANOVAs were used to analyze time blocks 1–4 (chamber habituation), 5–8 (saline habituation), and 9–16 (drug testing). When required, significant higher order interactions were further analyzed using one-way ANOVAs, while Tukey tests (P<0.05) were used for making post hoc comparisons. When the assumption of sphericity was violated, as determined by Mauchly's test of sphericity, the Huynh-Feldt epsilon statistic was used to adjust the degrees of freedom (Huynh and Feldt, 1976). Corrected degrees of freedom were rounded to the nearest whole number and are indicated by a superscripted “a”.
Separate 2 × 2 (drug × age) ANOVAs were used to analyze D1 and D2 binding site (Bmax) and affinity (KD) data from the homogenate ligand binding assays. Bmax and KD values were determined using nonlinear regression with Prism (GraphPad Software, San Diego, CA, USA). Post hoc analysis of receptor binding data was done using Tukey tests (P<0.05).
Litter effects were minimized by assigning one subject from each litter to a particular group (for a discussion of litter effects, see Zorrilla, 1997). In situations where this procedure was not possible (i.e., during the habituation phases), a single litter mean was calculated from multiple littermates assigned to the same group (Holson and Pearce, 1992; Zorrilla, 1997). Preliminary analyses indicated that DA agonist-induced behaviors did not differ according to sex, so subsequent analyses examining NPA's effects did not include sex as a factor. Interestingly, EEDQ differentially affected the baseline locomotor activity of male and female preweanling rats, so data from the EEDQ-treated control groups were assessed using a separate set of statistical analyses.
RESULTS
Experiment 1. Effects of EEDQ on NPA-induced behaviors
Adult rats
During the habituation phases (i.e., time blocks 1–4 and 5–8), EEDQ-treated adult rats had significantly smaller distance traveled scores than DMSO controls (Fig. 3, upper graph, left panel) [Pretreatment main effects, F(1, 31)=21.02, P<0.001; F(1, 31)=19.90, P<0.001]. Distance traveled scores declined across the second habituation phase and achieved a stable level of performance by time block 7 [aTime block main effect, F(3, 93)=38.68, P<0.001 and Tukey tests].
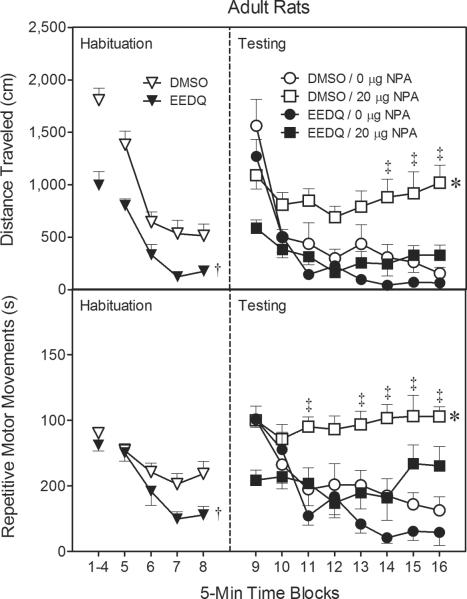
Fig. 3.
Mean distance traveled (±SEM) and repetitive motor movements (±SEM) during the 80-min behavioral testing session on PD 85 (n = 8–9 per group). At the conclusion of time block 8 (indicated by the second dashed line), EEDQ- and DMSO-treated adult rats received bilateral infusions of NPA (0 or 20 μg).
† Significantly different from DMSO-treated rats (collapsed across time blocks 5–8).
* Significantly different from all other groups (collapsed across time blocks 9–16).
‡ Significantly different from DMSO-treated rats infused with 0 μg NPA.
During the testing phase (i.e., time blocks 9–16), NPA significantly enhanced distance traveled scores [Agonist main effect, F(1, 29)=7.35, P<0.05], but only in adult rats pretreated with DMSO (Fig. 3, upper graph, right panel) [Pretreatment × Agonist interaction, F(1, 29)=5.73, P<0.05 and Tukey tests]. In other words, EEDQ-treated adults infused with 20 μg NPA performed no differently than rats given 0 μg NPA on the test day. Curiously, NPA reduced distance traveled scores on time block 10, but the DA agonist produced a subsequent increase in locomotion that was apparent on time blocks 14–16 [aAgonist × Time Block interaction, F(5, 152)=10.91, P<0.001 and Tukey tests].
During the initial component of the habituation phase (i.e., time blocks 1–4), the repetitive motor movements of the EEDQ- and DMSO-treated rats did not differ (Fig. 3, lower graph, left panel); however, rats given EEDQ exhibited a significant reduction in repetitive motor movements, relative to control rats, on time blocks 5–8 [Pretreatment main effect, F(1, 31)=8.74, P<0.05]. Repetitive motor movements declined across both components of the habituation phase until stabilizing on time block 7 [Time Block main effects, F(3, 93)=9.72, P<0.001; F(3, 93)=11.45, P<0.001 and Tukey tests].
During the testing phase, DMSO-treated rats given bilateral infusions of NPA (20 μg) had significantly more repetitive motor movements than all other groups (Fig. 3, lower graph, right panel) [Pretreatment × Agonist interaction, F(1, 29)=5.44, P<0.05 and Tukey tests]. EEDQ fully attenuated NPA-induced repetitive motor movements, because EEDQ-treated rats given NPA did not differ from vehicle controls. Overall, NPA-treated rats exhibited more repetitive motor movements than controls on time blocks 11 and 13–16 [Agonist × Time Block interaction, F(7, 203)=8.61, P<0.001 and Tukey tests].
Head-down sniffing (i.e., stereotyped sniffing) was infrequently observed during the habituation phases (< 1 occurrence per 5 min time block). Bilaterally infusing NPA (20 μg) into the dorsal CPu significantly increased head-down sniffing relative to vehicle controls (Table 1) [Agonist main effect, F(1, 29)=121.20, P<0.001], while EEDQ pretreatment reduced head-down sniffing [Pretreatment main effect, F(1, 29)=22.40, P<0.001]. NPA-induced head-down sniffing was partially blocked by EEDQ [Pretreatment × Agonist interaction, F(1, 29)=19.24, P<0.001]. More specifically, EEDQ-treated rats infused with NPA had fewer head-down sniffing counts than DMSO-treated rats infused with NPA, but more sniffing counts than the vehicle control group [Tukey tests].
Table 1.
Mean (±SEM) head-down sniffing counts of DMSO- and EEDQ-treated adult rats (n = 8–9 per group) on time blocks 9–16 (Experiment 1)
| Treatment Condition | Head-Down Sniffing |
|---|---|
| DMSO / 0 μg NPA | 0.64 (±0.19) |
| DMSO / 20 μg NPA | 10.38 (±0.86)*† |
| EEDQ / 0 μg NPA | 0.42 (±0.07) |
| EEDQ / 20 μg NPA | 4.61 (±0.84)* |
Head-down sniffing values represent mean scores per 5-min time block during the final 40-min testing phase.
