Abstract
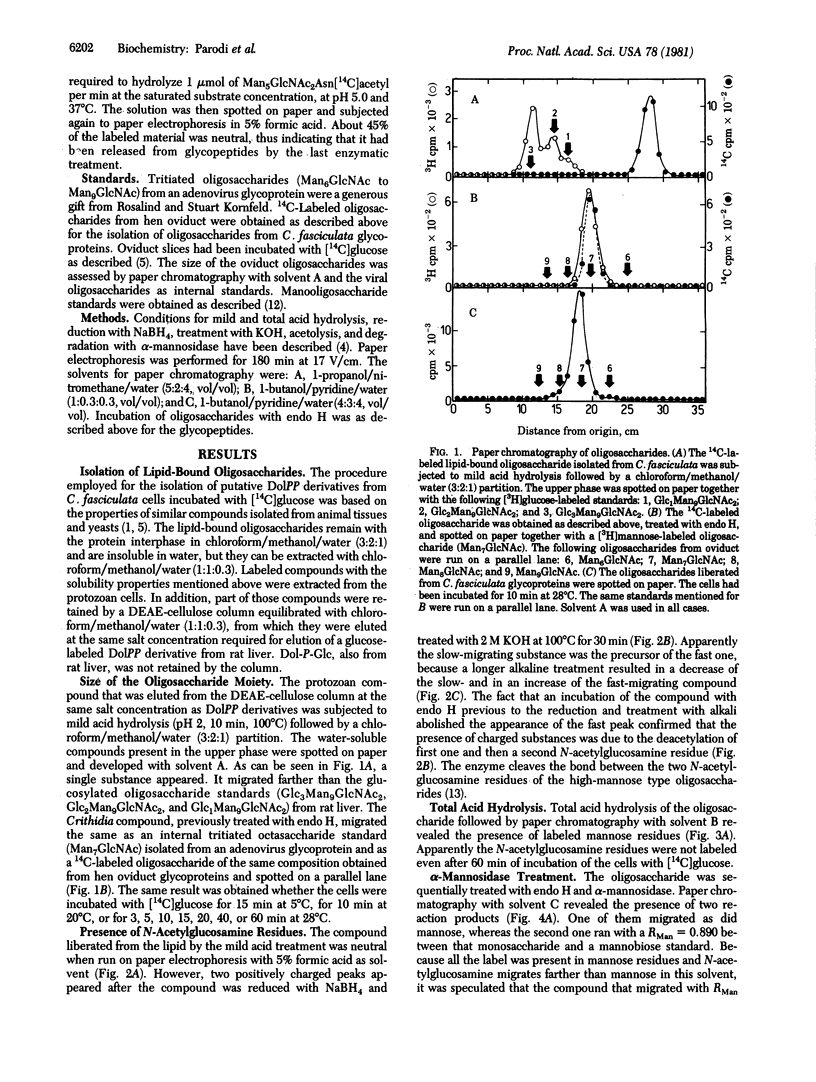
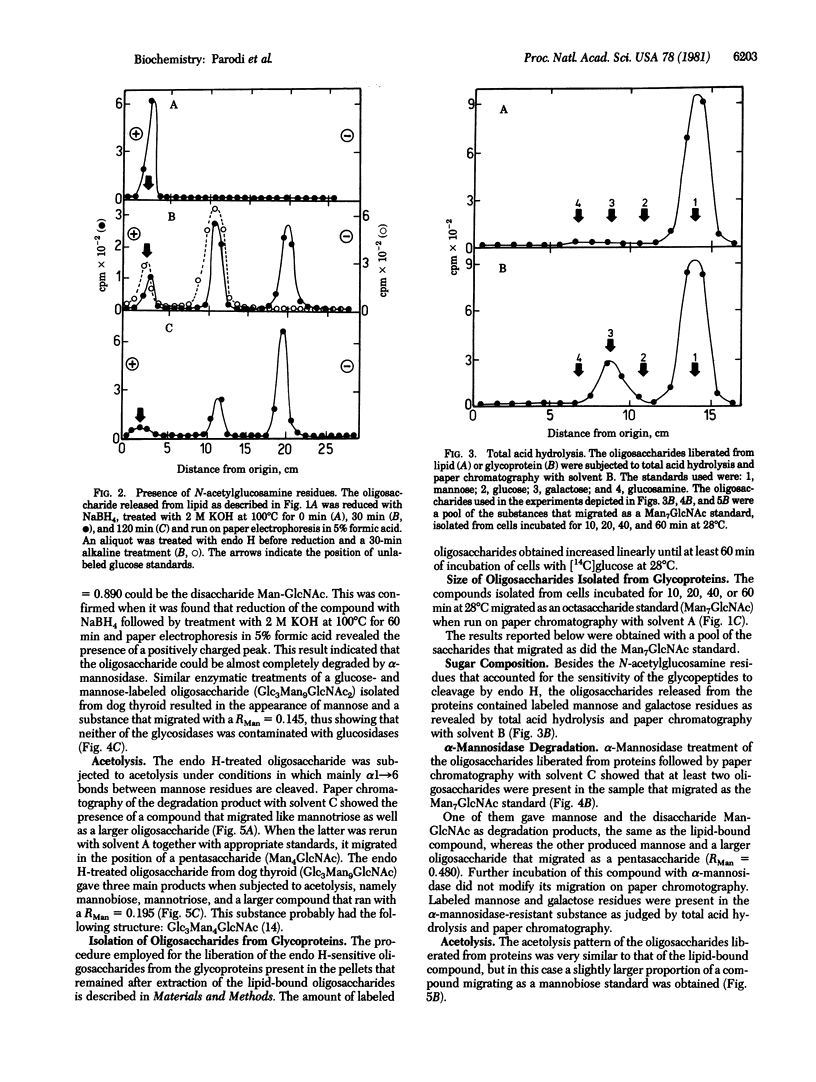
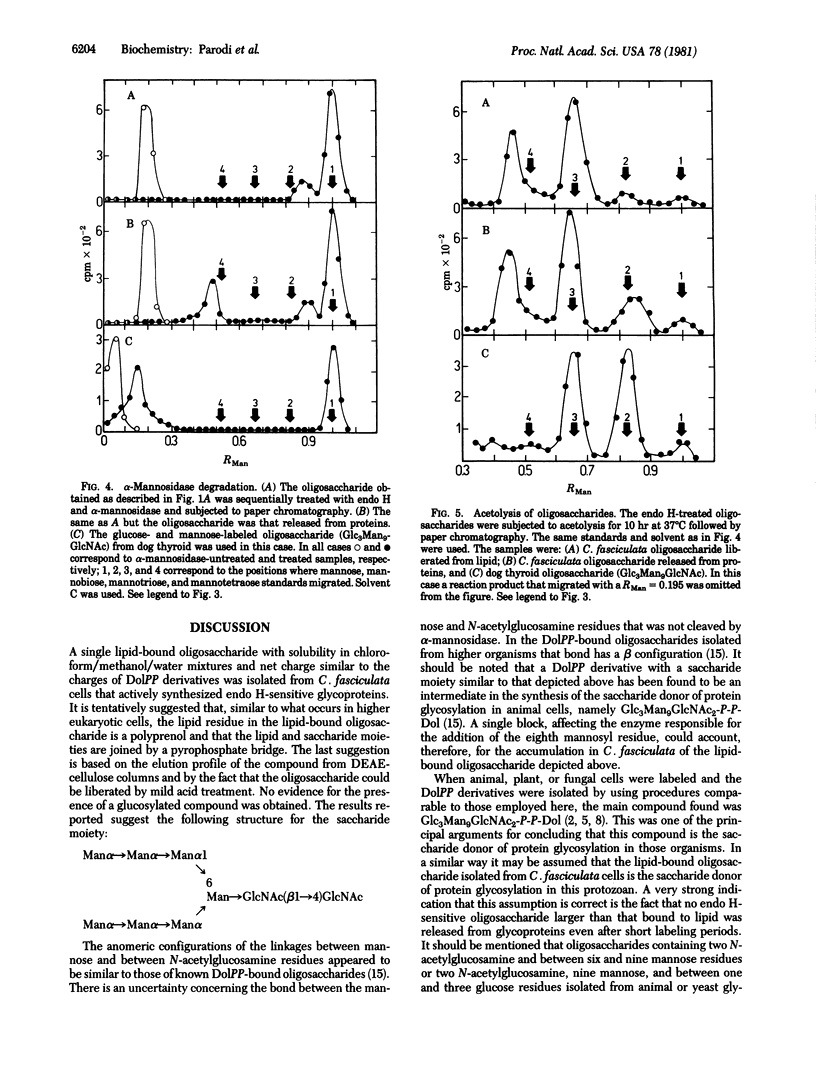
Cells of the insect parasite Crithidia fasciculata incubated with [14C]glucose were found to possess only one lipid-bound oligosaccharide with solubility in chloroform/methanol/water mixtures and net charge similar to the charges of dolichol pyrophosphate derivatives. The saccharide moiety could be released from lipid by mild acid hydrolysis. Several enzymatic and chemical treatments of the oligosaccharide indicated that the latter had the structure Man alpha leads to Man alpha leads to Man alpha leads to [Man alpha leads to Man alpha leads to Man (alpha 1 leads to 6)]Man leads to GlcNAc(beta 1 leads to 4)GlcNAc. Two labeled oligosaccharides were liberated from proteins by a sequential treatment with a protease and endo-beta-N-acetylglucosamindase H. One of the protein-bound oligosaccharides had the same structure as the lipid-linked compound, whereas in the second oligosaccharide some mannose residues had been replaced by galactose units, but both compounds migrated as did a Man7GlcNAc standard. These were the largest oligosaccharides obtained even after short labeling periods. It is suggested that glycosylation of proteins in the protozoan Crithidia fasciculata does not involved glucosylated lipid-bound oligosaccharides as intermediates.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bacchi J., Lambros C., Goldberg B., Hutner S. H., de Carvalho G. D. Susceptibility of an insect Leptomonas and Crithidia fasciculata to several established antitrypanosomatid agents. Antimicrob Agents Chemother. 1974 Dec;6(6):785–790. doi: 10.1128/aac.6.6.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman A., Li E., Kornfeld S. The biosynthesis of the major lipid-linked oligosaccharide of Chinese hamster ovary cells occurs by the ordered addition of mannose residues. J Biol Chem. 1979 Oct 25;254(20):10243–10249. [PubMed] [Google Scholar]
