Abstract
Hypocotyls from etiolated cucumber (Cucumis sativus L.) seedlings were gently abraded at their epidermal surface and cut segments were conditioned to develop competence for H2O2 elicitation. Alkaline hydrolysates of cutin from cucumber, tomato, and apple elicited H2O2 in such conditioned segments. The most active constituent of cucumber cutin was identified as dodecan-1-ol, a novel cutin monomer capable of forming hydrophobic terminal chains. Additionally, the cutin hydrolysates enhanced the activity of a fungal H2O2 elicitor, similar to cucumber surface wax, which contained newly identified alkan-1,3-diols. The specificity of elicitor and enhancement activity was further elaborated using some pure model compounds. Certain saturated hydroxy fatty acids were potent H2O2 elicitors as well as enhancers. Some unsaturated epoxy and hydroxy fatty acids were also excellent H2O2 elicitors but inhibited the fungal elicitor activity. Short-chain alkanols exhibited good elicitor and enhancer activity, whereas longer-chain alkan-1-ols were barely active. The enhancement effect was also observed for H2O2 elicitation by ergosterol and chitosan. The physiological significance of these observations might be that once the cuticle is degraded by fungal cutinase, the cutin monomers may act as H2O2 elicitors. Corrosion of cutin may also bring surface wax constituents in contact with protoplasts and enhance elicitation.
Fungal pathogens that attempt to penetrate into leaf surfaces have to cope with the plant cuticle, the structurally and chemically complex hydrophobic surface of all aerial plant parts. One component of the cuticle is cutin, an insoluble polymer built mainly from esterified C16 and C18 hydroxy and epoxy fatty acids. Layered onto and partly embedded into the polymer matrix is the so-called surface wax, a heterogenous mixture of lipids soluble in organic solvents. The composition of surface wax varies greatly among plant species, although the most common major components are longer-chain hydrocarbons and their oxygenated derivatives, namely primary and secondary alcohols, ketones, fatty acids, aldehydes, and esters. In addition, alkaline hydrolysis of isolated cuticle leaves a hydrophobic, insoluble residue (“cutan”), which greatly varies in amount among different plants and organs and may contain constituents of both cutin and wax, possibly connected by ether and C-C linkages (Jeffree, 1996; Kolattukudy, 1996; Riederer and Markstädter, 1996).
Cutin monomers generated by the action of fungal cutinase were shown to act as signals for fungal gene activation (Kolattukudy et al., 1995) and also to contribute to induction of fungal appressoria (Francis et al., 1996; Gilbert et al., 1996). Plant surface wax constituents can also trigger several steps in fungal development (Kolattukudy et al., 1995). Taken together, these results provide convincing evidence that constituents of the plant cuticle represent important potential signals for the recognition of plant surfaces by fungal pathogens.
In contrast, a signaling role of cutin monomers in the plant's defense strategy has only recently been recognized. Root pretreatment of rice plants with monoepoxy linoleic and linolenic acids was shown to induce resistance of the leaves against Pyricularia oryzae, the imperfect form of Magnaporthe grisea (Namai et al., 1993). It was also shown that a topical spray of cutin monomers on leaves of barley and rice enhanced resistance against Erysiphe graminis f. sp. hordei and M. grisea, respectively (Schweizer et al., 1994, 1996b). Since the cutin monomers used in these experiments exhibited no apparent fungicidal effect, the observed protection was taken as evidence that the cutin monomers induced acquired resistance in the plants. That plant cells can perceive free cutin monomers was shown in a model system consisting of suspension-cultured potato cells, which exhibited a transient alkalinization of the culture medium, similar to known elicitors of defense responses (Schweizer et al., 1996a).
We have recently used etiolated cucumber (Cucumis sativus L.) hypocotyls to correlate biochemically oriented, classical elicitor experiments with the resistance of epidermal cells against fungal infection. The epidermal cells acquired resistance to Colletotrichum lagenarium when 2,6-dichloroisonicotinic acid was applied to the roots or when cut hypocotyl segments were preincubated with salicylic acid (Siegrist et al., 1994). To allow application of elicitors directly to epidermal cells, the hypocotyls were gently abraded at their surface to make the cuticle permeable. Surprisingly, the pathogen-resistant epidermal cells of freshly abraded segments were barely competent for elicitation of H2O2 by a polymeric fungal elicitor, either ergosterol or chitosan (Fauth et al., 1996; Kauss and Jeblick, 1996). Such competence developed after abrasion in a time-dependent process requiring protein synthesis, and it was this process, referred to as conditioning, that was enhanced in segments exhibiting acquired resistance. Using partially acetylated chitosan as a universal H2O2 elicitor, the requirement for a conditioning period has recently been shown for etiolated hypocotyls or epicotyls from another six plant species, including soybean and bean, which in suspension cultures exhibit an apparently constitutive H2O2 elicitor competence (Kauss et al., 1997). Thus, in intact tissues an additional stimulus derived from surface abrasion is required to render the H2O2 elicitation system functional.
In the present report we demonstrate that cutin monomers can act as H2O2 elicitors and can also enhance the activity of other H2O2 elicitors in conditioned cucumber hypocotyls. This enhancement effect was also found for certain alkanols, which are models for constituents of surface wax.