Significantly different from the DMSO / 0 μg NPA vehicle group;
Significantly different from EEDQ-treated rats infused with 20 μg NPA (i.e., the EEDQ / 20 μg NPA group)
Preweanling rats
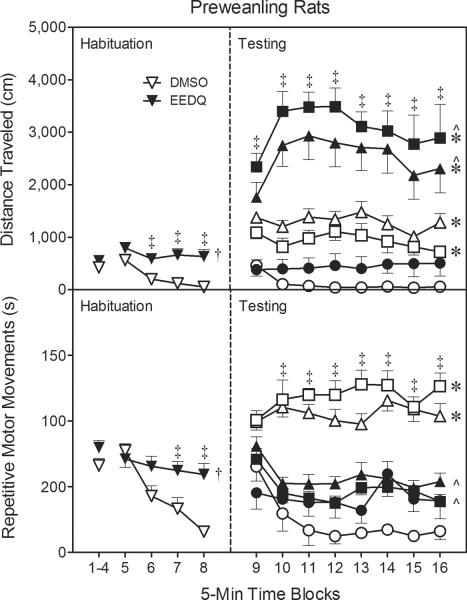
During the habituation phase, EEDQ-treated preweanling rats had greater distance traveled scores than DMSO controls (Fig. 4, upper graph, left panel) [Pretreatment main effect, F(1, 46)=15.41, P<0.001], with the differences between groups being statistically significant on time blocks 6–8 [aAgonist × Time Block interaction, F(2, 90)=5.36, P<0.001 and Tukey tests]. During the testing phase, EEDQ-treated preweanling rats infused with 10 or 20 μg NPA had significantly greater distance traveled scores than all other groups, including DMSO-treated rats injected with the identical dose of NPA (Fig. 4, upper graph, right panel) [Pretreatment × Agonist interaction, F(2 ,42)=6.69, P<0.01 and Tukey tests]. Thus, EEDQ potentiated NPA's locomotor activating effects in preweanling rats. DMSO- and EEDQ-treated rats infused with 0 μg NPA did not differ. Additional analyses showed that among the DMSO groups, preweanling rats infused with 10 or 20 μg NPA had greater distance traveled scores than rats given 0 μg NPA [Dose main effect, F(2, 21)=28.45, P<0.001 and Tukey tests]. The Pretreatment × Agonist × Time Block interaction was not statistically significant, but EEDQ-treated preweanling rats exhibited significantly more locomotor activity than DMSO controls on time blocks 9–16 [aPretreatment × Time Block interaction, F(4, 187)=8.52, P<0.001 and Tukey tests]. Separate statistical analyses showed that NPA-induced locomotor activity did not vary according to sex (data not shown), although EEDQ infusions did produce significantly greater distance traveled scores in male rats (M = 917 cm per time block, SEM = 192) than female rats (M = 179 cm per time block, SEM = 37) when collapsed across the testing session [Sex main effect, F(1, 12)=11.42, P<0.01; Sex × Pretreatment interaction, F(1, 12)=14.31, P<0.01]. The distance traveled scores of DMSO-treated male (M = 196 cm per time block, SEM = 42) and female (M = 238 cm per time block, SEM = 51) rats did not differ.
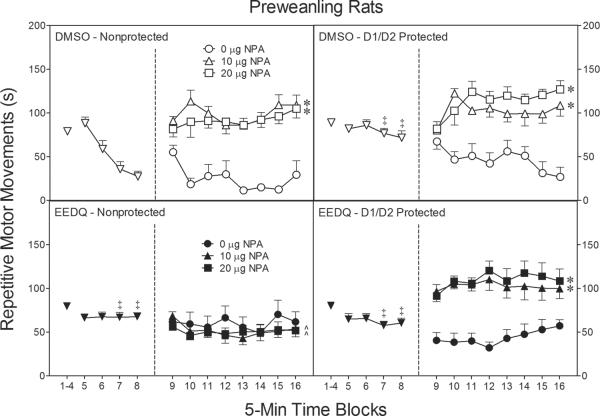
Fig. 4.
Mean distance traveled (±SEM) and repetitive motor movements (±SEM) during the 80-min behavioral testing session on PD 18 (n = 8 per group). At the conclusion of time block 8 (indicated by the second dashed line), EEDQ- and DMSO-treated preweanling rats received bilateral infusions of NPA (0, 10, or 20 μg). (◯) DMSO / 0 μg NPA; (▵) DMSO / 10 μg NPA; (◻) DMSO / 20 μg NPA; (●) EEDQ / 0 μg NPA; (▴) EEDQ / 10 μg NPA; (∎) EEDQ / 20 μg NPA.
† Significantly different from DMSO-treated rats (collapsed across time blocks 5–8).
‡ Significantly different from DMSO-treated rats.
* Significantly different from DMSO- or EEDQ-treated rats infused with 0 μg NPA (collapsed across time blocks 9–16).
^ Significantly different from DMSO-treated rats infused with the identical dose of NPA (collapsed across time blocks 9–16).
Repetitive motor movements of the DMSO- and EEDQ-treated preweanling rats did not vary during the first four time blocks of the habituation phase (Fig. 4, lower graph, left panel). During the second component of the habituation phase (i.e., time blocks 5–8), EEDQ-treated rats had significantly more repetitive motor movements than DMSO controls [Pretreatment main effect, F(1, 46)=9.24, P<0.01], with the differences between groups being apparent on time blocks 7–8 [Pretreatment × Time Block interaction, F(3, 138)=7.42, P<0.001 and Tukey tests]. Infusing NPA into the dorsal CPu did not affect the repetitive motor movements of EEDQ-treated preweanling rats (Fig. 4, lower graph, right panel); whereas, NPA (10 or 20 μg) significantly increased the repetitive motor movements of rats pretreated with DMSO [Pretreatment × Agonist interaction, F(2, 42)=33.38, P<0.001 and Tukey tests]. More specifically, DMSO-treated rats given bilateral infusions of 10 or 20 μg NPA had significantly more repetitive motor movements than all other groups on time blocks 10–16 [aPretreatment × Agonist × Time Block interaction, F(11, 225)=3.86, P<0.001 and Tukey tests]. The various groups of rats infused with 0 μg NPA did not differ. The repetitive motor movements of male and female preweanling rats were not differentially affected by EEDQ (P>0.05).
Experiment 2. Impact of dopamine receptor protection on the NPA-induced behaviors of preweanling rats
Distance Traveled
During both components of the habituation phase, distance traveled scores were elevated if rats had been treated with EEDQ or SCH23390/sulpiride, either alone or in combination, on PD 17 (compare the left panels of Fig. 5) [Pretreatment × Condition interactions, F(1, 92)=5.51, P<0.05; F(1, 92)=8.95, P<0.01 and Tukey tests]. Thus, reversibly or irreversibly antagonizing D1 and D2 receptors increased basal distance traveled scores when assessed 24 h later. The Pretreatment × Condition × Time Block interaction was nonsignificant, but EEDQ-treated rats had significantly greater distance traveled scores than DMSO controls on time blocks 6–8 [aPretreatment × Time Block interaction, F(2, 178)=20.98, P<0.001 and Tukey tests].
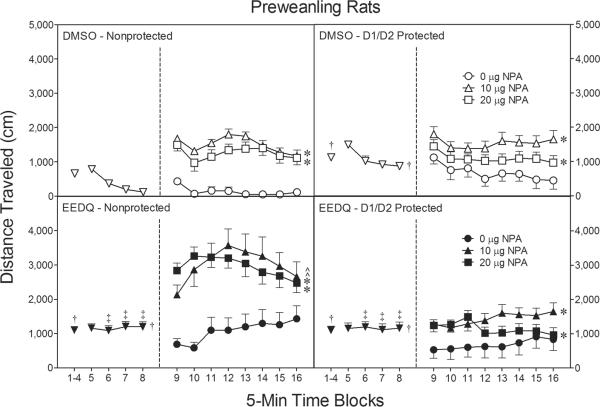
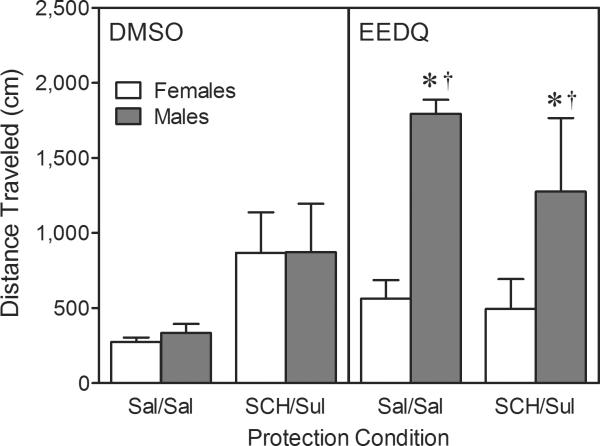
Fig. 5.
Mean distance traveled (±SEM) during the 80-min behavioral testing session on PD 18 (n = 8 per group). Rats were given IP injections of SCH23390/sulpiride (D1/D2 protected) or saline/saline (nonprotected) prior to EEDQ or DMSO infusions. At the conclusion of time block 8 (indicated by the second dashed line), EEDQ- and DMSO-treated preweanling rats received bilateral infusions of NPA (0, 10, or 20 μg).