- Hubbard S. C., Robbins P. W. Synthesis and processing of protein-linked oligosaccharides in vivo. J Biol Chem. 1979 Jun 10;254(11):4568–4576. [PubMed] [Google Scholar]
- Hunt L. A. CHO cells selected for phytohemagglutinin and con A resistance are defective in both early and late stages of protein glycosylation. Cell. 1980 Sep;21(2):407–415. doi: 10.1016/0092-8674(80)90477-8. [DOI] [PubMed] [Google Scholar]
- Kobata A. Use of endo- and exoglycosidases for structural studies of glycoconjugates. Anal Biochem. 1979 Nov 15;100(1):1–14. doi: 10.1016/0003-2697(79)90102-7. [DOI] [PubMed] [Google Scholar]
- Lehle L. Biosynthesis of the core region of yeast mannoproteins. Formation of a glucosylated dolichol-bound oligosaccharide precursor, its transfer to protein and subsequent modification. Eur J Biochem. 1980 Aug;109(2):589–601. doi: 10.1111/j.1432-1033.1980.tb04832.x. [DOI] [PubMed] [Google Scholar]
- Li E., Tabas I., Kornfeld S. The synthesis of complex-type oligosaccharides. I. Structure of the lipid-linked oligosaccharide precursor of the complex-type oligosaccharides of the vesicular stomatitis virus G protein. J Biol Chem. 1978 Nov 10;253(21):7762–7770. [PubMed] [Google Scholar]
- Parodi A. J. Biosynthesis of yeast glycoproteins. Processing of the oligosaccharides transferred from dolichol derivatives. J Biol Chem. 1979 Oct 25;254(20):10051–10060. [PubMed] [Google Scholar]
- Parodi A. J., Leloir L. F. The role of lipid intermediates in the glycosylation of proteins in the eucaryotic cell. Biochim Biophys Acta. 1979 Apr 23;559(1):1–37. doi: 10.1016/0304-4157(79)90006-6. [DOI] [PubMed] [Google Scholar]
- Parodi A. J. Lipid intermediates in the synthesis of the inner core of yeast mannan. Eur J Biochem. 1978 Feb 1;83(1):253–259. doi: 10.1111/j.1432-1033.1978.tb12090.x. [DOI] [PubMed] [Google Scholar]