MATERIALS AND METHODS
Cucumber (Cucumis sativus L. cv Mervita) seedlings were grown in the dark for 5 to 6 d (Fauth et al., 1996). The hypocotyls were abraded with a suspension (0.5 g mL−1) of SiC (800 mesh, Schriever, Hamburg, Germany) in water. A thumb and a forefinger were moistened with the suspension and the hypocotyl moved apically through these fingers under gentle pressure. After turning the seedlings by 90°, this treatment was repeated once and the seedlings were washed in water. This procedure rendered the surface around the hypocotyl permeable to water-soluble compounds but did not destroy epidermal cells, as monitored with staining procedures (Fauth et al., 1996). For conditioning, two segments (2 cm) were cut from each abraded hypocotyl and about 200 segments were gently shaken for 18 h in a 10-cm Petri dish with 30 mL of 10 mm Mes/KOH buffer, pH 6.5, containing 10 μg mL−1 each of chloramphenicol, penicillin G, and streptomycin. The conditioned segments were washed and used for the elicitor experiments in batches of 10 segments in 3.5-cm Petri dishes containing 3 mL of the above buffer without antibiotics. Under this condition the segments were just covered with liquid and were slowly rotated to avoid anoxia. At the indicated times, 100 μL of buffer was removed and H2O2 was measured by ferricyanide-catalyzed oxidation of luminol, as described previously (Fauth et al., 1996). In most cases, hydroxy fatty acids and cutin hydrolysates were added directly to the assay from a stock solution in DMSO (final solvent concentration 0.2%, v/v) or, in the case of ELA and ELAM, in acetone (final solvent concentration 0.03%). The C20-C30 alkanols were solubilized in warm chloroform and an aliquot was dried under N2, 0.5 mL of assay buffer was added, the suspension was sonicated, and the resulting emulsion was added as an elicitor to a 2.5-mL assay. All controls contained the same amount of respective solvent.
The preparation of a polymeric elicitor from cell walls of Phytophthora sojae was as described previously (Fauth et al., 1996) and is designated as a fungal elicitor throughout this report. Unless stated otherwise, materials were as described by Fauth et al. (1996) and Kauss and Jeblick (1996). Cutin layers and alkaline hydrolysates were prepared as described by Schweizer et al. (1996a). Material was examined by TLC (silica gel Merck 60; diethylether:hexane:methanol, 8:2:1, v/v) and eluted bands were analyzed by GC-MS as described by Schweizer et al. (1996a).
Surface wax was solubilized by dipping unwounded cucumber seedlings for about 1 min in chloroform. Composition of the wax extract was studied by capillary GC (model 5890, series II, Hewlett-Packard) with on-column injection (30 m DB-1 o.d., 0.32-mm film 0.1 μm, J & W Scientific, Folsom, CA) and a flame ionization or MS detector (70 eV, m/z 50–650, HP 5971). For this purpose hydroxyl- and carboxyl-containing substances in the sample were transformed to the corresponding trimethylsilyl derivatives by reaction with bis-N,N-trimethylsilyltrifluoroacetamide (Macherey-Nagel, Düren, Germany) in pyridine (30 min, 70°C). GC was carried out with the following temperature program: injection at 50°C, 2 min at 50°C, 40°C min−1 up to 200°C, 2 min at 200°C, 3°C min−1 up to 300°C, 30 min at 300°C. The inlet pressure of the carrier gases was adjusted to 50 kPa hydrogen and 10 kPa helium.
Commercial sources of lipids were: Aldrich (1-hexadecanol, 1 and 16-HDD), Fluka (1-DDEO and 1-DDO), Lancaster Laboratories (Lancaster, PA) (1,2-HDDO, 1,2-DDDO, and 1,12-DDDO), and Sigma (1-HDEO, PA, HPA, SA, HSA, e-DHSA, t-DHSA, ETEA, EPEA, cis,cis-linoleic acid, and trans,trans-linoleic acid). The two isomers of ELA and the respective methyl esters were prepared as described by Namai et al. (1993). The unsaturated hydroxy fatty acids occur in free form in sunflower seeds from which they were isolated and identified according to a protocol described for rice plants (Kato et al., 1993).
RESULTS
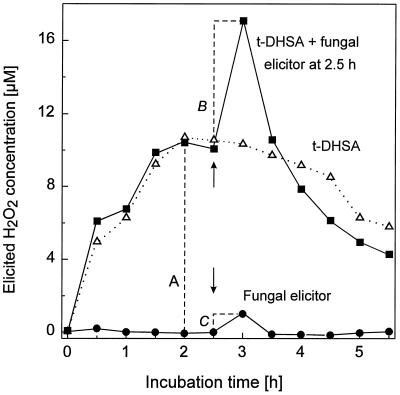
The experimental protocol for the studies reported here is shown for t-DHSA in Figure 1. This hydroxy fatty acid induced the production of H2O2 in the abraded and conditioned hypocotyl segments for much longer than did the fungal elicitor (Fig. 1), ergosterol, chitosan, or mastoparan (Fauth et al., 1996; Kauss and Jeblick, 1996). H2O2 production was evident at a concentration of 20 μm t-DHSA, increased with the dose and was still not saturated at 100 μm (data not shown). It should be noted, however, that the lipids were barely soluble in the assay buffer, evident from the observation that the assay buffer became visibly turbid upon application to more than 50 μm of most of the oxygenated hydrocarbons (exception: alkanols <C14). Therefore, the difficulties in saturating the H2O2 response might be explained by limited passage of the precipitated compounds across the abraded cuticle and outer cell wall toward the epidermal plasma membrane. It should also be noted that, in contrast to fungal elicitor, ergosterol, and chitosan (Fauth et al., 1996; Kauss and Jeblick, 1996), H2O2 elicitation by t-DHSA was only increased 1.2-fold when salicylic acid was present during conditioning (data not shown). Therefore, we performed all of the experiments reported here with abraded segments that had been conditioned in the absence of salicylic acid.
Figure 1.