† Significantly different from rats in the DMSO-nonprotected group (collapsed across time blocks).
‡ Significantly different from DMSO-treated rats.
* Significantly different from rats in the same treatment conditions infused with 0 μg NPA (collapsed across time blocks 9–16).
^ Significantly different from rats in the other treatment conditions infused with 10 or 20 μg NPA (collapsed across time blocks 9–16).
When collapsed across time blocks 9–16 (i.e., the testing phase), EEDQ-treated rats continued to have greater distance traveled scores than DMSO controls (compare the right panels of Fig. 5) [Pretreatment main effect, F(1, 84)=28.68, P<0.001]. The locomotor enhancing effects of EEDQ were only apparent if D1 and D2 receptors were left unprotected (i.e., if DA receptors were inactivated). Specifically, EEDQ-treated rats in the nonprotected condition had significantly greater distance traveled scores than the various DMSO groups as well as rats given SCH23390/sulpiride protection prior to EEDQ infusions [Pretreatment × Condition interaction, F(1, 84)=25.40, P<0.001 and Tukey tests].
Overall, microinjecting NPA (10 or 20 μg) into the dorsal CPu increased distance traveled scores [Agonist main effect, F(2, 84)=32.09, P<0.001 and Tukey tests], with this effect varying according to treatment condition [Agonist × Condition interaction, F(2, 84)=5.07, P<0.01]. Separate statistical analyses showed that NPA (10 or 20 μg) induced significantly more locomotor activity in EEDQ-treated rats from the nonprotected condition (Fig. 5, lower left graph) when compared to either DMSO controls (Fig. 5, upper graphs) or EEDQ-treated rats given SCH23390/sulpiride protection (Fig. 5, lower right graph) [Pretreatment × Condition interaction, F(6, 84)=2.32, P<0.05 and Tukey tests]. The various groups of rats infused with 0 μg NPA exhibited similar amounts of locomotor activity. Most of the interactions involving time block were nonsignificant; however, the pretreatment variable (i.e., EEDQ vs DMSO) did interact with time block to affect distance traveled scores [aPretreatment × Time Block interaction, F(3, 288)=8.51, P<0.001]. In summary, NPA (10 or 20 μg) caused a potentiated locomotor response on PD 18, but only if EEDQ inactivated D1 and D2 receptors.
Similar to what occurred in Experiment 1, EEDQ infusions produced significantly more locomotor activity in male rats, as compared to females, when assessed across the 80-min testing session (Fig. 6, right panel) [Sex main effect, F(1, 24)=8.97, P<0.01; Sex × Pretreatment interaction, F(1, 24)=7.81, P<0.01]. The latter effect was apparent regardless of protection condition.
Fig. 6.
Mean distance traveled (±SEM) per 5-min time block during the behavioral testing session on PD 18 (these are a subset of the rats shown in Fig. 5). The male and female rats (n = 4 per group) included on this figure were injected with 0 μg NPA on the test day. On PD 17, rats were given IP injections of SCH23390/sulpiride (D1/D2 protected) or saline/saline (nonprotected) prior to EEDQ or DMSO infusions.
† Significantly different from EEDQ-treated female rats.
* Significantly different from DMSO-treated male or female rats.
Repetitive Motor Movements
On time blocks 1–4 of the habituation phase, repetitive motor movements of the various groups did not differ (left panels, Fig. 7); however, on time blocks 5–8 the repetitive motor movements of control rats (i.e., the DMSO-nonprotected group) declined across the habituation phase, whereas rats treated with EEDQ or SCH23390/sulpiride maintained a stable level of performance across time blocks 5–8 [Pretreatment × Condition interaction, F(1, 92)=12.55, P<0.001; Pretreatment × Condition × Time Block, F(3, 257)=6.46, P<0.001]. The end result, is that the DMSO-nonprotected group exhibited significantly fewer repetitive motor movements than all other groups on time blocks 7 and 8 [Tukey tests].
Fig. 7.
Mean repetitive motor movements (±SEM) during the 80-min behavioral testing session on PD 18 (n = 8 per group). Rats were given IP injections of SCH23390/sulpiride (D1/D2 protected) or saline/saline (nonprotected) prior to EEDQ or DMSO infusions. At the conclusion of time block 8 (indicated by the second dashed line), EEDQ- and DMSO-treated preweanling rats received bilateral infusions of NPA (0, 10, or 20 μg).
‡ Significantly different from rats in the DMSO-nonprotected group.
* Significantly different from rats in the same treatment conditions infused with 0 μg NPA (collapsed across time blocks 9–16).
^ Significantly different from rats in the other treatment conditions infused with 10 or 20 μg NPA (collapsed across time blocks 9–16).
During the testing phase, EEDQ did not affect repetitive motor movements in the same manner as distance traveled scores (see right panels, Fig. 7). Specifically, EEDQ significantly reduced, rather than potentiated, the repetitive motor movements of preweanling rats [Pretreatment main effect, F(1, 84)=5.49, P<0.05]. D1/D2 receptor inactivation was responsible for this effect [Condition main effect, F(1, 84)=33.26, P<0.001], because the EEDQ-induced reduction in repetitive motor movements was only apparent in the nonprotected condition (Fig. 7, lower left graph) [Pretreatment × Condition interaction, F(3, 96)=5.09, P<0.01 and Tukey tests]. Interestingly, basal levels of repetitive motor movements were not affected by EEDQ (i.e., rats infused with 0 μg NPA did not show depressed levels of repetitive motor movements relative to controls). Instead, EEDQ fully attenuated the repetitive motor movements of rats infused with 10 or 20 μg NPA, but only when D1 and D2 receptors were left unprotected [Pretreatment × Condition × Agonist interaction, F(2, 84)=11.17, P<0.001 and Tukey tests]. In other words, rats given SCH23390 and sulpiride prior to EEDQ infusions showed a normal pattern of responsiveness after NPA treatment (i.e., if DA receptors were intact), whereas NPA did not increase the repetitive motor movements of EEDQ-treated rats in the nonprotected condition (i.e., if DA receptors were inactivated). The four-way interaction involving time block (i.e., the Pretreatment × Condition × Agonist × Time Block interaction) was nonsignificant, although NPA did induce a slight increase in locomotor activity across the testing session [aAgonist × Time Block interaction, F(9, 397)=2.38, P<0.05 and Tukey tests], but only in DMSO-treated rats [aPretreatment × Agonist × Time Block interaction, F(9, 397)=2.85, P<0.01 and Tukey tests]. Separate analyses showed that EEDQ-treated male rats exhibited more repetitive motor movements than EEDQ-treated female rats, but only when rats were infused with 0 or 20 μg NPA on the test day [Pretreatment × Agonist × Sex interaction, F(2, 72)=3.80, P<0.05 and Tukey tests].
Head-Down Sniffing
Head-down sniffing was infrequently observed during the habituation phases (< 1 occurrence per 5 min time block). During the testing phase, the head-down sniffing and repetitive motor movement data produced a generally similar pattern of results (Table 2). Overall, NPA caused a robust dose-dependent increase in head-down sniffing [Agonist main effect, F(2, 84)=383.18, P<0.001 and Tukey tests] that was particularly evident in DMSO-treated rats and EEDQ-treated rats given SCH23390/sulpiride protection [Pretreatment × Condition × Agonist interaction, F(2, 84)=21.86, P<0.001]. Preweanling rats in the EEDQ-nonprotected condition exhibited significantly fewer NPA (10 and 20 ug) induced sniffing counts than rats from the other treatment conditions [Pretreatment × Condition × Agonist interaction, F(2, 84)=21.86, P<0.001 and Tukey tests]. Thus, DA receptor inactivation significantly reduced both the NPA-induced head-down sniffing and repetitive motor movements of preweanling rats. Rats infused with 0 ug NPA had few sniffing counts and were not affected by EEDQ or SCH23390/sulpiride protection. Head-down sniffing did not vary according to sex.
Table 2.