- Parodi A. J. The mechanism of synthesis of the polysaccharide part of mannan in Saccharomyces cerevisiae. Arch Biochem Biophys. 1981 Aug;210(1):372–382. doi: 10.1016/0003-9861(81)90200-9. [DOI] [PubMed] [Google Scholar]
- Quesada Allue L. A. The biosynthesis of glucose containing insect lipid linked oligosaccharide and its possible role in glycoprotein assembly. Mol Cell Biochem. 1980 Dec 16;33(3):149–155. doi: 10.1007/BF00225287. [DOI] [PubMed] [Google Scholar]
- Spiro M. J., Spiro R. G., Bhoyroo V. D. Lipid-saccharide intermediates in glycoprotein biosynthesis. I. Formation of an oligosaccharide-lipid by thyroid slices and evaluation of its role in protein glycosylation. J Biol Chem. 1976 Oct 25;251(20):6400–6408. [PubMed] [Google Scholar]
- Staneloni R. J., Tolmasky M. E., Petriella C., Ugalde R. A., Leloir L. F. Presence in a plant of a compound similar to the dolichyl diphosphate oligosaccharide of animal tissue. Biochem J. 1980 Oct 1;191(1):257–260. doi: 10.1042/bj1910257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staneloni R. J., Ugalde R. A., Leloir L. F. Addition of glucose to dolichyl diphosphate oligosaccharide and transfer to protein. Eur J Biochem. 1980 Apr;105(2):275–278. doi: 10.1111/j.1432-1033.1980.tb04498.x. [DOI] [PubMed] [Google Scholar]
- Tai T., Yamashita K., Ogata-Arakawa M., Koide N., Muramatsu T., Iwashita S., Inoue Y., Kobata A. Structural studies of two ovalbumin glycopeptides in relation to the endo-beta-N-acetylglucosaminidase specificity. J Biol Chem. 1975 Nov 10;250(21):8569–8575. [PubMed] [Google Scholar]
- Trimble R. B., Maley F., Tarentino A. L. Characterization of large oligosaccharide-lipids synthesized in vitro by microsomes from Saccharomyces cerevisiae. J Biol Chem. 1980 Nov 10;255(21):10232–10238. [PubMed] [Google Scholar]