The hydroxy fatty acid t-DHSA elicits H2O2 in abraded and conditioned cucumber hypocotyl segments, and enhances H2O2 elicitation of a polymeric fungal elicitor. Three batches each of 10 conditioned segments were either left as a control (•) or induced with 100 μm t-DHSA (▪ and ▵). One of the t-DHSA batches and the control batch received fungal elicitor (10 μg mL−1) 2.5 h after the t-DHSA application (arrows). Values from another control batch run in parallel without any elicitor were subtracted. The elicitor activity of t-DHSA is shown as distance A. The factor for enhancement of the fungal elicitor activity caused by lipids was defined as division of distance B by distance C. In the above example, a 6-fold enhancement was calculated.
In addition to direct H2O2 elicitation, t-DHSA also enhanced the H2O2 elicitation by a polymeric fungal elicitor that was applied subsequently to the hydroxy fatty acid (Fig. 1). It should be noted that the concentration of fungal elicitor used throughout this report was only about 75% saturating, since at near-saturating doses (about 20 μg mL−1) enhancement was lower (data not shown). Both the enhancement effect and the elicitor activity were calculated in most experiments relative to the activity of 10 μg mL−1 fungal elicitor (see Fig. 1), allowing a better comparison of different batches of segments, which, for unknown reasons, varied in their absolute potential for H2O2 production.
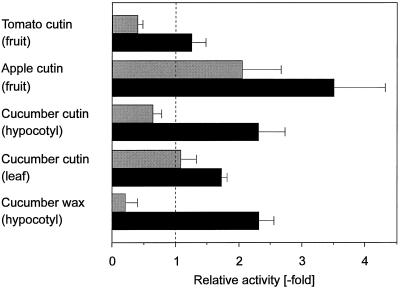
Using the protocol outlined in Figure 1, the H2O2 elicitation by alkaline hydrolysates of cutin from various plants was also studied (Fig. 2). The elicitation potential of hydrolysates from tomato and apple fruit, as well as from etiolated cucumber hypocotyl and green leaves, differed considerably. This indicates some specificity of the response with respect to the monomer composition of the hydrolysates. A low H2O2 elicitor activity was also found for the cuticular wax from cucumber hypocotyls. This material greatly enhanced the activity of fungal elicitor, similar in degree to the cutin hydrolysates (Fig. 2).
Figure 2.
Alkaline hydrolysates of cutin from various plants elicit H2O2 production and also enhance the H2O2 activity of a fungal H2O2 elicitor, similar to cucumber surface wax. All activities were calculated relative to the activity of fungal elicitor (10 μg mL−1) determined in the same batch of abraded and conditioned hypocotyl segments (see Fig. 1). Gray bar, Elicitor activity; black bar, enhancement. Note that for enhancement a relative activity of 1.0 indicates no effect, whereas in the case of elicitor activity a value of 1.0 indicates the same activity as fungal elicitor. Means ± sd from three independent determinations are shown. Cutin hydrolysates from tomato, apple, and cucumber hypocotyl, as well as surface wax, were used at 100 μg mL−1, whereas cucumber leaf cutin hydrolysate was used at 50 μg mL−1 to allow comparison with fractions isolated by TLC.
The cuticular wax from etiolated cucumber hypocotyls consisted of alkan-1,3-diols (C20-C30, mainly C22) and alkan-1-ols (C20-C34, mainly C26) with predominantly even chain lengths. Free 1-DDO was not detected in the wax fraction. Since alkan-1,3-diols of a given chain length and the respective alkan-1-ols with a chain length of two carbon units longer were not separated chromatographically, only the sum of both substance classes (52% of the total peak area) can be given. Alkanoic acids (C10-C30) with even chain lengths represented 11% of the wax fraction. They exhibited a bimodal chain-length distribution, with maxima at C16 and C28, and contained monounsaturated (C16 and C18) and diunsaturated (C18) species comparable in amount to the corresponding saturated acids. Of the n-alkanes, only one chain length (C29) was detected and amounted to 4%. About 33% of the wax fraction consisted of unidentified compounds; the most frequent, unidentified single component had a portion of 2.5% of total peak area. Long-chain esters were not detected in the cucumber wax fraction.
The overall composition of the cucumber cutin hydrolysate from etiolated hypocotyls and leaves was similar on TLC and GC-MS (data not shown). Since greater amounts of cutin could be prepared from leaves, we further analyzed only this material. After alkaline hydrolysis 70% of this cutin was not solubilized, whereas from tomato fruit cutin only 20% to 30% was alkali resistant. GC-MS of the total alkaline hydrolysate revealed as a major component 1-DDO (about 40%), in addition to some SA, PA, and two major and some minor unidentified compounds. Only low amounts of dihydroxypalmitic acid were detected. These results show that the alkaline hydrolysate of the cucumber cutin fraction mainly contains monomers capable of terminating chains in the proposed structure for cutin (Jeffree, 1996; Kolattukudy, 1996). Some polymer could be formed from the low amounts of dihydroxypalmitic acid and perhaps from the unidentified monomers. However, the high portion of alkali-resistant material, together with the unusual monomer composition, suggests that in cucumber cutin a major portion exists as “cutan.”