Mean (±SEM) head-down sniffing counts of DMSO- and EEDQ-treated preweanling rats (n = 8 per group) on time blocks 9–16 (Experiment 2)
| DMSO Condition | |||
|---|---|---|---|
| Nonprotected | SCH/Sul Protection | ||
| 0 μg NPA | 0.39 (±0.20) | 0 μg NPA | 0.72 (±0.31) |
| 10 μg NPA | 9.17 (±0.55)*‡ | 10 μg NPA | 11.03 (±0.64)*‡ |
| 20 μg NPA | 11.76 (±0.66)*†‡ | 20 μg NPA | 13.45 (±0.46)*†‡ |
| EEDQ Condition | |||
|---|---|---|---|
| Nonprotected | SCH/Sul Protection | ||
| 0 μg NPA | 0.95 (±0.39) | 0 μg NPA | 1.03 (±0.46) |
| 10 μg NPA | 4.17 (±0.59)* | 10 μg NPA | 11.05 (±0.41)*‡ |
| 20 μg NPA | 2.17 (±0.88) | 20 μg NPA | 13.33 (±0.28)*†‡ |
Head-down sniffing values represent mean scores per 5-min time block during the final 40-min testing phase.
Significantly different from the 0 μg NPA group in the same treatment condition;
Significantly different from the 10 μg NPA group in the same treatment condition;
Significantly different from rats in the EEDQ-nonprotected condition receiving the identical dose of NPA.
Experiments 1 and 2. Effects of EEDQ on body weight
Overall, preweanling rats weighed significantly less than adult rats (Table 3) [Age main effect, F(1, 173)=19517.32, P<0.001], with separate analyses showing that the body weights of preweanling rats did not differ according to sex and were not affected by SCH23390/sulpiride pretreatment (data not shown). Although the Pretreatment × Age interaction was not statistically significant (P>0.05), it was evident that infusing EEDQ into the dorsal CPu caused a significant reduction in body weight when measured 24 h later (i.e., immediately prior to behavioral testing) [Pretreatment main effect, F(1, 173)=4.90, P<0.05]. The behavioral ramifications of these EEDQ-induced reductions in body weight are uncertain; however, they probably did not influence the overall pattern of results, since EEDQ decreased the body weights of both age groups, yet differentially affected the locomotor activity of young and adult rats.
Table 3.
Mean (+SEM) body weights (g) measured 24 h after infusing DMSO or EEDQ into the dorsal CPu of preweanling (n = 72 per group) and adult rats (n = 16–17 per group).
| Postnatal Day (PD) | Pretreatment Group |
|
|---|---|---|
| DMSO | EEDQ | |
| PD 18 | 44.7 g (+0.65) | 39.98 g (+0.62)* |
| PD 85 | 401.0 g (+6.81) | 394.1 g (+7.42)* |
Significantly different from DMSO-treated rats of the same age (P < 0.05).
Effects of EEDQ on D1 and D2 binding sites
PD 85 rats had significantly more D1 and D2 binding sites (Bmax) in the CPu than PD 18 rats (Table 4) [Age main effects, F(1, 19)=5.82, P<0.05; F(1, 20)=5.06, P<0.05, respectively]. Infusing EEDQ into the CPu caused a significant reduction in both D1 and D2 binding sites [Pretreatment main effects, F(1, 19)=70.17, P<0.001; F(1, 20)=9.78, P<0.01, respectively], with EEDQ having a relatively greater impact on D1 receptors. Although a nonsignificant trend was apparent, the percentage of D1 (PD 18, 22%; PD 85, 29%) and D2 (PD 18, 46%; PD 85, 65%) receptors remaining after EEDQ infusions did not differ according to age (P>0.05). The affinity (KD) of D1 binding sites was marginally elevated after EEDQ treatment (Table 4) [Pretreatment main effect, F(1, 19)=4.12, P=.057]. In terms of D2 receptors, EEDQ did not affect the KD values of homogenates from preweanling rats; however, EEDQ did significantly increase the affinity of D2 binding sites in adult rats [Age × Pretreatment interaction, F(1, 20)=5.88, P<0.05 and Tukey tests]. The D1 and D2 binding of preweanling rats did not differ according to sex (data not shown), although the small number of male (n = 3) and female (n = 3) rats per treatment group may have precluded the ability to detect sex differences.
Table 4.
Effects of DMSO and EEDQ infusions on D1 and D2 receptor density (Bmax) and affinity (KD) in the CPu of preweanling and adult rats (n = 6 per group)
| D1 Receptor Binding |
|||||
|---|---|---|---|---|---|
| Age |
B
max
|
K
D
|
|||
| DMSO | EEDQ | % DMSO | DMSO | EEDQ | |
| PD 18 | 3,631 (±484)† | 802 (±111)*† | 22% | 0.699 (±0.98) | 0.981 (±0.26) |
| PD 85 | 4,772 (±459) | 1,413 (±235)* | 29% | 0.684 (±0.06) | 1.069 (±0.16) |
| D2 Receptor Binding |
|||||
|---|---|---|---|---|---|
| Age |
B
max
|
K
D
|
|||
| DMSO | EEDQ | % DMSO | DMSO | EEDQ | |
| PD 18 | 606 (±84)† | 276 (±62)*† | 46% | 0.140 (±0.01) | 0.124 (±0.03) |
| PD 85 | 801 (±148) | 520 (±73)* | 65% | 0.114 (±0.02) | 0.289 (±0.07)‡ |
Significantly different from DMSO-treated rats of the same age;
Significantly different from PD 85 rats;
Significantly different from all other groups.
DISCUSSION
In adult rats, administering the full DA agonist NPA into the dorsal CPu caused a substantial increase in both stereotypy and locomotor activity. The latter result was somewhat surprising because nonselective DA agonists often cause intense stereotypies and a concomitant reduction in locomotion (Bordi and Meller, 1989; Bordi et al., 1989; Waszczak et al., 2002). The CPu is not a homogeneous structure (Kelley et al., 1982; Gerfen, 1992), thus it is appears that selectively stimulating DA receptors in the dorsal portion of the CPu produces both locomotor activity and moderately intense stereotypy in adult rats (see also Dickson et al., 1994; Carrera et al., 1998; Dias et al., 2006). Infusing EEDQ into the dorsal CPu of adult rats caused a significant reduction in D1 and D2 receptors, while decreasing basal and NPA-induced locomotor activity, repetitive motor movements, and head-down sniffing. Therefore, inactivating a substantial complement of DA receptors in adult rats was able to attenuate the behavior-inducing properties of a full DA agonist. Similar results have been reported many times before (Arnt et al., 1988; Double and Crocker, 1990; Giorgi and Biggio, 1990a, 1990b; Neisewander et al., 1995; Hemsley and Crocker, 2001), with researchers often attributing the absence of agonist-induced behavioral effects to an insufficient number of functional DA receptors. In a few cases, the DA-mediated behaviors of adult rats (e.g., repetitive jaw movements) were unaffected by EEDQ pretreatment, presumably because the underlying neuroanatomical structure had a sufficient receptor reserve to compensate for the loss of DA receptors (Rosengarten et al., 1989, 1993; see also Arnt et al., 1988).
Microinjecting NPA into the dorsal CPu of preweanling rats also increased locomotor activity, repetitive motor movements, and head-down sniffing on PD 18 (see also Charntikov et al., 2008, 2011). Similar to what was observed in adult rats, DA receptor inactivation almost completely eliminated the stereotyped responding of NPA-treated preweanling rats. More specifically, infusing EEDQ into the CPu fully attenuated NPA-induced repetitive motor movements on PD 18 and partially attenuated NPA-induced head-down sniffing. In striking contrast, EEDQ caused a significant potentiation of NPA's locomotor activating effects on PD 18. Therefore, inactivating DA receptors in the CPu affected the locomotor activity of preweanling and adult rats in a qualitatively different manner: EEDQ blocked NPA-induced locomotor activity in adult rats, while potentiating locomotion in preweanling rats. EEDQ alkylates a variety of different receptor types, including muscarinic M1 and M2, α2-adrenergic, GABAA, 5-HT1A and 5-HT2A receptors (Norman et al., 1989; Miller et al., 1991; Sallés et al., 1993; Ribas et al., 1998; Kettle et al., 1999; Vinod et al., 2001); however, the protection experiment clearly showed that EEDQ's ability to enhance NPA-induced locomotor activity was due to the inactivation of DA receptors. More specifically, behavioral potentiation was not evident if D1 and D2 receptors were protected by SCH23390/sulpiride pretreatment. Only when D1 and D2 receptors were inactivated by EEDQ did NPA produce a potentiated locomotor response.
Three central questions arise when considering these results: (1) Why did EEDQ differentially affect the locomotor activity and stereotyped behaviors of preweanling rats; (2) why did EEDQ potentiate the locomotor activity of preweanling rats; and (3) why did preweanling and adult rats exhibit different patterns of NPA-induced locomotor activity after EEDQ treatment? One of the most interesting features of these data is that EEDQ increased the NPA-induced locomotor activity of preweanling rats, while attenuating NPA-induced stereotypy. A possible explanation is that locomotor activity and stereotypy may rely on identical populations of D1/D2 receptors, but the relative amount of receptor stimulation determines which class of behavior will be expressed (Bordi et al., 1989). According to this view, stimulating progressively more receptors would cause behavioral expression to move through a continuum from nonstereotyped behaviors (e.g., locomotor activity) to low, then moderate, and finally intense stereotypies (Bordi et al., 1989). In terms of the preweanling rat, EEDQ may have inactivated enough receptors in the dorsal CPu so that NPA produced an attenuated stereotypic response; however, a sufficient number of receptors remained to produce a largely unimpeded locomotor response. In other words, a potentiated locomotor response may have been evident because the behavioral competition from stereotypic responding was reduced relative to the control (normosensitive) condition.
Another explanation for the dichotomy between NPA-induced locomotor activity and stereotypy is that these two classes of behavior may be mediated by distinct subpopulations of receptors within the dorsal CPu. If true, EEDQ may inactivate receptor subpopulations at different rates (Arnt et al., 1988; Rosengarten et al., 1993) or the receptors mediating locomotor activity (as compared to stereotypy) may have a larger receptor reserve. In terms of the latter suggestion, the size of individual receptor reserves differs not only according to receptor type (e.g., D1 vs. D2; Arnt et al., 1988), but also according to the response being measured (Meller et al., 1988; Yokoo et al., 1988; see also Meller et al., 1989). Thus, there is ancillary evidence suggesting that locomotor activity and head-down sniffing may be mediated by distinct populations of DA receptors, and these receptor subpopulations are differentially affected by EEDQ.
Both of these explanations leave unclear why DA receptor inactivation caused a potentiated locomotor response in preweanling rats. One possibility is that a sufficient number of functional receptors remained after EEDQ treatment to mediate locomotor activity and these receptors were supersensitive. A related idea is that DA receptors repopulate at a faster rate in EEDQ-treated preweanling rats than adults (Leff et al., 1984; Kula et al., 1992) and these newly synthesized receptors may be supersensitive. In either case, it is likely that a transient receptor supersensitivity is responsible for the potentiated response to NPA. In a somewhat analogous situation, reserpine and 6-OHDA treatment dramatically reduce dopaminergic tone in preweanling and adult rats, thereby causing both receptor supersensitivity and a potentiated behavioral response to DA-acting drugs (Arnt and Hyttel, 1984; Jaber et al., 1992; Farley et al., 2006; Harrison and LaHoste, 2006). In fact, manipulations that produce DA supersensitivity are almost always associated with increased levels of the D2High receptor (Seeman et al., 2005). All of this being said, there is little pre-existing evidence to suggest that EEDQ supersensitizes DA receptors. In one study, treating adult rats with very low doses of EEDQ (0.8 mg/kg) caused D1 receptor supersensitivity in the prefrontal cortex, but this effect became undetectable when higher doses of EEDQ were administered (Trovero et al., 1992). EEDQ has also been reported to potentiate the NPA-induced head-down sniffing of adult rats, but Meller et al. (1989) concluded that this effect was due to the lack of behaviors competing with the sniffing response rather than a “rebound supersensitivity.” Finally, EEDQ significantly reduces DA levels in the CPu (Crawford et al., 1992, 1994), thus receptor inactivation produces a state often associated with the induction of receptor supersensitivity.
Data from the habituation phase are relevant to the issue of supersensitivity, because both EEDQ infusions and systemic SCH23390/sulpiride injections enhanced the basal locomotor activity and repetitive motor movements of preweanling rats, but not adults. Curiously, the locomotor enhancing effects of EEDQ, but not SCH23390/sulpiride, were more prominent in male rats than female rats. Regardless, the most obvious explanation is that both of these pharmacological manipulations caused a receptor supersensitivity that was expressed as an increase in the baseline levels of these behaviors. Age-dependent differences in the absolute amount of receptor inactivation (Crawford et al., 1992, 1994) or receptor plasticity (e.g., the rate of receptor synthesis; Leff et al., 1984; Kula et al., 1992) may explain why only EEDQ-treated preweanling rats showed an increase in behavioral responsiveness during the habituation phase. Although less likely, it is possible that the irreversible and reversible DA antagonists caused a failure to habituate. Whatever the precise reason, EEDQ increased the basal motoric responding of preweanling rats and, when combined with later NPA administration, was responsible for a potentiated locomotor response.
Lastly, it is difficult to explain why infusing EEDQ into the dorsal CPu blocked the NPA-induced locomotor activity of adult rats, while potentiating the locomotion of preweanling rats. When administered systemically, EEDQ (7.5 mg/kg) reduced D1 and D2 receptors in the CPu of preweanling rats by 69% and 61%; whereas, the D1 and D2 receptors of adult rats were reduced by 86% and 80% (Crawford et al., 1992, 1994). Thus, it is possible that preweanling rats exhibit a full locomotor response because, unlike adult rats, they have a sufficient number of functional DA receptors remaining after EEDQ treatment. On first analysis, the homogenate binding data from the present study are not consistent with this interpretation, since EEDQ infusions left fewer functional D1 and D2 binding sites in preweanling rats than adults (Table 3). These data may be somewhat misleading, however, because the same volume of EEDQ was microinjected into both age groups. Diffusion characteristics and the physical size of neuroanatomical structures varies across ontogeny, so by keeping injection volume constant it is almost certain that EEDQ affected a larger proportion of the CPu in the younger animal. In other words, it is likely that adult rats had more functional DA receptors than preweanling rats because EEDQ infusions left more of the CPu intact in older animals. To ensure that the DA agonist did not diffuse outside of EEDQ-affected regions, EEDQ (0.75 μl) was administered at a relatively greater volume than NPA (0.5 μl). By infusing a large volume of EEDQ it is possible, especially in preweanling rats, that brain structures adjacent to the CPu were affected by the irreversible DA antagonist. D1 and D2 receptor autoradiography showed that EEDQ did not diffuse to ventral regions of the CPu or the nucleus accumbens (Fig. 1), but this methodology cannot exclude the possibility that EEDQ affected nondopaminergic cortical structures overlying the CPu. Thus, it is not known whether cortical damage might have contributed to the age-dependent behavioral differences observed in the present study.
In adult rats, DA agonists often have pronounced sex-dependent effects, with female rats evidencing more locomotor activity than male rats (Schindler and Carmona, 2002; Festa et al., 2004; Milesi-Hallé et al., 2007). In contrast, prepubescent rats do not typically exhibit sex differences after treatment with DA agonist compounds (e.g., Bowman et al., 1997; Snyder et al., 1998; McDougall et al., 2007). Consistent with this general result, we found that the NPA-induced locomotor activity of preweanling rats, as well as repetitive motor movements, stereotyped sniffing, and Bmax values, did not vary according to sex on PD 18. Some sex differences were apparent, however, since EEDQ enhanced the baseline locomotor activity of male preweanling rats to a greater extent than female rats. This EEDQ-induced effect was not mediated by DA receptors because it was evident in both the nonprotection and protection conditions. For this reason, it is uncertain why EEDQ's locomotor enhancing effects varied according to sex. Importantly, these experiments were not designed to assess sex differences (n = 3–4 of each sex per group), thus these particular results need to be confirmed by studies employing more male and female rats.