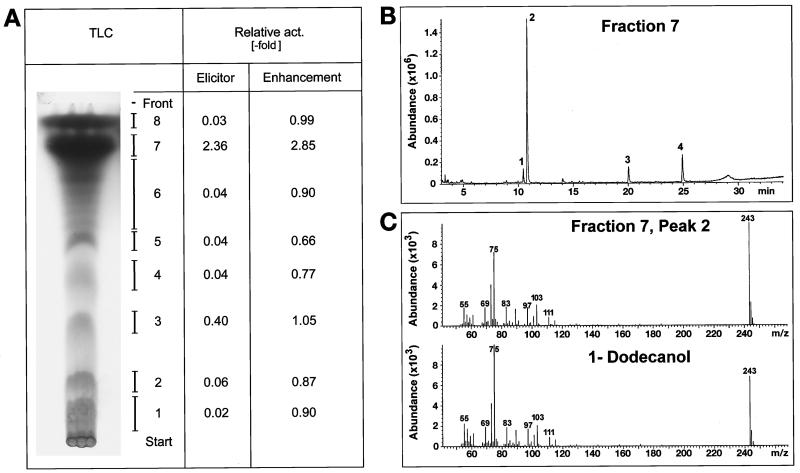
The TLC of the cucumber cutin hydrolysate revealed two major bands migrating near the solvent front, in addition to a number of minor more polar components (Fig. 3A). Most of the H2O2 elicitor and enhancer activities resided in band 7 (Fig. 3A). GC-MS analysis of this band showed that it was mainly composed of 1-DDO (Fig. 3B), a shorter-chain alkanol, which, to our knowledge, has not been reported before as a cutin constitutent. The other more polar TLC fractions were clearly less active. GC-MS analysis of these minor amounts of material was not conclusive even though some dihydroxy fatty acids were present in bands 3 and 4.
Figure 3.
Composition and biological activities of cucumber leaf cutin hydrolysate. H2O2 elicitor activity and enhancement of fungal H2O2 elicitor activity are given relative to the activity of fungal elicitor (see Fig. 1). A, Charred TLC sample. From TLC separations run in parallel, bands were eluted and solubilized at the same concentration as that present in the original hydrolysate. Since the activities of band 7 are higher compared with the values for unfractionated cucumber leaf cutin hydrolysate (Fig. 2), the active compound was obviously enriched by the TLC separation. B, GC-elution profile of TLC band 7 (peak 1 = unknown; peak 2 = 1-DDO, 75%; peak 3 = PA, 9%; and peak 4 = SA, 14%). C, Mass spectrum of peak 2 of fraction 7 and of authentic 1-DDO. The TLC bands 3 and 4 moved in the RF range of dihydroxy fatty acids but could not be satisfactorily analyzed by GC-MS even though both bands exhibited mass fragments characteristic of hydroxy fatty acids. Note that enhancement factors <1 (especially bands 2, 4, and 5) indicate that inhibitory materials might also be present.
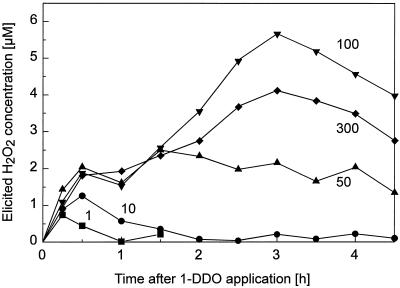
The profile of H2O2 elicitation by authentic 1-DDO was concentration dependent (Fig. 4). At low concentrations a more rapid H2O2 burst prevailed, whereas a second later phase of H2O2 production became prominent above 50 μm but decreased again above 100 μm. At 100 μm 1-DDO, neither the early nor the late H2O2 production was observed in the presence of 10 units mL−1 of catalase, indicating that the elicited product monitored with the luminol assay indeed was H2O2 (data not shown). Whether these two phases of H2O2 production result from the same mechanism has yet to be shown.
Figure 4.
Time course of H2O2 elicitation by 1-DDO at various concentrations. The results from one typical experiment are shown. In this experiment the H2O2 peak induced by 100 μm of 1-DDO was at the 3-h time point, 2.9-fold higher than at 0.5 h. In seven experiments performed similarly for over 3 months, this factor varied between 1.6- and 2.9-fold. In these experiments, the decrease between 3 and 4 h was sometimes either not observed or rather small.
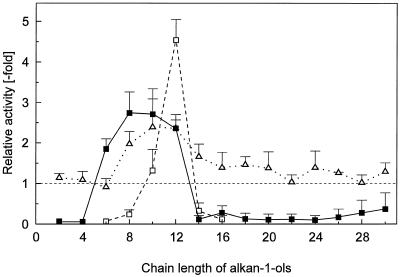
The H2O2 elicitor and enhancer activity of some commercial pure alkan-1-ols is compared in Figure 5. At a concentration of 100 μm, alkanols with a chain length between C6 and C10 exhibited mainly the early H2O2 elicitor activity (0.5 h). In contrast, the longer-lasting H2O2 burst (4 h) was almost exclusively confined to C12, the alkan-1-ol (1-DDO) naturally occurring in cucumber cutin (Fig. 3). Enhancement of fungal elicitor activity was similar in degree between C8 and C12. The alkan-1-ols with a chain length >14 were barely active (Fig. 5). It should be noted that long-chain-length alkanols were barely soluble even in warm chloroform. Thus, an emulsion had to be used as an elicitor, and presumably only a small portion of free compound was available for the epidermal cells.
Figure 5.
Specificity of various alkan-1-ols for elicitation of H2O2 and for enhancement of the H2O2 elicitation by a fungal elicitor. All activities were calculated relative to fungal elicitor (see Fig. 1). The elicitor activity was determined either 0.5 h (▪) or 4 h (□) after addition of 100 μm of the lipids. For determination of enhancement (▵), fungal elicitor was added 1.5 h after the lipids (see Fig. 1). The variability with long-chain alkanols may relate to the fact that emulsions were added as elicitor. Means ± sd from three independent experiments performed within 1 week are shown.