In conclusion, when given systemically or intracerebrally DA-acting drugs often affect preweanling and adult rats in a quantitatively different manner (Moody and Spear, 1982; Charntikov et al., 2011). In the present study, we report that EEDQ caused qualitative behavioral differences in preweanling and adult rats. Specifically, infusing EEDQ into the dorsal CPu blocked the NPA-induced locomotor activity of adult rats, while causing a potentiated locomotor response in preweanling rats. The neuropharmacological bases for this ontogenetic difference have not been determined, but they may involve age-dependent changes in the effectiveness of EEDQ alkylation. In addition, much of the behavioral data from the preweanling rat are consistent with the hypothesis that DA receptors remaining after EEDQ treatment are supersensitive.
Research Highlights
-
>
Age-dependent behavioral effects of depleting DA receptors in the CPu were assessed.
-
>
DA receptor depletion paradoxically increased the locomotor activity of young rats.
-
>
Receptor inactivation may cause prolonged receptor supersensitivity in young rats.
Acknowledgements
This research was supported by NIGMS research grant GM073842 (SAM) and NIDA training grant DA025319 (TD and FAV).
Abbreviations
- ANOVA
Analysis of variance
- CPu
caudate-putamen
- DA
dopamine
- DMSO
dimethyl sulfoxide
- EEDQ
N-ethoxycarbonyl-2-ethoxy-1,2-dihydroquinoline
- IP
intraperitoneal
- NPA
R-propylnorapomorphine
- PD
postnatal day
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Andersen SL. Stimulants and the developing brain. Trends Pharmacol Sci. 2005;26:237–243. doi: 10.1016/j.tips.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Arnt J, Hyttel J. Postsynaptic dopamine agonistic effects of 3-PPP enantiomers revealed by bilateral 6-hydroxy-dopamine lesions and by chronic reserpine treatment in rats. J Neural Transm. 1984;60:205–223. doi: 10.1007/BF01249094. [DOI] [PubMed] [Google Scholar]
- Arnt J, Hyttel J, Meier E. Inactivation of dopamine D-1 or D-2 receptors differentially inhibits stereotypies induced by dopamine agonists in rats. Eur J Pharmacol. 1988;155:37–47. doi: 10.1016/0014-2999(88)90400-1. [DOI] [PubMed] [Google Scholar]
- Bordi F, Meller E. Enhanced behavioral stereotypies elicited by intrastriatal injection of D1 and D2 dopamine agonists in intact rats. Brain Res. 1989;504:276–283. doi: 10.1016/0006-8993(89)91368-1. [DOI] [PubMed] [Google Scholar]
- Bordi F, Carr KD, Meller E. Stereotypies elicited by injection of N-propylnorapomorphine into striatal subregions and nucleus accumbens. Brain Res. 1989;489:205–215. doi: 10.1016/0006-8993(89)90852-4. [DOI] [PubMed] [Google Scholar]
- Bowman BP, Blatt B, Kuhn CM. Ontogeny of the behavioral response to dopamine agonists after chronic cocaine. Psychopharmacology (Berl) 1997;129:121–127. doi: 10.1007/s002130050171. [DOI] [PubMed] [Google Scholar]
- Boyson SJ, McGonigle P, Molinoff PB. Quantitative autoradiographic localization of the D1 and D2 subtypes of dopamine receptors in rat brain. J Neurosci. 1986;6:3177–3188. doi: 10.1523/JNEUROSCI.06-11-03177.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron DL, Crocker AD. Alkylation of striatal dopamine receptors abolishes stereotyped behavior but has no effect on dopamine adenylate cyclase activity. Neurosci Lett. 1988;90:165–171. doi: 10.1016/0304-3940(88)90805-1. [DOI] [PubMed] [Google Scholar]
- Cameron DL, Crocker AD. Localization of striatal dopamine receptor function by central injection of an irreversible receptor antagonist. Neuroscience. 1989;32:769–778. doi: 10.1016/0306-4522(89)90297-2. [DOI] [PubMed] [Google Scholar]
- Cameron DL, Crosbie J, Crocker AD. A fixed interval momentary sampling method for assessing on-going behaviours induced by dopamine receptor agonists. Prog Neuro-Psychopharmacol Biol Psychi. 1988;12:595–606. doi: 10.1016/0278-5846(88)90005-x. [DOI] [PubMed] [Google Scholar]
- Campbell A, Baldessarini RJ. Effects of maturation and aging on behavioral responses to haloperidol in the rat. Psychopharmacology (Berl) 1981;73:219–222. doi: 10.1007/BF00422406. [DOI] [PubMed] [Google Scholar]
- Canales JJ, Iversen SD. Behavioural topography in the striatum: differential effects of quinpirole and D-amphetamine microinjections. Eur J Pharmacol. 1998;362:111–119. doi: 10.1016/s0014-2999(98)00752-3. [DOI] [PubMed] [Google Scholar]
- Carrera PM, Brunhara FC, Schwarting RK, Tomaz C. Drug conditioning induced by intrastriatal apomorphine administration. Brain Res. 1998;790:60–66. doi: 10.1016/s0006-8993(98)00047-x. [DOI] [PubMed] [Google Scholar]
- Charntikov S, Halladay LR, Herbert MS, Marquez EM, McDougall SA. Effects of dorsal striatal infusions of R(–)-propylnorapomorphine (NPA) on κ-opioid-mediated locomotor activity in the young rat: possible role of the indirect pathway. Neuroscience. 2008;155:603–612. doi: 10.1016/j.neuroscience.2008.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charntikov S, Der-Ghazarian T, Herbert MS, Horn LR, Widarma CB, Gutierrez A, Varela FA, McDougall SA. Importance of D1 and D2 receptors in the dorsal caudate-putamen for the locomotor activity and stereotyped behaviors of preweanling rats. Neuroscience. 2011;183:121–133. doi: 10.1016/j.neuroscience.2011.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cory-Slechta DA, O'Mara DJ, Brockel BJ. Nucleus accumbens dopaminergic mediation of fixed interval schedule-controlled behavior and its modulation by low-level lead exposure. J Pharmacol Exp Ther. 1998;286:794–805. [PubMed] [Google Scholar]
- Cory-Slechta DA, Brockel BJ, O'Mara DJ. Lead exposure and dorsomedial striatum mediation of fixed interval schedule-controlled behavior. Neurotoxicology. 2002;23:313–327. doi: 10.1016/s0161-813x(02)00059-1. [DOI] [PubMed] [Google Scholar]
- Crawford CA, McDougall SA, Rowlett JK, Bardo MT. Depletion of dopamine binding sites and dopamine levels and changes in dihydroxyphenylacetic acid levels in the 17- and 90-day-old rat striatum after irreversible receptor antagonism. Neurosci Lett. 1992;137:265–269. doi: 10.1016/0304-3940(92)90419-8. [DOI] [PubMed] [Google Scholar]
- Crawford CA, Rowlett JK, McDougall SA, Bardo MT. Age-dependent differences in the rate of recovery of striatal dopamine D1 and D2 receptors after inactivation with EEDQ. Eur J Pharmacol. 1994;252:225–231. doi: 10.1016/0014-2999(94)90601-7. [DOI] [PubMed] [Google Scholar]
- Crawford CA, Akopian G, Ring J, Jakowec MW, Petzinger GM, Andersen JK, Vittozzi-Wong P, Wang K, Farley CM, Charntikov S, Mitroi D, Beal MF, Chow R, Walsh JP. Acute and long-term response of dopamine nigrostriatal synapses to a single, low-dose episode of 3-nitropropionic acid-mediated chemical hypoxia. Synapse. 2011;65:339–350. doi: 10.1002/syn.20852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias FR, Carey RJ, Carrera MP. Conditioned locomotion induced by unilateral intrastriatal administration of apomorphine: D2 receptor activation is critical but not the expression of the unconditioned response. Brain Res. 2006;1083:85–95. doi: 10.1016/j.brainres.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Dickson PR, Lang CG, Hinton SC, Kelley AE. Oral stereotypy induced by amphetamine microinjection into striatum: an anatomical mapping study. Neuroscience. 1994;61:81–91. doi: 10.1016/0306-4522(94)90062-0. [DOI] [PubMed] [Google Scholar]
- Double KL, Crocker AD. Effects of inactivation of D1 dopamine receptors on stereotypic and thermic responses to quinpirole (LY 171555) Neurosci Lett. 1990;115:81–85. doi: 10.1016/0304-3940(90)90521-a. [DOI] [PubMed] [Google Scholar]
- Farley CM, Baella SA, Wacan JJ, Crawford CA, McDougall SA. Pre- and postsynaptic actions of a partial D2 receptor agonist in reserpinized young rats: longevity of agonistic effects. Brain Res. 2006;1124:37–44. doi: 10.1016/j.brainres.2006.09.068. [DOI] [PubMed] [Google Scholar]
- Festa ED, Russo SJ, Gazi FM, Niyomchai T, Kemen LM, Lin SN, Foltz R, Jenab S, Quinones-Jenab V. Sex differences in cocaine-induced behavioral responses, pharmacokinetics, and monoamine levels. Neuropharmacology. 2004;46:672–687. doi: 10.1016/j.neuropharm.2003.11.017. [DOI] [PubMed] [Google Scholar]
- Gerfen CR. The neostriatal mosaic: multiple levels of compartmental organization. Trends Neurosci. 1992;15:133–139. doi: 10.1016/0166-2236(92)90355-c. [DOI] [PubMed] [Google Scholar]
- Giorgi O, Biggio G. Selective unilateral inactivation of striatal D1 and D2 dopamine receptor subtypes by EEDQ: turning behavior elicited by D2 dopamine receptor agonists. Brain Res. 1990a;533:53–59. doi: 10.1016/0006-8993(90)91794-h. [DOI] [PubMed] [Google Scholar]
- Giorgi O, Biggio G. Unilateral inactivation of dopamine receptors after intrastriatal injection of N-ethoxy-carbonyl-2-ethoxy-1,2-dihydroquinoline (EEDQ): a novel rotational model to investigate dopamine receptor interactions. Pharmacol Biochem Behav. 1990b;35:877–884. doi: 10.1016/0091-3057(90)90374-q. [DOI] [PubMed] [Google Scholar]
- Gnanalingham KK, Hunter AJ, Jenner P, Marsden CD. An autoradiographic study of the differential effects of N-ethoxycarbonyl-2-ethoxy-1,2-dihydroquinoline (EEDQ) on striatal and extrastriatal D-1 and D-2 dopamine receptors in the rat. Neuropharmacology. 1994;33:647–655. doi: 10.1016/0028-3908(94)90170-8. [DOI] [PubMed] [Google Scholar]
- Hamblin MW, Creese I. Behavioral and radioligand binding evidence for irreversible dopamine receptor blockade by N-ethoxycarbonyl-2-ethoxy-1,2-dihydroquinoline. Life Sci. 1983;32:2247–2255. doi: 10.1016/0024-3205(83)90423-x. [DOI] [PubMed] [Google Scholar]
- Harrison LM, LaHoste GJ. Rhes, the Ras homolog enriched in striatum, is reduced under conditions of dopamine supersensitivity. Neuroscience. 2006;137:483–492. doi: 10.1016/j.neuroscience.2005.08.017. [DOI] [PubMed] [Google Scholar]
- Hemsley KM, Crocker AD. Changes in muscle tone are regulated by D1 and D2 dopamine receptors in the ventral striatum and D1 receptors in the substantia nigra. Neuropsychopharmacology. 2001;25:514–526. doi: 10.1016/S0893-133X(01)00245-7. [DOI] [PubMed] [Google Scholar]
- Hemsley KM, Farrall EJ, Crocker AD. Dopamine receptors in the subthalamic nucleus are involved in the regulation of muscle tone in the rat. Neurosci Lett. 2002;317:123–126. doi: 10.1016/s0304-3940(01)02460-0. [DOI] [PubMed] [Google Scholar]
- Holson RR, Pearce B. Principles and pitfalls in the analysis of prenatal treatment effects in multiparous species. Neurotoxicol Teratol. 1992;14:221–228. doi: 10.1016/0892-0362(92)90020-b. [DOI] [PubMed] [Google Scholar]
- Huynh H, Feldt LS. Estimation of the Box correction for degrees of freedom from sample data in randomized block and split-plot designs. J Educ Stat. 1976;1:69–82. [Google Scholar]
- Jaber M, Fournier MC, Bloch B. Reserpine treatment stimulates enkephalin and D2 dopamine receptor gene expression in the rat striatum. Mol Brain Res. 1992;15:189–194. doi: 10.1016/0169-328x(92)90108-n. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Domesick VB, Nauta WJ. The amygdalostriatal projection in the rat--an anatomical study by anterograde and retrograde tracing methods. Neuroscience. 1982;7:615–630. doi: 10.1016/0306-4522(82)90067-7. [DOI] [PubMed] [Google Scholar]
- Kettle CJ, Cheetham SC, Martin KF, Prow MR, Heal DJ. The effects of the peptide-coupling agent, EEDQ, on 5-HT2A receptor binding and function in rat frontal cortex. Neuropharmacology. 1999;38:1421–1430. doi: 10.1016/s0028-3908(99)00061-1. [DOI] [PubMed] [Google Scholar]
- Kreipke CW, Walker PD. NMDA receptor blockade attenuates locomotion elicited by intrastriatal dopamine D1-receptor stimulation. Synapse. 2004;53:28–35. doi: 10.1002/syn.20035. [DOI] [PubMed] [Google Scholar]
- Krolewski DM, Bishop C, Walker PD. Intrastriatal dopamine D1 receptor agonist-mediated motor behavior is reduced by local neurokinin 1 receptor antagonism. Synapse. 2005;57:1–7. doi: 10.1002/syn.20148. [DOI] [PubMed] [Google Scholar]
- Kula NS, George T, Baldessarini RJ. Rate of recovery of D1 and D2 dopaminergic receptors in young vs. adult rat striatal tissue following alkylation with ethoxycarbonylethoxy-dihydroquinoline (EEDQ) Dev Brain Res. 1992;66:286–289. doi: 10.1016/0165-3806(92)90095-e. [DOI] [PubMed] [Google Scholar]
- Lee CY, Double KL, Crocker AD. Expression of stereotyped behaviour requires stimulation of nigral D1 dopamine receptors. Brain Res. 1995;681:205–208. doi: 10.1016/0006-8993(95)00263-p. [DOI] [PubMed] [Google Scholar]
- Leff SE, Gariano R, Creese I. Dopamine receptor turnover rates in rat striatum are age-dependent. Proc Natl Acad Sci USA. 1984;81:3910–3914. doi: 10.1073/pnas.81.12.3910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepekhina LM, Tsitsurina EA. Stereotyped behavior in the ontogeny of rats. Bull Exp Biol Med. 2007;144:349–351. doi: 10.1007/s10517-007-0330-5. [DOI] [PubMed] [Google Scholar]
- McDougall SA, Crawford CA, Nonneman AJ. Effects of irreversible dopamine receptor inactivation on locomotor activity and grooming in the 17- and 90-day-old rat. Psychopharmacology (Berl) 1992;106:502–510. doi: 10.1007/BF02244822. [DOI] [PubMed] [Google Scholar]
- McDougall SA, Crawford CA, Nonneman AJ. Behavioral effects of selective and nonselective dopamine agonists on young rats after irreversible antagonism of D1 and/or D2 receptors. Psychopharmacology (Berl) 1993;111:225–232. doi: 10.1007/BF02245528. [DOI] [PubMed] [Google Scholar]