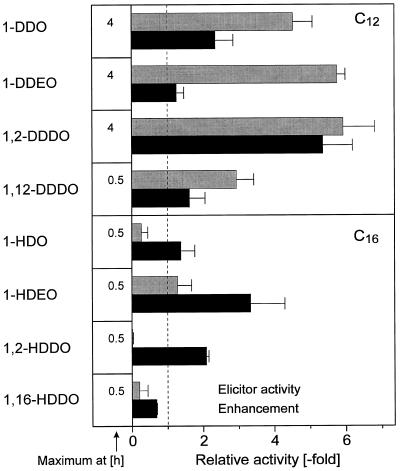
The effect of some additional features of alkanols on the two biological activities studied are shown in Figure 6. If one (1-HDEO) or two (1-DDEO) double bonds were present in the alkanols, the elicitor activity increased when compared with the respective saturated alkanols. In contrast, the two double bonds in 1-DDEO decreased, whereas the one double bond in 1-HDEO increased the enhancer activity. A second hydroxyl group on the carbon atom adjacent to that carrying the primary one (1,2-DDDO; 1, 2-HDDO) markedly increased the enhancer activity, whereas a hydroxyl group at the opposite end of the molecule caused a decrease (1,12-DDDO; 1,16-HDDO).
Figure 6.
Influence of double bonds and hydroxyl group position in some alkanols on the H2O2 elicitor activity and enhancement of fungal H2O2 elicitor. The time course of H2O2 elicitation differed for the various compounds even though all were used at the same concentration (100 μm). The maximal peak height reached at the indicated time was used, therefore, to calculate the activity relative to fungal elicitor. Means ± sd from three experiments are shown.
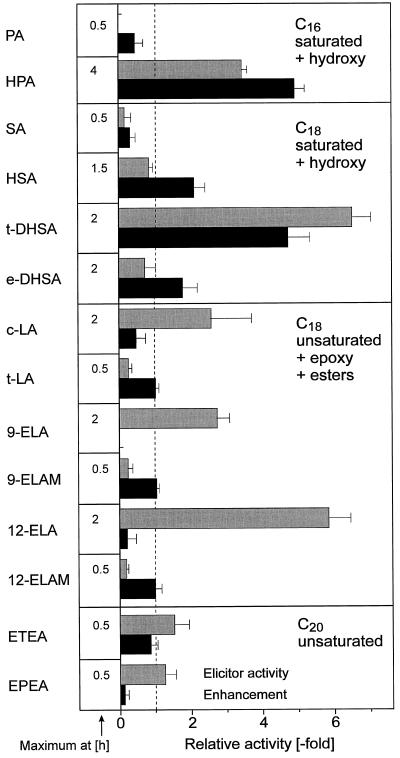
The H2O2 elicitation and elicitor-enhancement potential of some oxygenated fatty acids were also studied. Introduction of a ω-hydroxyl function in PA resulted in an excellent H2O2 elicitor (HPA), whereas one additional hydroxyl group in the middle of a fatty acid molecule resulted only in a moderately active molecule (HSA, Fig. 7). When two hydroxyl groups were present in SA, the threo-isomer (t-DHSA) was much more effective than the corresponding erythro-isomer (e-DHSA). Among the unsaturated epoxy fatty acids available, cis,cis-9-ELA exhibited higher elicitor activity than cis,cis-12-ELA, whereas the corresponding methyl esters showed low elicitor activity (Fig. 7). Two unsaturated mono- and one dihydroxy fatty acid were the most active elicitors found, whereas an unsaturated trihydroxy C18 fatty acid exhibited no activity (Table I). Of the two available isomers of linoeic acid, only the cis-cis form had elicitor activity. Both C20 polyunsaturated fatty acids were moderate H2O2 elicitors (Fig. 7).
Figure 7.
Specificity of various fatty acids and some methyl esters for elicitation of H2O2 and for enhancement of H2O2 elicitation by a fungal elicitor. Fatty acids were used at 47 μg mL−1. Elicitor activity and enhancement are given relative to the activity of the fungal elicitor (see Fig. 1). Note that enhancement values <1 indicate inhibition. The time course was different for the various compounds. The elicitor activity is given, therefore, as the highest H2O2 concentration reached at the time indicated. Means ± sd from three independent experiments are shown. c-LA, cis,cis-Linoleic acid; t-LA, trans,trans-linoleic acid; 9-ELA and 12-ELA, cis,cis-9-ELA and cis,cis-12-ELA, respectively; 9-ELAM and 12-ELAM, cis,cis-9-ELAM and cis,cis-12-ELAM, respectively.
Table I.
H2O2 elicitation and influence on the fungal elicitor activity of some unsaturated C18 hydroxy fatty acids
| Fatty Acid Added | Elicitor Activity | Enhancement |
|---|---|---|
| -folda | ||
| 9-Hydroxy-10E, 12Z-octadecadienoic | 13.6 ± 1.5 | 0 ± 0 |
| 13-Hydroxy-9Z, 11E-octadecadienoic | 12.2 ± 2.2 | 0 ± 0 |
| 9,10-threo Dihydroxy-12Z-octadecenoic | 9.4 ± 0.6 | 2.3 ± 0.3 |
| 12,13-threo Dihydroxy-9Z-octadecenoic | 4.0 ± 1.7 | 0.9 ± 0.6 |
| 9,12,13-Trihydroxy-10E-octadecenoic | 0.2 ± 0.2 | 1.1 ± 0.2 |
Activities were determined with 100 μm of the compounds and are given relative to fungal elicitor (see Fig. 7). Maximum H2O2 production was reached at 2 h for the first four and at 0.5 h for the last compound. Means ± sd from three experiments are given.