- McDougall SA, Baella SA, Stuebner NM, Halladay LM, Crawford CA. Cocaine-induced behavioral sensitization in preweanling and adult rats: effects of a single drug-environment pairing. Psychopharmacology (Berl) 2007;193:323–332. doi: 10.1007/s00213-007-0788-x. [DOI] [PubMed] [Google Scholar]
- Meller E, Enz A, Goldstein M. Absence of receptor reserve at striatal dopamine receptors regulating cholinergic neuronal activity. Eur J Pharmacol. 1988;155:151–154. doi: 10.1016/0014-2999(88)90413-x. [DOI] [PubMed] [Google Scholar]
- Meller E, Bordi F, Bohmaker K. Behavioral recovery after irreversible inactivation of D-1 and D-2 dopamine receptors. Life Sci. 1989;44:1019–1026. doi: 10.1016/0024-3205(89)90553-5. [DOI] [PubMed] [Google Scholar]
- Meller E, Bohmaker K, Namba Y, Goldstein M, Friedhoff AJ. Inactivation of D1 and D2 dopamine receptors by N-ethoxycarbonyl-2-ethoxy-1,2-dihydroquinoline in vivo: selective protection by neuroleptics. J Pharmacol Exp Ther. 1985;233:656–662. [PubMed] [Google Scholar]
- Mestlin M, McDougall SA. Ontogenetic differences in the effects of EEDQ on dopamine-mediated behaviors. Pharmacol Biochem Behav. 1993;45:797–802. doi: 10.1016/0091-3057(93)90123-b. [DOI] [PubMed] [Google Scholar]
- Milesi-Hallé A, McMillan DE, Laurenzana EM, Byrnes-Blake KA, Owens SM. Sex differences in (+)-amphetamine- and (+)-methamphetamine-induced behavioral response in male and female Sprague-Dawley rats. Pharmacol Biochem Behav. 2007;86:140–149. doi: 10.1016/j.pbb.2006.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller LG, Lumpkin M, Galpern WR, Greenblatt DJ, Shader RI. Modification of γ-aminobutyric acid, receptor binding and function by N-ethoxycarbonyl-2-ethoxy-1,2-dihydroquinoline in vitro and in vivo: effect of aging. J. Neurochem. 1991;56:1241–1247. doi: 10.1111/j.1471-4159.1991.tb11417.x. [DOI] [PubMed] [Google Scholar]
- Moody CA, Spear LP. Ontogenetic differences in the psychopharmacological responses to separate and combined stimulation of D1 and D2 dopamine receptors during the neonatal to weanling age period. Psychopharmacology. 1992;106:161–168. doi: 10.1007/BF02801967. [DOI] [PubMed] [Google Scholar]
- Neisewander JL, Ong A, McGonigle P. Anatomical localization of SKF-38393-induced behaviors in rats using the irreversible monoamine receptor antagonist EEDQ. Synapse. 1995;19:134–143. doi: 10.1002/syn.890190209. [DOI] [PubMed] [Google Scholar]
- Norman AB, Eubanks JH, Creese I. Irreversible and quaternary muscarinic antagonists discriminate multiple muscarinic receptor binding sites in rat brain. J Pharmacol Exp Ther. 1989;248:1116–1122. [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain: in stereotaxic coordinates. 4th ed Academic Press; San Diego: 1998. [Google Scholar]
- Ribas C, Miralles A, Escribá PV, García-Sevilla JA. Effects of the alkylating agent EEDQ on regulatory G proteins and recovery of agonist and antagonist α2-adrenoceptor binding sites in rat brain. Eur J Pharmacol. 1998;351:145–154. doi: 10.1016/s0014-2999(98)00295-7. [DOI] [PubMed] [Google Scholar]
- Rosengarten H, Schweitzer JW, Friedhoff AJ. A full repetitive jaw movement response after 70% depletion of caudate D1 receptors. Pharmacol Biochem Behav. 1989;34:895–897. doi: 10.1016/0091-3057(89)90290-6. [DOI] [PubMed] [Google Scholar]
- Rosengarten H, Schweitzer JW, Friedhoff AJ. A subpopulation of dopamine D1 receptors mediate repetitive jaw movements in rats. Pharmacol Biochem Behav. 1993;45:921–924. doi: 10.1016/0091-3057(93)90140-o. [DOI] [PubMed] [Google Scholar]
- Sallés J, Wallace MA, Fain JN. Differential effects of alkylating agents on the multiple muscarinic receptor subtypes linked to activation of phospholipase C by carbachol in rat brain cortical membranes. J Pharmacol Exp Ther. 1993;264:521–529. [PubMed] [Google Scholar]
- Schindler CW, Carmona GN. Effects of dopamine agonists and antagonists on locomotor activity in male and female rats. Pharmacol Biochem Behav. 2002;72:857–863. doi: 10.1016/s0091-3057(02)00770-0. [DOI] [PubMed] [Google Scholar]
- Seeman P, Weinshenker D, Quirion R, Srivastava LK, Bhardwaj SK, Grandy DK, Premont RT, Sotnikova TD, Boksa P, El-Ghundi M, O'Dowd BF, George SR, Perreault ML, Männistö PT, Robinson S, Palmiter RD, Tallerico T. Dopamine supersensitivity correlates with D2High states, implying many paths to psychosis. Proc Natl Acad Sci USA. 2005;102:3513–3518. doi: 10.1073/pnas.0409766102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwood NM, Timiras P. A stereotaxic atlas of the developing rat brain. University of California Press; Berkeley: 1970. [Google Scholar]
- Snyder KJ, Katovic NM, Spear LP. Longevity of the expression of behavioral sensitization to cocaine in preweanling rats. Pharmacol Biochem Behav. 1998;60:909–914. doi: 10.1016/s0091-3057(98)00078-1. [DOI] [PubMed] [Google Scholar]
- Spear LP. The use of psychopharmacological procedures to analyse the ontogeny of learning and retention: issues and concerns. In: Spear NE, Campbell BA, editors. Ontogeny of learning and memory. Lawrence Erlbaum Associates; Hillsdale, NJ: 1979. pp. 135–156. [Google Scholar]
- Spear LP, Brick J. Cocaine-induced behavior in the developing rat. Behav Neural Biol. 1979;26:401–415. doi: 10.1016/s0163-1047(79)91410-9. [DOI] [PubMed] [Google Scholar]
- Trovero F, Hervé D, Blanc G, Glowinski J, Tassin JP. In vivo partial inactivation of dopamine D1 receptors induces hypersensitivity of cortical dopamine-sensitive adenylate cyclase: permissive role of α1-adrenergic receptors. J Neurochem. 1992;59:331–337. doi: 10.1111/j.1471-4159.1992.tb08908.x. [DOI] [PubMed] [Google Scholar]
- Van Hartesveldt C, Cottrell GA, Potter T, Meyer ME. Effects of intracerebral quinpirole on locomotion in rats. Eur J Pharmacol. 1992;214:27–32. doi: 10.1016/0014-2999(92)90091-h. [DOI] [PubMed] [Google Scholar]
- Vinod KY, Subhash MN, Srinivas BN. Differential protection and recovery of 5-HT1A receptors from N-ethoxycarbonyl-2-ethoxy-1,2-dihydroquinoline (EEDQ) inactivation in regions of rat brain. Neurochem Res. 2001;26:113–120. doi: 10.1023/a:1011038510723. [DOI] [PubMed] [Google Scholar]
- Waszczak BL, Martin LP, Finlay HE, Zahr N, Stellar JR. Effects of individual and concurrent stimulation of striatal D1 and D2 dopamine receptors on electrophysiological and behavioral output from rat basal ganglia. J Pharmacol Exp Ther. 2002;300:850–861. doi: 10.1124/jpet.300.3.850. [DOI] [PubMed] [Google Scholar]
- Yokoo H, Goldstein M, Meller E. Receptor reserve at striatal dopamine receptors modulating the release of [3H]dopamine. Eur J Pharmacol. 1988;155:323–327. doi: 10.1016/0014-2999(88)90523-7. [DOI] [PubMed] [Google Scholar]
- Zorrilla EP. Multiparous species present problems (and possibilities) to developmentalists. Dev Psychobiol. 1997;30:141–150. doi: 10.1002/(sici)1098-2302(199703)30:2<141::aid-dev5>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]