Most saturated hydroxy fatty acids (HPA, HSA, and t-DHSA) exhibited a pronounced enhancer effect, whereas PA and SA, as well as most of the unsaturated fatty acid derivatives, did not enhance but even inhibited the fungal elicitor activity (Fig. 7; Table I). The exception from this tendency is represented by ETEA (Fig. 7) and the two unsaturated dihydroxy fatty acids (Table I), one of the latter representing even a moderate enhancer (Table I).
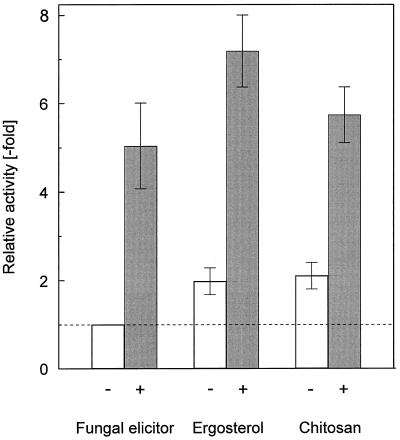
The enhancement effect of t-DHSA shown above (Figs. 1 and 7) for elicitation of H2O2 by the fungal elicitor in which a glucan appears to represent at least part of the active portion (Fauth et al., 1996) was also observed for two other H2O2 elicitors of fungal origin, namely ergosterol and chitosan (Fig. 8).
Figure 8.
Comparison of H2O2 induction by fungal elicitor, ergosterol, and chitosan as influenced by t-DHSA. H2O2 induction by fungal elicitor (10 μg mL−1) in the absence (−) or presence (+) of 100 μm t-DHSA was determined as in Figure 1, ergosterol (10 nm) or chitosan (10 μg mL−1) were used instead of fungal elicitor. Means ± sd from three independent experiments are shown. The results are given relative to the activity of fungal elicitor in the absence of t-DHSA.
DISCUSSION
Considering the production of H2O2 as an easily detectable parameter presumed to be associated with pathogen defense, the present report shows that in conditioned, abraded cucumber hypocotyls the crude cutin hydrolysates can act as H2O2 elicitors (Fig. 2). The hydrolysates from tomato and apple cutin are known to contain hydroxy fatty acids (for citations, see Schweizer et al., 1996a, 1996b) and these compounds were active in the cucumber assay (Fig. 7), suggesting that they may be the active components in these hydrolysates. In contrast, we found only comparatively low amounts of hydroxy fatty acids in cucumber leaf cutin hydrolysate, even though cucumber fruit cutin was reported to contain 8,16-dihydroxyhexadecanoic acid as a major component (Gérard et al., 1994). Most of the H2O2 elicitor and enhancer activity in the hydrolysate of cucumber leaf cutin resided in the major TLC band, which was composed mainly of 1-DDO (Fig. 3). Authentic 1-DDO was also very active as an elicitor (Figs. 4 and 5). Therefore, it appears likely that both the elicitor and enhancer activities of the crude cucumber cutin hydrolysate are mainly due to 1-DDO. This substance does not occur in a free form in the wax fraction and, thus, appears to represent a true cutin monomer, which is esterified into the polymer but does not offer further functional groups for ester or ether cross-linkages. It should be noted, however, that 1-DDO likely does not represent the only cucumber cutin monomer of potential elicitor activity. The compounds in band 3 of Figure 3 also exhibit some activity that might result from the presence of some hydroxy fatty acids. In addition, indirect evidence shows that cucumber cutin contains epoxy groups (Riederer and Schönherr, 1986). Epoxy fatty acids are active as elicitors (Fig. 7) but are at least partly destroyed by alkaline hydrolysis.
The surface wax in cucumber consists mainly of a series of long-chain alkan-1-ols and alkan-1,3-diols. This complex composition prevented separation and functional testing of individual components. In addition, alkan-1,3-diols were not commercially available. Therefore, the elicitor and enhancer activity in the total wax fraction (Fig. 2) cannot yet be attributed to individual compounds. The available pure long-chain alkan-1-ols exhibited a low level of both activities (Fig. 5). However, a second proximal hydroxyl group in other alkanols increased their enhancer activity (1,2-HDDO and 1,2-DDDO, Fig. 6). Therefore, it is possible that the newly described long-chain 1,3-diols exhibit higher activities than the corresponding 1-monools. In addition, some unsaturated fatty acids are also present in the wax and these compounds exhibit some elicitor activity (Fig. 7). Therefore, it might be that several compounds contribute to the observed low elicitor activity, as well as to the more prominent enhancer activity of the cucumber surface wax.
The structural features of oxygenated hydrocarbons that are important for H2O2 elicitation in the cucumber hypocotyl system became only partly clear with the selection of authentic compounds used in this study. In saturated fatty acids at least one hydroxyl group is required (HPA and HSA, Fig. 7) for activity, preferentially located at the ω-position (HPA > HSA). Alternatively, cis-double bonds are favorable (cis-linoleic acid, ETEA, and EPEA, Fig. 7). In this respect, our results are in agreement with the observation that arachidonic acid (ETEA) can elicit several defense responses in potato (for references, see Choi et al., 1994). Hydroxyl and epoxy groups in some cases enhance but in others decrease elicitor activity of fatty acids, depending on their number and relative position (Fig. 7; Table I). It should be noted that hydroxyl and epoxy groups, as well as double bonds in lipid molecules, have a great influence on the overall molecule shape, which, therefore, appears to be of importance for the H2O2 elicitor activity of the oxygenated hydrocarbons. Taken together, it remains questionable whether for such a broad range of active oxygenated hydrocarbons complementary receptors exist. A more likely assumption of indirect effects resulting from lipid interactions will be discussed below in the context of the enhancement effect.
Many of the oxygenated hydrocarbons, which are directly moderate or potent H2O2 elicitors, can also enhance the activity of other H2O2 elicitors (Figs. 1 and 5–7). This enhancement effect was not strictly correlated with the ability to directly cause H2O2 production as exemplified by the good enhancer 1,2-HDDO, which exhibited a rather low elicitor activity (Fig. 6). Similarly, both activities can change in opposite directions comparing related compounds (t-DHSA versus e-DHSA, Fig. 7; 1-DDO versus 1-DDEO, Fig. 6). Thus, the enhancement by oxygenated fatty acids may be due mainly to the molecule part bearing the hydroxyl functions. The above-mentioned H2O2 elicitor enhancers are lipophilic molecules and, therefore, might exert their action at the level of membrane-bound constituents of the H2O2-eliciting system. Because a comparatively broad array of compounds was found active, a possible mode of action may be a change in the lipid neighborhood of either the receptors for the three chemically different elicitors or the plasma membrane-located NAD(P)H oxidase complex assumed to be responsible for reducing O2 (Hammond-Kosack and Jones, 1996; Mehdy et al., 1996; Lamb and Dixon, 1997).
It has been demonstrated that fungal pathogens can secrete cutinases during early stages of the germination process (Coleman et al., 1993). It remains controversial and may depend on the actual case studied whether the cutinases secreted indeed represent essential fungal virulence factors (recently discussed by Kolattukudy et al., 1995; van Kan et al., 1997). Nevertheless, the presence of cutinases in the attacked surface area is likely to result in the production of free cutin monomers, and it appears likely that cutinases or similar unspecific esterases will also liberate the 1-DDO esterified to cucumber cutin. In the case of cucumber hypocotyls, which acquired resistance to C. lagenarium by root pretreatment with 2,6-dichloroisonicotinic acid, we have observed that fungal attack of the epidermal surface can induce deposition of cell wall phenolics, which possibly involves H2O2 production for polymerization (Siegrist et al., 1994). Indeed, in barley a localized H2O2 production has been shown below appressorial germ tubes and appressoria of powdery mildew (Thordal-Christensen et al., 1997). The incorporation of phenolic esters into the cell wall and the expression of apoplastic chitinase in epidermal cells of resistant cucumber hypocotyls attacked by C. lagenarium are both prepenetration events (Siegrist et al., 1994; Kästner et al., 1998). These two early defense responses possibly may relate to the localized action of cutinases that were shown to be produced by C. lagenarium (Bonnen and Hammerschmidt, 1989; Huang and Kuc, 1995). Even if putatively liberated cutin monomers do not turn out to be the actual elicitors for the above-cited two early defense responses, cutin monomers could exert their action by enhancing the activity of other unknown fungal elicitors possibly diffusing through the corroded cuticle. Alternatively, such an enhancement could occur by components of the surface wax layer, which may become mobilized in the course of spore adhesion and/or cuticle corrosion by cutinase.
ACKNOWLEDGMENTS
We would like to thank Raimund Tenhaken and Uwe Conrath for stimulating discussions.
Abbreviations:
- 1-DDEO
8,10-dodecadien-1-ol
- 1-DDO
1-dodecanol
- 1-HDEO
cis-9-hexadecen-1-ol
- DDDO (1,2- or 1,12-)
dodecandiol
- e-DHSA
erythro-9,10-dihydroxystearic acid
- ELA
epoxylinoleic acid
- ELAM
ELA methyl ester
- EPEA
all-cis-5,8,11,14,17-eicosapentaenoic acid
- ETEA
all-cis-5,8,11,14-eicosatetraenoic or arachidonic acid
- HDDO (1,2- or 1,16-)
hexadecandiol
- HPA
16-hydroxypalmitic acid
- HSA
12-hydroxystearic acid
- PA
palmitic acid
- SA
stearic acid
- t-DHSA
threo-9,10-dihydroxystearic acid
Footnotes
This work was supported by the Deutsche Forschungsgemeinschaft and Fonds der Chemischen Industrie.
LITERATURE CITED
- Bonnen AM, Hammerschmidt R. Role of cutinolytic enzymes in infection of cucumber by Colletotrichum lagenarium. Physiol Mol Plant Pathol. 1989;137:475–481. [Google Scholar]
- Choi D, Bostock RM, Avdiushko S, Hildebrand DF. Lipid-derived signals that discrimate wound- and pathogen-responsive isoprenoid pathways in plants: methyl jasmonate and the fungal elicitor arachidonic acid induce different 3-hydroxy-3-methylglutaryl-coenzyme A reductase genes and antimicrobial isoprenoids in Solanum tuberosum L. Proc Natl Acad Sci USA. 1994;91:2329–2333. doi: 10.1073/pnas.91.6.2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman JOD, Hiscock SJ, Dewey FM. Monoclonal antibodies to purified cutinase from Fusarium solani f. sp. pisi. Physiol Mol Plant Pathol. 1993;43:391–401. [Google Scholar]
- Fauth M, Merten A, Hahn MG, Jeblick W, Kauss H. Competence for elicitation of H2O2 in hypocotyls of cucumber is induced by breaching the cuticle and is enhanced by salicylic acid. Plant Physiol. 1996;110:347–354. doi: 10.1104/pp.110.2.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis SA, Dewey FM, Gurr SJ. The role of cutinase in germling development and infection by Erysiphe graminis f. sp. hordei. Physiol Mol Plant Pathol. 1996;49:201–211. [Google Scholar]
- Gérard JC, Pfeffer PE, Osman SF. 8,16-Dihydroxyhexadecanoic acid, a major component from cucumber cutin. Phytochemistry. 1994;35:818–819. [Google Scholar]
- Gilbert RD, Johnson AM, Dean RA. Chemical signals responsible for appressorium formation in the rice blast fungus Magnaporthe grisea. Physiol Mol Plant Pathol. 1996;48:335–346. [Google Scholar]
- Hammond-Kosack KE, Jones JDG. Resistance gene-dependent plant defense responses. Plant Cell. 1996;8:1773–1791. doi: 10.1105/tpc.8.10.1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Q, Kuc J. Cutin, cutinase and esterase as related to the induced systemic resistance of cucumber against Colletotrichum lagenarium. Physiol Mol Plant Pathol. 1995;46:215–226. [Google Scholar]
- Jeffree CE. Structure and ontogeny of plant cuticles. In: Kerstiens G, editor. Plant Cuticles: an Integrated Functional Approach. Oxford, UK: Bios Scientific Publishers; 1996. pp. 33–82. [Google Scholar]
- Kästner B, Tenhaken R, Kauss H. Chitinase in cucumber hypocotyls is induced by germinating fungal spores and by fungal elicitor in synergism with inducers of acquired resistance. Plant J. 1998;13:447–454. [Google Scholar]
- Kato T, Yamaguchi Y, Namai T, Hirukawa T. Oxygenated fatty acids with anti-rice blast fungus activity in rice plants. Biosci Biotech Biochem. 1993;57:283–287. doi: 10.1271/bbb.57.283. [DOI] [PubMed] [Google Scholar]
- Kauss H, Jeblick W. Influence of salicylic acid on the induction of competence for H2O2 elicitation. Comparison of ergosterol with other elicitors. Plant Physiol. 1996;111:755–763. doi: 10.1104/pp.111.3.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauss H, Jeblick W, Domard A, Siegrist J (1997) Partial acetylation of chitosan and a conditioning period are essential for elicitation of H2O2 in surface-abraded tissues from various plants. In A Domard, GAF Roberts, KM Vårum, eds, Advances in Chitin Science, Vol II. J. André Publisher, Lyon, France, pp 94–101
- Kolattukudy PE. Biosynthetic pathways of cutin and waxes, and their sensitivity to environmental stresses. In: Kerstiens G, editor. Plant Cuticles: an Integrated Functional Approach. Oxford, UK: Bios Scientific Publishers; 1996. pp. 83–108. [Google Scholar]
- Kolattukudy PE, Rogers LM, Li D, Hwang C-S, Flaishman MA. Surface signaling in pathogenesis. Proc Natl Acad Sci USA. 1995;92:4080–4087. doi: 10.1073/pnas.92.10.4080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb C, Dixon RA. The oxidative burst in plant disease resistance. Annu Rev Plant Physiol Plant Mol Biol. 1997;48:251–275. doi: 10.1146/annurev.arplant.48.1.251. [DOI] [PubMed] [Google Scholar]
- Mehdy MC, Sharma YK, Sathasivan K, Bays NW. The role of activated oxygen species in plant disease resistance. Physiol Plant. 1996;98:365–374. [Google Scholar]
- Namai T, Kato T, Yamaguchi Y, Hirukawa T. Anti-rice blast activity and resistance induction of C-18 oxygenated fatty acids. Biosci Biotech Biochem. 1993;57:611–613. doi: 10.1271/bbb.57.283. [DOI] [PubMed] [Google Scholar]
- Riederer M, Markstädter C. Cuticular waxes: a critical assessment of current knowledge. In: Kerstiens G, editor. Plant Cuticles: an Integrated Functional Approach. Oxford, UK: Bios Scientific Publishers; 1996. pp. 189–200. [Google Scholar]
- Riederer M, Schönherr J. Covalent binding of chlorophenoxyacetic acids to plant cuticles. Arch Environ Contam Toxicol. 1986;15:97–105. [Google Scholar]
- Schweizer P, Felix G, Buchala A, Müller C, Métraux J-P. Perception of free cutin monomers by plant cells. Plant J. 1996a;10:331–341. [Google Scholar]
- Schweizer P, Jeanguénat A, Mösinger E, Métraux J-P. Plant protection by free cutin monomers in two cereal pathosystems. In: Daniels MJ, Downie JA, Osbourn AE, editors. Advances in Molecular Genetics of Plant-Microbe Interactions. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1994. pp. 371–374. [Google Scholar]
- Schweizer P, Jeanguénat A, Whitacre D, Métraux J-P, Mösinger E. Induction of resistance in barley against Erysiphe graminis f. sp. hordei by free cutin monomers. Physiol Mol Plant Pathol. 1996b;49:103–120. [Google Scholar]
- Siegrist J, Jeblick W, Kauss H. Defense responses in infected and elicited cucumber (Cucumis sativus L.) hypocotyl segments exhibiting acquired resistance. Plant Physiol. 1994;105:1365–1374. doi: 10.1104/pp.105.4.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thordal-Christensen H, Zhank Z, Wei Y, Collinge DB. Subcellular localization of H2O2 in plants: H2O2 accumulation in papillae and hypersensitive response during the barley-powdery mildew interaction. Plant J. 1997;11:1187–1194. [Google Scholar]
- van Kan JAL, van't Klooster JW, Wagemakers CAM, Dees DCT, van der Vlugt-Bergmans CJB. Cutinase A of Botrytis cinerea is expressed, but not essential, during penetration of gerbera and tomato. Mol Plant-Microbe Interact. 1997;10:30–38. doi: 10.1094/MPMI.1997.10.1.30. [DOI] [PubMed] [Google Scholar]